Introduction
Since it’s discovery as an effector of PI 3-K (phosphoinositide 3-kinase) (Franke et al., 1995), the serine/threonine kinase Akt (also known as PKB, protein kinase B) has emerged as a critical signal transducer of oncogenic signals in virtually all human solid tumors as well as hematological malignancies. Most cancers display elevated Akt activity and this is achieved by growth factor signaling or through oncogenic mutations in the PI 3-K pathway. In this context, the PI 3-K/Akt pathway has received considerable attention from a therapeutic perspective since proteins that regulate or transduce the PI 3-K signal harbor some of the most frequent genetic lesions in human cancers, including activating mutations in oncogenes as well as LOH (loss of heterozygosity) in tumor suppressors (Engelman, 2009). Similarly, activating oncogenic mutations in the Akt genes have recently been described in various human solid tumors, and small molecule Akt inhibitors are currently being evaluated in clinical trials (Carpten et al., 2007). Akt mediates downstream signaling by phosphorylating substrate proteins that in turn initiate secondary pathways that modulate numerous phenotypes associated with malignancy, including cellular proliferation, evasion from apoptosis, invasive migration, angiogenesis and metabolic reprogramming. Close to 200 Akt substrate proteins have been uncovered either by candidate screening approaches or by whole phospho-proteome mass spectrometry sequencing technologies (Manning and Cantley, 2002, 2007; Moritz et al., 2010). The challenge remains to ascribe a particular cellular function of each identified substrate to a distinct cellular function and its relevance in human pathophysiology.
Importantly, there exist three Akt isoforms in humans, Akt1, Akt2 and Akt3, that are derived from distinct genes (Akt1/PKBα, AKT1; Akt2/PKBβ, AKT2; Akt3/PKBγ, AKT3). Recent studies have clearly demonstrated that rather than functioning in cellular signaling in a redundant manner, Akt isoforms have very distinct functions in specific cell lineages with important consequences for cellular physiology. Specific functions of Akt isoforms appear not to be simply due to differential expression patterns or activation profiles, since all three proteins are expressed in virtually all cells and tissues. Similarly, in most examined cells and cancerous tissues, all three Akt isoforms appear to be hyperactive as a result of oncogenic activation of PI 3-K, and therefore any differential, non-redundant signaling via specific Akt isoforms must be derived from more complex mechanisms that result in regulation of a specific isoform. Understanding the precise mechanism(s) that result in activation and signal relay through specific Akt isoforms is of critical importance as it is predicted to have profound consequences for targeted therapy in the PI 3-K/Akt pathway in cancer and other pathophysiologies. Numerous reviews have focused on the details of the mechanisms leading to activation of PI 3-K and in turn regulation of Akt (Engelman et al., 2006; Mora et al., 2004; Vanhaesebroeck and Alessi, 2000). Here, I will focus on the differential functions of Akt isoforms in cancer cell signaling and review current efforts aimed at identifying mechanisms by which Akt isoforms contribute to malignancy in specific, rather than general, settings.
