Abstract
The contemporary neural understanding of motivation is based almost exclusively on the neural mechanisms of incentive motivation. Recognizing this as a limitation, we used event-related functional magnetic resonance imaging (fMRI) to pursue the viability of expanding the neural understanding of motivation by initiating a pioneering study of intrinsic motivation by scanning participants’ neural activity when they decided to act for intrinsic reasons versus when they decided to act for extrinsic reasons. As expected, intrinsic reasons for acting more recruited insular cortex activity while extrinsic reasons for acting more recruited posterior cingulate cortex (PCC) activity. The results demonstrate that engagement decisions based on intrinsic motivation are more determined by weighing the presence of spontaneous self-satisfactions such as interest and enjoyment while engagement decisions based on extrinsic motivation are more determined by weighing socially-acquired stored values as to whether the environmental incentive is attractive enough to warrant action.
Introduction
Motivation concerns the reasons why people do what they do. One primary reason people act is because they expect doing so will bring an attractive consequence. For instance, they work on a project because they anticipate receiving money once the project has been completed. Hence, the reason for working on the project is to attain an attractive contingent reward. How extrinsic reasons energize and direct behavior is well understood in neuroscience research under the heading of incentive motivation (Berridge, 2004; Cardinal et al., 2002; Mogenson et al., 1980). Incentive-based reasons for action are associated with neuronal responses in the (a) amygdala and striatum that process the rewarding properties of directly experienced environmental stimuli and (b) dorsolateral prefrontal cortex, orbitofrontal cortex, and cingulate cortex that process the learned reinforcement value of various environmental stimuli (Hampton and O’Doherty, 2007; Hayden et al., 2008; McClure et al., 2004; O’Doherty, 2004; Schultz, 2000). But there is a second primary reason why people act—namely, because they expect doing so will bring spontaneously satisfying experiences. For instance, they work on a project because that project is able to generate in them feelings of interest and enjoyment. Hence, the reason for working on the project is to feel interest and to enjoy doing the activity for its own sake. How intrinsic reasons for action energize and direct behavior is poorly understood in neuroscience research1.
The purpose of the present study was to pursue the viability of expanding the contemporary neural understanding of motivation beyond an exclusive focus on incentive motivation by initiating a pioneering study of intrinsic motivation. Intrinsic motivation is the inherent desire to engage one’s interests, to explore, and to exercise one’s capacities and, in doing so, to seek out and master optimal challenges (Deci and Ryan, 1985; Ryan and Deci, 2000). When intrinsically motivated, people act out of personal interest and because they find the task at hand to be inherently enjoyable and capable of producing spontaneous self-satisfactions such as “That’s interesting” and “I enjoy it”. Social and educational psychologists argue that action energized and directed by intrinsic reasons such as intrinsic motivation is qualitatively different from action energized and directed by extrinsic reasons (Ryan and Deci, 2000). This means that intrinsic motivation is a fundamentally different type of motivation than is incentive motivation. Intrinsic motivation is an inherent and task-endogenous type of motivation, one that orients people toward an activity because of the anticipation of experiencing spontaneous self-satisfactions during task engagement. Extrinsic motivation (e.g., incentive-based motivation), on the other hand, is an acquired and task-exogenous type of motivation that orients people toward an activity because they have learned that its engagement in the past has been associated with an attractive but separate environmental consequence (Deci and Ryan, 1985). The impetus for the present study was that virtually all contemporary neuroimaging investigations exclude inherent and task-endogenous types of motivational concepts in their conceptual understanding of the nature of motivation.