Mechanisms of Akt Activation
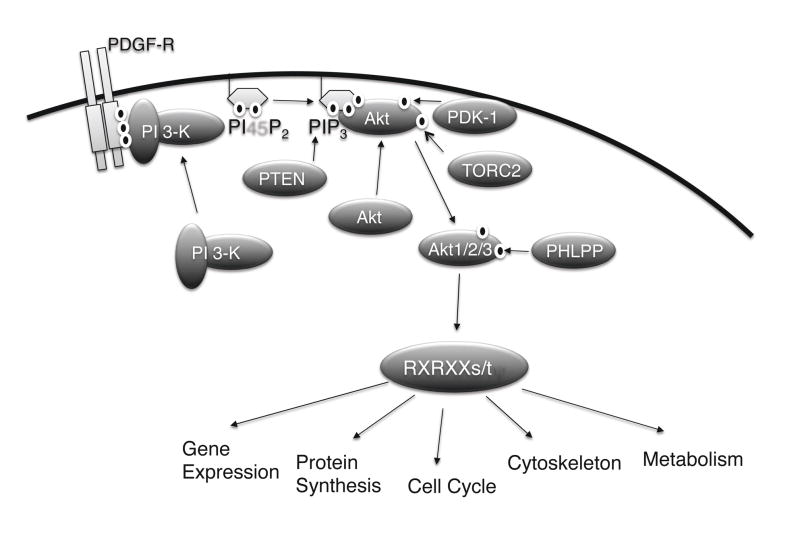
All three Akt isoforms are activated by essentially identical mechanisms downstream of PI 3-K. Upon stimulation by growth factors such as IGF-1 (insulin-like growth factor-1) and PDGF (platelet-derived growth factor), the regulatory p85 subunit of PI 3-K is recruited to phosphotyrosine-containing sequences within either cytoplasmic domains of activated receptor tyrosine kinases (RTK), or adapter molecules such as IRS-1 (insulin-receptor substrate-1) and GAB1 (GRB2-associated-binding protein 1) (Fig. 1). This binding co-localizes the p110 catalytic subunit of PI 3-K proximal to its substrate, PI45P2 (phosphatidylinositol-4,5-bisphosphate) which is then phosphorylated at the 3′ position of the inositol head ring to generate PIP3 (phosphatidylinositol-3,4,5-trisphosphate). PIP3 in turns serves to recruit effector molecules such as Akt through binding of the PH (pleckstrin homology) domain, effectively recruiting the kinase to the plasma membrane. This binding induces a conformational change in Akt which facilitates the phosphorylation of two critical residues. Thr308 in the activation loop is phosphorylated by PDK-1 (phosphoinositide-dependent kinase-1), which is also recruited to membranes through a PH:PIP3 interaction (Alessi, 2001; Alessi et al., 1997; Stokoe et al., 1997). Phosphorylation of the carboxyl-terminal Ser473 residue in mediated by the mTOR (mammalian target of rapamycin) kinase comprising the TORC2 (target of rapamycin complex 2) complex (Sarbassov et al., 2005), although a number of other kinases may also function in specific settings (Toker, 2002). Once fully phosphorylated, Akt is locked in the catalytically-competent conformation, loses the PIP3 requirement, and translocates to a variety of intracellular locations including the cytoplasm, mitochondria and nucleus where it phosphorylates specific substrates, many of which are directly implicated in cancer-associated phenotypes (Fig. 1).
Fig. 1.
Regulation of Akt Activation by the PI 3-K pathway. The figure shows the major upstream regulators of Akt. Receptor tyrosine kinases such as the PDGF receptor stimulate recruitment of PI 3-K to the membrane where it interconverts PI45P2 into PIP3. This stimulates recruitment of inactive Akt to the cell surface, concomitant with recruitment of PDK-1. PDK-1 and TORC2 phosphorylate Akt at T308 and S473, respectively, and lock the enzyme in the catalytically-competent conformation. Akt can relocalize to intracellular locations and phosphorylate a large number of substrates, which harbor the consensus motif RxRxxs/t. The pathway is terminated by two critical negative regulators, both of which are tumor suppressors in cancer. PTEN dephosphorylates PIP3 back into PI45P2 and PHLPP dephosphorylates Akt at S473.