Our research strategy was to scan participants’ neural activity when they decided to act on a task for intrinsic reasons versus when they decided to act on the same task but for extrinsic reasons using event-related functional magnetic resonance imaging (fMRI). Our prediction for the neural bases of extrinsic motivation (i.e., incentive motivation) is not a novel one. Instead, it reflects the well-established findings that the valuation system, such as ventromedial prefrontal cortical activity (e.g., the orbitofrontal cortex; OFC) and anterior and posterior cingulate cortical activity, would be more recruited by decision making based on weighing attractive extrinsic reasons to act (Bray et al., 2010; Britton et al., 2006; Hayden et al., 2008; Maddock et al., 2003; Plassmann et al., 2007). Our prediction for the neural bases of intrinsic motivation, however, is a novel one. It represents a key open question in the study of affective neuroscience. As people become aware of how a task affects their subjective feelings—as they formulate a conscious experience of “my feelings about that thing”—they show greater insular cortex activity (Craig, 2009, p. 65). Hence, we predicted that the insular cortex would be more recruited by decision making based on weighing inherent feelings that serve as intrinsic reasons to act. We expected to observe greater insular cortex activity as people weighed their intrinsic reasons to act because insular cortex activity is related to practically all inherent feelings (Craig, 2009) but it is particularly related to feelings of inherent need satisfactions (Cardinal et al., 2002; Naqvi et al., 2007; Singer et al., 2009). Feelings that arise from the satisfaction versus frustration of need states are important in the social psychological study of intrinsic motivation because feelings of interest and enjoyment are said to arise as spontaneous satisfactions from the psychological needs for autonomy and competence during one’s interactions with the environment (as proposed by self-determination theory; Deci and Ryan, 2000; Ryan and Deci, 2000).
Method
Participants
Ten undergraduates (6 females and 4 males; mean age: 19.7 ± 0.87), who were recruited from introductory educational psychology classes at the University of Iowa, participated. They were neurologically healthy, right-handed, native English speakers who had normal or corrected-to-normal vision. All participants provided informed consent in accordance with the regulations of the Institutional Review Board of the University of Iowa.
Task and Procedure
In this study, phrases were used to describe situations from the following three conditions: intrinsic motivation, extrinsic motivation, and a neutral condition. The phrases were developed based on self-determination theory’s conceptual and operational definitions of intrinsic motivation and extrinsic motivation (Ryan and Deci; 2000; see Table 1). Sixty familiar situations (e.g., writing a paper, working on a computer, participating in a project) were specified and three different reasons for doing each task were inserted to characterize the activity as motivated by the type of motivation unique to the experimental condition. In the intrinsic motivation condition, the phrases described situations that motivate people due to internal causalities, such as interest or enjoyment (e.g., writing an enjoyable paper, working on the computer out of curiosity, participating in a fun project). In the extrinsic motivation condition, the phrases described situations that motivate people due to attractive extrinsic incentives (e.g., writing an extra-credit paper, working on the computer for bonus points, participating in a money-making project). In the neutral condition (a control comparison), the phrases described neutral situations that mildly unmotivate people or at least fail to generate intrinsic motivation or extrinsic motivation (e.g., writing an assigned paper, working on the computer to meet a deadline, participating in a required project). The neutral phrases functioned as filler items to avoid participants’ skewed “yes” responses. The 60 sets of phrases were selected from a larger pool of 90 sets of phrases based on a pilot test in which a separate group of participants rated the phrases using a computer presentation. The phrases across the three conditions were matched not only in the situation depicted but also in terms of sentence structure and number of words.
Table 1.
Examples of phrases from three experimental conditions used in the experimental task
| Intrinsic motivation phrases | Extrinsic motivation phrases | Neutral phrases |
|---|---|---|
| Writing an enjoyable paper | Writing an extra-credit paper | Writing an assigned paper |
| Working on the computer out of curiosity | Working on the computer for bonus points | Working on the computer to meet a deadline |
| Participating in a fun project | Participating in a money-making project | Participating in a required project |
| Pursuing my personal interests in class | Pursuing an attractive reward in class | Pursuing a routine task in class |
| Working with freedom | Working for incentives | Working with pressure |
| Having options and choices | Having prizes and awards | Having pressures and obligations |
| Working because its fun | Working because I want money | Working because I have to |
| Feeling interested | Anticipating a prize | Feeling frustrated |
An event-related fMRI experiment, which consisted of three runs, was performed. Each run lasted 10 minutes and consisted of 60 trials, which were taken randomly from each of the three conditions (20 trials per condition) and presented in a random order. In each trial (see Fig. 1), a phrase was presented for three seconds to describe a situation related to one of the three conditions. During those three seconds, participants were asked to read the phrase and make a decision, “Do you want to do this?”—yes or no?, by pressing the left button with the forefinger (for yes) or the right button with the middle finger (for no). Following this response, there was a jitter of 2–12 seconds (mean = 7 seconds) between each trial. Then, the next trial began, which presented a phrase describing another one of the three conditions.