Oncogenic mutations in the PI 3-K pathway also lead to hyperactivation of Akt. The most frequent of these include somatic activating mutations in PIK3CA, the p110α subunit of PI 3-K, that is mutated in approximately 30% of human breast cancers, particularly those that are ER (estrogen receptor) positive. The PIK3CA gene is also amplified in many human cancers. Conversely, mutations of other class I p110 isoforms such as PIK3CB (p110β), PIK3CD (p110δ) and PIK3CG (p110γ) are not found, yet have been shown to contribute to malignant cell signaling. Recent studies have also identified somatic mutations in the PI 3-K class I p85 regulatory isoforms (which include p85α, p85β, p55α, p50α, and p55γ, encoded by three distinct genes that gives rise to the five splice variants: PIK3R1, PIK3R2 and PIK3R3) (Cheung et al., 2011). For a recent review on specific functions of p85 and p110 isoforms, see (Okkenhaug and Vanhaesebroeck, 2001; Vanhaesebroeck et al., 2010). PIK3CB and PIK3CD appear to be particularly important in prostate cancer in which PTEN (phosphatase and tensin homolog on chromosome ten) is lost by LOH or by inactivating mutations and deletions. PTEN is a negative regulator of the PI 3-K pathway as it dephosphorylates PIP3 back to PI45P2, effectively terminating signal relay (Li et al., 1997; Maehama and Dixon, 1998). Thus, LOH or mutations in PTEN that lead to excessive accumulation of PIP3 stimulate constitutive signaling to Akt and other effectors (Li et al., 1998). As such, PTEN is one of the most frequently mutated tumor suppressors in human cancers, particularly glioblastoma and prostate cancer (Carracedo et al., 2011). A second tumor suppressor in the PI 3-K pathway is PHLPP (PH domain and leucine rich protein phosphatase), a serine/threonine phosphatase that dephosphorylates Akt at pSer473, a residue required for maximal catalytic activity (Gao et al., 2005). Again, mutations or LOH in PHLPP would be predicted to relieve tumor suppression leading to constitutive Ser473 phosphorylation and Akt activation. Consistent with this, a recent study provided compelling evidence for the absolute requirement for both PTEN and PHLPP loss in prostate cancer progression (Chen et al., 2011).
Akt phosphorylates substrates in a sequence-specific context, and as a basophilic-directed kinase it has a an absolute requirement for an Arg residue at the −3 position relative to the Ser or Thr phosphoacceptor (Obata et al., 2000). In most cases an Arg at −5 is also required, although there are examples of Akt substrates that lack −5 Arg. In many cases, a +1 hydrophobic residue is also preferred, and can be followed by a +2 Pro residue that provides an optimal motif for binding to 14-3-3 proteins upon phosphorylation (Manning and Cantley, 2007). In some cases several consensus motifs are found on Akt substrates, ensuring specificity and fidelity for efficient signal relay through PI 3-K/Akt. Many of the identified substrates of Akt have orthologs in other mammals and lower invertebrates such as flies and worms, and in some cases this has both validated the importance of a given substrate in Akt signaling, and also highlighted its function in mediating signal relay to phenotypes such as growth and survival.
Akt Isoform-specific Signaling
The first hint that Akt isoforms function non-redundantly came when the Birnbaum laboratory generated Akt1, Akt2 and Ak3 knockout mice (Bae et al., 2003; Cho et al., 2001a; Cho et al., 2001b). Although all three knockout lines are viable, Akt1 null mice revealed growth retardation and perinatal lethality (Chen et al., 2001; Cho et al., 2001b). In contrast, Akt2 null mice develop insulin-resistant diabetes and it is now established that Akt2 is the primary Akt isoform that contributes to metabolic signaling in the liver (Cho et al., 2001a; Garofalo et al., 2003). In contrast Akt3 null mice reveal a reduced brain size, consistent with an enrichment of this isoform in neuronal cells and tissues (Easton et al., 2005; Tschopp et al., 2005). The non-overlapping phenotypes evident in the individual Akt isoform null mice immediately suggested that differences must exist at the level of Akt1, Ak2 and Akt3 signal relay. The use of Akt null mice and MEFs (mouse embryonic fibroblasts) derived from these animals has begun to shed some light on some of these specific signaling mechanisms. As discussed in detail below, the first realization of non-redundant signaling by Akt proteins was the discovery that they have specific functions in the regulation of epithelial cell and carcinoma cell migration, invasion and metastasis. However, a number of other specific signaling mechanisms have also been attributed to one or more Akt isoform. These have largely been possible through the use of specific RNAi tools, as well as the development of Akt isoform-specific inhibitors. Researchers at Merck Research Laboratories first developed a series of allosteric inhibitors Akt, which interfere with both the PH domain as well as the catalytic domain, allowing greater specificity for inhibition (Barnett et al., 2005; DeFeo-Jones et al., 2005). These first generation inhibitors termed Akti-1, Akti-2 and Akti1-/2 displayed some selectivity towards Akt1 and Akt2 both in vitro and in vivo. The most recent iteration of these inhibitors is MK-2206, a pan-Akt inhibitor currently in phase I clinical trials (Hirai et al., 2010). Importantly, the use of MK-2206 and other PI 3-K pathway inhibitors, including rapamycin that blocks the TORC1 complex downstream of Akt, has revealed the existence of multiple negative feedback loops that normally serve to attenuate PI 3-K signaling to Akt and other effectors, such that long term inhibitor treatment alleviates negative feedback and thus enhances signaling, with obvious consequences for targeted therapy (Carracedo et al., 2008; Chandarlapaty et al., 2011; O’Reilly et al., 2006). For additional information concerning PI 3-K/Akt signaling to TORC1 and negative feedback loops, see the review by (Baselga, 2011).
Although close to 200 substrates of Akt have been identified in many cell types and tissues, only a small handful of these have been evaluated for Akt isoform-specificity. The cell cycle regulator p21 is phosphorylated exclusively by Akt1 and this negatively regulates cell cycle progression and proliferation (Heron-Milhavet et al., 2006). Similarly, the E3 ubiquitin ligase Skp2 is exclusively phosphorylated by Akt1 and not by Akt2, and this event controls Skp2 stability by preventing degradation by the APC-Cdh1 ubiquitin ligase complex (Gao et al., 2009). In turn, this affects cell cycle progression and cellular transformation, phenotypes associated with deregulated Akt and Skp2 signaling. Akt2-specific substrates have also been identified. For example AS160, a protein that modulates glucose transport in insulin-responsive cells and tissues through the GLUT4 transporter, appears to be regulated primarily by Akt2 (Ng et al., 2008). Similarly, myosin5a is an Akt2-specific substrate that also functions to modulate GLUT4 vesicle translocation (Yoshizaki et al., 2007). An important role for differential Akt isoform signaling in myogenic differentiation and myoblast function has also emerged (Rotwein and Wilson, 2009). In this context, Ankrd2/ARPP is an Akt2-specific substrate that regulates myogenic differentiation in cells exposed to oxidative stress (Cenni et al., 2011). Similarly, Akt2 appears to be exclusively required for osteoblast differentiation and bone development (Mukherjee and Rotwein, 2009), though specific substrates that account for this phenotype have yet to be described. In summary, primarily through candidate screening approaches, a number of Akt isoform-specific substrates have been identified, and in some cases phosphorylation has been directly linked to the regulation of one or more cellular response.
Regulation of Invasive Migration and Metastasis by Akt
Arguably much information concerning isoform-specific signaling through Akt isoforms has come from the analysis of the regulation of cell migration in a variety of cell lineages. Initial studies using expression of activated alleles in vitro revealed that signaling through Akt enhances cell migration, for example in fibroblasts and fibrosarcoma cells (Enomoto et al., 2005; Kim et al., 2001). Similarly, expression of activated Akt1 promotes EMT (epithelial to mesenchymal transition) of squamous carcinoma cells, concomitant with reduced cellular adhesion and enhanced migration (Grille et al., 2003). Similarly, Akt1 and Akt2 promotes the expression of the IGF-1 receptor leading to increased pancreatic cancer cell motility (Tanno et al., 2001). These studies indicated that various Akt isoforms can stimulate motility, at least as measured using in vitro assays, primarily under conditions of overexpression of Akt proteins.