Figure 1.
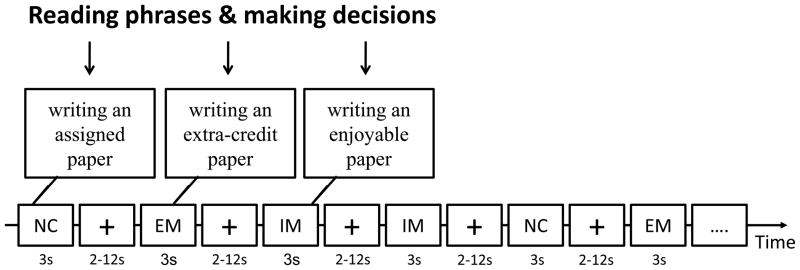
The experimental task and the experimental design are presented. 180 phrases (60 sets of phrases depicting the same situation) were randomly presented. During a three-second presentation of each phrase, participants were asked to make a decision. Between phrases, there were jitters which were randomized from 2 to 12 seconds. Note. IM: intrinsic motivation; EM: extrinsic motivation; NC: neutral condition.
During the experimental session, participants first received the task instruction and practiced the experimental task outside the scanner before performing the real task during the brain image scans. Participants’ anatomic images were first acquired and then functional images were scanned while participants performed the experimental task. After the brain imaging, participants were debriefed about the experiment and received compensation for their participation.
fMRI Data Acquisition
Imaging was performed with a 3T Trio MRI scanner (Siemens, Erlangen, Germany). First, T1-weighted anatomic images (TR = 1590 ms, TE = 3.58 ms, flip angle = 10°, FOV = 256 × 256, and slice thickness = 2 mm) were acquired for anatomical localization using a MP-RAGE sequence in order to facilitate the precise determination of the structures corresponding to the functional activation foci. After obtaining anatomic images, 16-slice functional images were acquired using a T2*-weighted gradient-echo echo planar imaging (EPI) sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 220 × 220, 64 × 64 matrix, and slice thickness = 5 mm with 1mm gap).
fMRI Data Analysis
Imaging preprocessing, individual analyses, and group analyses were performed using AFNI (Cox, 1996; http://afni.nimh.nih.gov). The first eight images of each run were discarded to allow hemodynamics and MRI signals to reach a steady state. In preprocessing, the functional images were temporally realigned for timing correction and spatially realigned for head motion correction. These temporally and spatially realigned brain images were spatially smoothed with a Gaussian kernel of 4 mm full-width at half-maximum (FWHM). After the values of background voxels (i.e., voxels outside the brain) were excluded, these time-series data were scaled as a percent of the mean for running future statistical analyses. The functional images of each run were separately preprocessed, and then the three runs of each participant were concatenated before individual analyses.
In individual analyses, the time-series data were analyzed by a general linear model (GLM) using nine regressors of individual participants which were convoluted with hemodynamic response functions (HRF). Three regressors were for the time points that individual participants made decisions in experimental conditions (i.e., intrinsic motivation, extrinsic motivation, neutral condition) and the six regressors were for head motion parameters of individual participants which were included as covariates to partial out the effects of head motion artifacts. Responses inconsistent with our intended manipulation (i.e., unmotivated (no) responses for intrinsic motivation and incentive motivation phrases) were discarded in the further analyses2. For the group analyses, each individual’s statistical data were transformed to MNI space using each individual’s standardized high-resolution anatomic images and were resampled to 2 × 2 × 2 mm3 voxels.