The use of more specific tools such as RNAi as well as in vivo assays for invasive migration and metastasis painted a rather different picture as to the role of Akt isoforms in modulating motility, especially in breast carcinoma. The first study to suggest that Akt1 might function as a metastasis suppressor came from the Muller laboratory who engineered a constitutively active Akt1 transgene in the mouse mammary gland in the background of ERB2, and showed that while it shortens the latency of multifocal mammary tumor development, it actually suppresses tumor invasion into surrounding tissues (Hutchinson et al., 2004). Interestingly, in a preceding study, expression of constitutively active Akt in the mouse mammary gland in the background of mutant polyoma middle T (PyV mT, known to signal through PI 3-K), did not rescue the highly metastatic phenotype displayed by wild-type PyV mT (Hutchinson et al., 2001).
Subsequent studies performed using in vitro assays also demonstrated Akt isoform-specificity in the regulation of breast cancer invasive migration. Our group showed that Akt1 suppresses breast cancer cell migration by enhancing the proteasomal degradation of the NFAT (nuclear factor of activated T cells) transcription factor (Yoeli-Lerner et al., 2009; Yoeli-Lerner et al., 2005), which promotes the transcriptional induction of pro-migration and invasion genes, including COX2 (cyclooxygenase-2), autotaxin and glypican-6 (Chen and O’Connor K, 2005; Yiu et al., 2011; Yiu and Toker, 2006). Similar results were published by the Brugge laboratory, who further showed that while Akt1 can block migration, in the same cells Akt2 actually enhances this phenotype (Irie et al., 2005). They further showed that the mechanism for differential regulation of MCF10A cell migration by Akt1 and Akt2 is in part through modulation of ERK (extracellular-regulated kinase) activity. Similarly, the Bissell laboratory also showed the Akt1 can block cell migration through TSC2 (tuberous sclerosis complex 2) (Liu et al., 2006). More recent studies have shown that the EMT phenotype induced by Akt1 silencing in MCF10A cells correlates with downregulation of the mIR-220 family of micro-RNAs (Iliopoulos et al., 2009). These findings are consistent with earlier studies which showed that expression of only Akt2 can phenocopy the invasive phenotype of PI 3-K-expressing breast cancer cells (Arboleda et al., 2003). Similarly, Akt2 overexpression upregulates β1 integrin expression and thus enhances cell migration and metastasis in vivo (Arboleda et al., 2003). More recent studies have added more mechanistic insight as to how Akt isoforms differentially control cell migration, at least in breast cancer cells. Our own studies identified palladin as an Akt1-specific substrate that mediates the inhibitory activity of this isoform on breast cancer cell migration (Chin and Toker, 2010). Palladin is an actin bundling protein that is ubiquitously expressed and its bundling activity is absolutely required for efficient cell migration. Our studies showed that palladin is an exclusive Akt1 substrate which is not phosphorylated by Akt2, and moreover interfering with palladin phosphorylation by Akt1 subverts the inhibitory function of Akt1 on breast cancer cell migration (Chin and Toker, 2010). The specific substrates of Akt2 that are responsible for enhancing cell migration and metastatic dissemination remain presently unidentified.
In vivo studies using either Akt knockout mice or transgenic lines harboring activated Akt alleles in the mammary epithelium have provided physiological evidence of the non-redundant effects of Akt isoforms on invasion and metastasis, albeit with not entirely consistent results. As discussed above, an activated Akt1 transgene in an ERB2 background accelerates tumorigenesis, but with decreased metatstatic lesions (Dillon et al., 2009; Dillon et al., 2007; Hutchinson et al., 2001; Hutchinson et al., 2004). Similarly, germline knockout of Akt1 results in severely impaired tumor induction as revealed by two separate studies (Ju et al., 2007; Maroulakou et al., 2007). However, one of these studies noted that Akt1 knockout resulted in fewer metastases, with the conclusion that Akt1 signaling is positively associated with invasion leading to metastasis, although whether this is secondary to the consequence of Akt1 ablation or effects of Akt1 loss in the tumor microenvironment has not been determined (Ju et al., 2007). In contrast, a separate study did note suppression of metastases in ERB2/Akt1-deficient tumors, consistent with Akt1 functioning as a metastasis suppressor (Maroulakou et al., 2007). This same study also noted that knockout of Akt2 in MMTV-ERB2 mice decreases metastasis, consistent with Akt2 functioning as a metastasis enhancer. In summary, there is now overwhelming evidence that Akt1 and Akt2 have opposing functions in modulating phenotypes associated with migration and invasion. What has remained mysterious are the specific molecular mechanisms that account for these distinctions.
Mechanisms for Akt Isoform Selectivity in Signaling
There exist a number of competing possibilities as to how specificity is achieved by Akt1, Akt2 and Akt3 proteins in relying the PI 3-K signal to cellular responses, and it should be noted that these are not mutually exclusive. It appears that both growth factors, oncogenic PI 3-K and PTEN loss all activate Akt isoforms to the same extent in all cell types thus far examined, at least as measured in whole cell lysates. Therefore, if there is specific activation of Akt’s by distinct upstream signals, this likely occurs at discrete cellular locations such as the nucleus or endomembranes. A systematic analysis of the specific contributions of p110 and p85 isoforms in the regulation of Akt1, Akt2 and Akt3 in various cells and tissues has not been performed. Presently, such an analysis is hampered by the fact that current tools that serve as surrogates for Akt activation, such as phospho-specific antibodies, do not discriminate between the three isoforms. This is clearly a technological hurdle that will hopefully be overcome in the future. In addition to upstream regulators, termination of Akt signaling may also afford specificity. Termination of Akt signaling is achieved by a number of mechanisms including ubiqitylation and proteasomal degradation, although whether this is isoform selective or not is unknown (Oh et al., 2010; Suizu et al., 2009; Wu et al., 2011). In contrast, dephosphorylation of S473 by PHLPP does indicate some selectivity, whereby the PHLPP1 isoform appears to dephosphorylate Akt2 and Akt3, whereas PHLPP2 targets Akt1 and 3 (Brognard et al., 2007).
An additional mechanism that likely accounts for isoform-selective signaling is compartmentalization. Distinct cellular localization of total Akt proteins has been detected, for example Akt2 colocalizes at sites adjacent to the extracellular matrix during adhesion, whereas Akt1 does not (Arboleda et al., 2003). However, identification of distinct intracellular pools of Akt isoforms does not necessarily imply that any given pool is actually required for transducing the signal to a specific phenotype. Interfering with a specific Akt pool and observing the phenotypic consequence would provide cause-and-effect demonstration, and to this end a recent study used a new technology in which cellular compartment-directed Akt pseudosubstrate inhibitors were used to attenuate plasma membrane, cytoplasmic and nuclear Akt pools, individually (Maiuri et al., 2010). This approach revealed that nuclear and plasma membrane Akt pools are required for adipocyte differentiation, whereas the cytoplasmic pool appears to be dispensable. While this approach does not discriminate between Akt isoforms, presumably it could be coupled with expression of Akt alleles, or silencing of Akt isoforms using RNAi, to begin to probe the contribution of distinct subcellular pools of Akt isoforms in physiology and pathophysiology.