In the group analyses, subtraction analyses were performed to examine the neural differences between the two different types of motivation (intrinsic motivation vs. extrinsic motivation). For correcting multiple comparison inferences in whole brain analyses, the cluster-wise threshold was employed based on Monte-Carlo simulations (Forman et al., 1995), which set a p value of .043 determined by a conjoined voxel-wise threshold (p < .005), a connectivity radius of 2.0 mm, and a minimum volume of 272 mm3 (34 contiguous voxels). The significant activations for these subtraction analyses were reported by Talairach coordinates (Talairach and Toumoux, 1988) after the MNI coordinates converted to the Talairach space by using a mni2tal algorithm (Lacadie et al., 2008). In order to confirm the neural difference results from the subtraction analyses, time-series BOLD signal changes of regions of interests (ROIs), which were set from the subtraction analyses, were compared between the two different types of motivation.
Results
Behavioral Results
Participants’ yes/no finger-press responses to the action question (Do you want to do this?) served as a behavioral indicator of approach-based motivated action. The mean percentages and the standard errors of participants’ motivated (yes) responses for phrases of the intrinsic motivation and extrinsic motivation conditions were 92.4 ± 2.09 % and 85.8 ± 5.52 % respectively, which were not significantly different from each other, but only 23.8 ± 3.24 % for phrases in the neutral condition, which were significantly lower than both experimental conditions, F(2,8) = 444.42, p < .05, with neutral condition < intrinsic motivation = extrinsic motivation, using Student-Newman-Keuls post hoc tests. Participants’ responses were therefore consistent with the experimental manipulation—approach-oriented motivated responses for the intrinsic motivation and extrinsic motivation phrases and unmotivated responses for the neutral phrases—thereby confirming that the experimental manipulation was successful.
Means and standard errors for the reaction time (RT) responses for phrases of the intrinsic motivation, extrinsic motivation, and neutral conditions were 1576.7 ± 62.5 ms, 1870.5 ± 72.0 ms, and 1791.6 ± 63.1 ms respectively. Results revealed that participants showed significantly shorter RTs in the intrinsic motivation condition than in both the extrinsic motivation and neutral conditions, F(2,8) = 48.10, p < .05, with intrinsic motivation < extrinsic motivation = neutral condition, using Student-Newman-Keuls post hoc tests.
fMRI Results
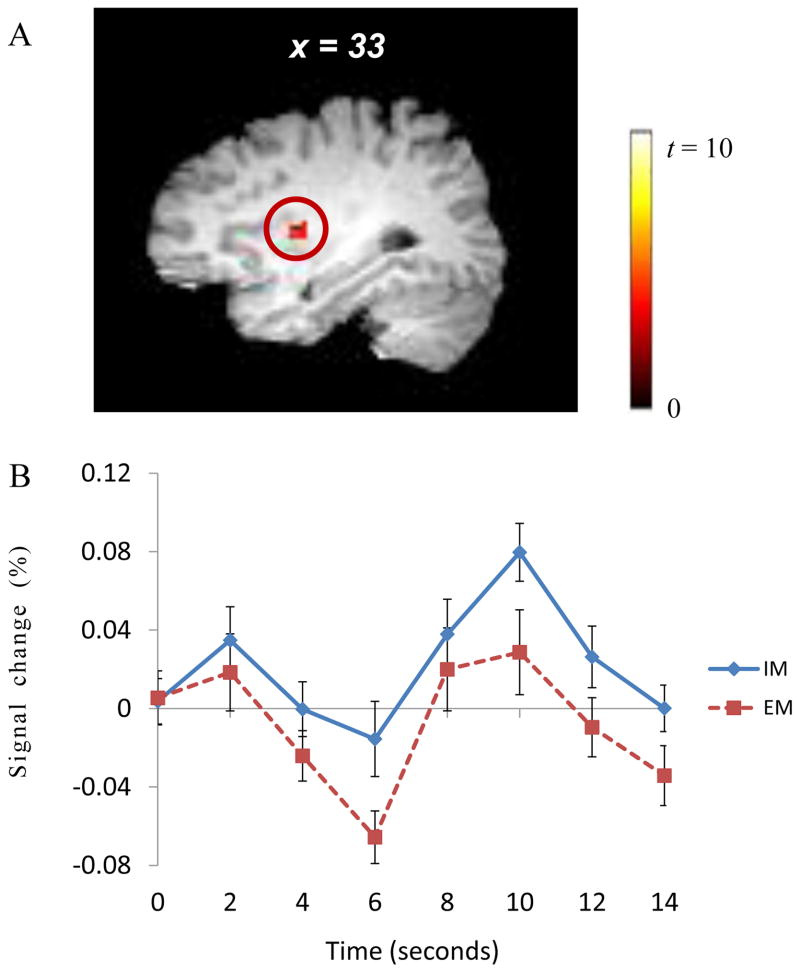
Results from the subtraction analyses between the intrinsic motivation and extrinsic motivation conditions showed that the right insular cortex was more activated in the intrinsic motivation condition than in the extrinsic motivation condition (peak activations: 33, −2, 9; maximum t = 5.88; volume: 632 mm3; corrected p < .043; Fig. 2. A). We also extracted time-series BOLD signal change patterns of this right insular cortex activity between the intrinsic motivation and extrinsic motivation conditions, and these data also showed increased activations in the intrinsic motivation condition that were consistent with the results of the subtraction analysis (see Fig. 2. B).
Figure 2.
The insular cortex was more activated in the intrinsic motivation condition than in the extrinsic motivation condition (A). The time-series BOLD signal changes of the insular cortex are presented (B). Note. IM: intrinsic motivation; EM: extrinsic motivation. The time 0 means the time point of decision making.
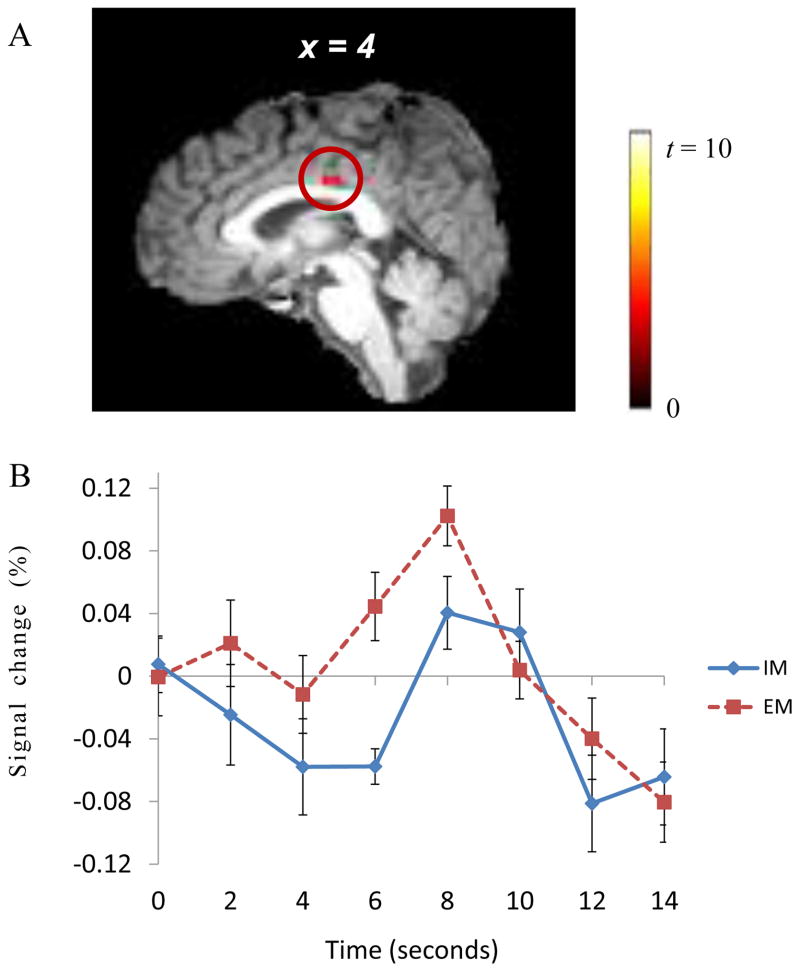
In contrast, the extrinsic motivation condition showed greater neural activity of the right posterior cingulate cortex (PCC), which is a brain region of the valuation system, than did the intrinsic motivation condition (peak activations: 4, −15, 29; maximum t = 5.20; volume: 328 mm3; corrected p < .043; Fig. 3. A). We again extracted time-series BOLD signal change patterns of this right PCC activity between the intrinsic motivation and extrinsic motivation conditions, and these data also showed increased activations in the extrinsic motivation condition that were consistent with the results of the subtraction analysis (see Fig. 3. B).
Figure 3.
The posterior cingulate cortex (PCC) was more activated in the extrinsic motivation condition than in the intrinsic motivation condition (A). The time-series BOLD signal changes of the PCC are presented (B). Note. IM: intrinsic motivation; EM: extrinsic motivation. The time 0 means the time point of decision making.
Discussion
The purpose of this study was to answer the new question of “Are the neural bases of intrinsic motivation different from the neural bases of extrinsic motivation?” To address this question, we identified neural differences as people made decisions whether to engage in familiar activities but for the very different reasons that related either to intrinsic motivation or to extrinsic motivation. In doing so, we sought to provide the evidence necessary to extend the neuroscientific conception of motivation beyond an exclusive focus on extrinsic motivation to include intrinsic motivation as well.
Participants recruited different patterns of neural activity during the decision making process depending on intrinsic versus extrinsic reasons for doing. The insular cortex was more recruited during the decision making process weighing intrinsic reasons for doing. The general function of the insular cortex is emotional processing (Craig, 2009; Damasio et al., 2000; Pessoa, 2008; Phan et al., 2002). In studies on decision making, insular cortex activity has been frequently observed, which suggests that emotional processing influences decision making (Bechara and Damasio, 2005; Damasio, 1999). In addition, in studies on addiction and craving, the insular cortex is suggested to be associated with hedonic feelings generated by bodily need satisfactions (Brody et al., 2002; Goldstein et al., 2009; Naqvi et al., 2007; Pelchat et al., 2004). In contrast, the PCC was more recruited during the decision making process weighing extrinsic reasons for doing. The PCC has been consistently reported to be activated in studies on decision making, particularly in studies on reward-based decision making (Fujiwara et al., 2009; McCoy et al., 2003; Smith et al., 2009). This PCC activity is generally interpreted as weighing the stored (learned) value of external stimuli (Hayden et al., 2008; Maddock et al., 2003). Within the valuation system, the PCC is particularly known to be related to subjective value informed by social knowledge (Johnson et al., 2006; Schiller et al., 2009).
Based on these results, we can infer that, in this study, participants in the intrinsic motivation condition decided that they wanted to engage in the activities based on the presence of spontaneous self-satisfactions (e.g., enjoyment, interest, feeling free), while participants in the extrinsic motivation condition decided that they wanted to engage in the activities based on socially-acquired values (e.g., incentive, extra-credit, prize). These inferences are supported by the RT results showing that participants engaged in faster responses to the intrinsic motivation phrases than to the extrinsic motivation phrases. This supports the interpretation that participants made relatively quick “gut felt” decisions about intrinsic reasons for acting while they made calculated cost-benefit decisions (e.g., is this consequence attractive enough to be worth the effort?) about extrinsic reasons for acting (Bechara and Damasio, 2005).
Intrinsic motivation theorists propose that human motivation is not singular (Ryan and Deci, 2000). They argue that qualitatively different types of motivation exist. In particular, they distinguish intrinsic motivation, which is generated by inherent processes, from extrinsic motivation, which is generated through environmental contingencies (Deci and Ryan, 1985). Neural evidence from the present study supports these assumptions. When participants in the present study imagined the intrinsic motivation situations, they were assumed to decide to engage in the situations based on their inherent-feeling need satisfaction processing. This is an important point, because intrinsic motivation theorists define intrinsic motivation as that which arises from the satisfaction of inherent psychological needs (for autonomy and competence; Ryan and Deci, 2000). If the situations were perceived as inherently need satisfying, positive feelings led participants to freely want to approach the described situation. In contrast, when participants imagined the extrinsic motivation situations, they made their decision to engage in the situations based on the learned value of whether the offered environmental incentive was attractive (i.e., “valued”) enough benefit to warrant action. This means that intrinsic motivation is produced more by the presence of endogenous positive feelings, which emanate out of the intuitive processing of spontaneously experienced self-satisfactions (following Ryan & Deci, 2000), while extrinsic motivation is produced more by the environmentally-associated benefits that task engagement is expected to generate, which emanate out of the processing of stored values and environmental contingencies (following Bray et al., 2010). Now that the present study has confirmed neural differences between intrinsic versus extrinsic reasons for doing, we encourage future research to investigate the neural activity occurring during different intrinsic and extrinsic reasons for doing.
Acknowledgments
This research was supported by the NIH grant awarded to Jinhu Xiong (Grant no. 1 R21 MH 082187-01A1) and also, for Johnmarshall Reeve, by the WCU (World Class University) Program funded by the Korean Ministry of Education, Science and Technology, consigned to the Korea Science and Engineering Foundation (Grant no. R32-2008-000-20023-0).
Footnotes
Some neuroscience studies have examined the concept of intrinsic motivation in their investigations, but these investigations have only discovered the shared reward-related neural bases between intrinsic motivation and incentive motivation (Kang et al., 2009; Mizuno et al., 2008; Murayama et al., 2010).
We recognize that discarding participants’ responses that were inconsistent with the intended experimental manipulation might produce a data-driven bias within the findings. Recognizing this, we also analyzed the data after pooling all participant responses—those consistent and those inconsistent with the intended experimental manipulation. Results with consistent and inconsistent responses showed the same neural activation patterns as did the results reported in the “fMRI Results” section that excluded participants’ inconsistent responses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games Econ Behav. 2005;52:336–372. [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bray S, Shimojo S, O’Doherty JP. Human medial orbitofrontal cortex is recruited during experience of imagined and real rewards. J Neurophysiol. 2010;103:2506–2512. doi: 10.1152/jn.01030.2009. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: an fMRI study. Neuroimage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Brody A, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LB, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiat. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav R. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The feeling of what happens: Body and emotion in the making of consciousness. Harcourt Brace; New York: 1999. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behavior. Plenum; New York: 1985. [Google Scholar]
- Deci EL, Ryan RM. The ‘what’ and ‘why’ of goal pursuits: Human needs and the self-determination of behavior. Psychol Inq. 2000;11:227–268. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnet Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fujiwara J, Tobler PN, Taira M, Iijima T, Tsutsui KI. Segregated and integrated coding of reward and punishment in the cingulated cortex. J Neurophysiol. 2009;101:3284–3293. doi: 10.1152/jn.90909.2008. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton AN, O’Doherty JP. Decoding the neural substrates of reward-related decision making with functional MRI. P Natl Acad Sci USA. 2007;104:1377–1382. doi: 10.1073/pnas.0606297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Nair AC, McCoy AN, Platt ML. Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008;60:19–25. doi: 10.1016/j.neuron.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc Cogn Affect Neurosci. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MJ, Hsu M, Krajbich IM, Loewenstein G, McClure SM, Wang JT, Camerer CF. The wick in the candle of learning: epistemic curiosity activates reward circuitry and enhances memory. Psychol Sci. 2009;20:963–973. doi: 10.1111/j.1467-9280.2009.02402.x. [DOI] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using nonlinear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AN, Crowley JC, Haghighian G, Dean HL, Platt ML. Saccade reward signals in posterior cingulate cortex. Neuron. 2003;40:1031–1040. doi: 10.1016/s0896-6273(03)00719-0. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in human: the modern role of fMRI. Neurosceintist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Tanaka M, Ishii A, Tanabe HC, Onoe H, Sadato N, Watanabe Y. The neural basis of academic achievement motivation. Neuroimage. 2008;42:369–378. doi: 10.1016/j.neuroimage.2008.04.253. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Murayama K, Matsumoto M, Izuma K, Matsumoto K. Neural basis of the undermining effect of monetary reward on intrinsic motivation. P Natl Acad Sci USA. 2010;107:20911–20916. doi: 10.1073/pnas.1013305107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nature Neurosci. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy, and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Smith BW, Mitchell DG, Hardin MG, Jazbec S, Fridberg D, Blair RJ, Ernst M. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;44:600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical; New York: 1988. [Google Scholar]