Consistent with the notion that distinct Akt isoforms modulate specific phenotypes in a spatially restricted manner, it has been shown that membrane recruitment of Akt2 in insulin-stimulated adipocytes appears to be more robust than Akt1, and to be dependent on the PH domain and the Akt2 linker region (Gonzalez and McGraw, 2009). In this context, it is interesting to note that our studies showed that the Akt1 linker region determines the selectivity of Akt1 over Akt2 in the phosphorylation of palladin (Chin and Toker, 2010). This is reminiscent of studies in MEFs derived from Akt null mice, in which chimeric constructs bearing the PH and linker domains of either Akt1 or Akt2, in various combinations, were introduced. This analysis revealed that the defect in MEF cell migration could be rescued by a chimera harboring the Akt2 PH domain and Akt2 linker region (Zhou et al., 2006). Whether the linker region contains specific residues or microdomains that confer Akt substrate selectivity remains to be determined. Similarly, whether additional determinants exist on Akt isoforms that dictate substrate selectivity is not known. The POSH (plenty of SH3 domains) protein is an Akt substrate, however it only directly interacts with Akt2 and not Akt1 (Figueroa et al., 2003). However since POSH is involved in apoptotic signaling, it is unlikely that it accounts for the differential role of Akt’s in cell migration and metastasis. Regardless, specific substrates of Akt isoforms that are responsible for transducing distinct phenotypes clearly do exist, and the challenge is now to discover the subset of these proteins that are exclusively regulated by Akt1, Akt2 or Akt3.
Screening for Akt Substrates in Human Cancers
The identification of novel substrates of protein kinases, including Akt, using quantitative mass spectrometry sequencing approaches such as SILAC (stable isotope labeling of amino acids in cell culture) or KESTREL (kinase substrate tracking and elucidation) has significantly contributed to our understanding of the mechanisms by which the PI 3-K/Akt pathway mediates downstream signaling (Cohen and Knebel, 2006; Manning and Cantley, 2002). These studies have combined the use of Akt consensus motif substrate-directed antibodies to enrich for phosphopeptides from complex mixtures including cell lysates and whole organ extracts, followed by mass spectrometry sequencing and database analysis. The first of these approaches made use of standard chromatography techniques to enrich phosphopeptides from HeLa cell nuclear lysates followed by sequencing (Beausoleil et al., 2004). From this emerged numerous novel phosphopeptides that could be attributed to one or more protein kinase subfamilies, including Akt. Subsequent approaches using mouse liver extracts and Akt substrate-directed antibodies (Rxxs/t or RxRxxs/t) identified thousands of novel phosphopeptides that have since been curated on publicly available databases and made available to the research community (Villen et al., 2007). Many groups, including our own, have made use of these databases to identify putative novel Akt substrates which can subsequently be validated using standard biochemical approaches (Manning and Cantley, 2002). It should be noted that the use of the Akt substrate-directed antibody has also proven instrumental in substrate identification using standard biochemical assays (Zhang et al., 2002), beginning with the discovery of the TSC2 protein as a substrate of Akt, and major regulator of the TORC1 complex (Manning et al., 2002; Tee et al., 2002). The most recent iteration of this technology has used second generation substrate-directed antibodies, highly specific to the Akt consensus (which should be noted is also shared by other AGC kinases including S6-kinases, SGKs (serum and glucocorticoid-inducible kinases) and RSK (ribosomal S6 kinase)) (Moritz et al., 2010). This has been combined with specific pathway inhibitors in cells harboring relevant pathway mutations. The resulting mass spectrometry analysis has revealed thousand of additional phosphopeptides that can be attributed to one or more protein kinase, including Akt, and once again curated on public databases (Moritz et al., 2010). Similar approaches have also used tissues from human tumors do identify specific phosphorylation events that might be associated with disease etiology and progression (Rikova et al., 2007). While none of these approaches to date have addressed Akt isoform-specificity with respect to substrate phosphorylation, this presumably can be achieved using either Akt isoform-specific inhibitors or RNAi to discover the complement of pan-Akt, Akt1-, Akt2-, Akt3-specific substrates.
Conclusions
Subsequent to the generation of Akt isoform-specific knockout mice and the realization that Akt1, Akt2 and Akt3 function non-redundantly in the regulation of cellular responses, the development and use of tools to interfere specifically with Akt isoforms has provided unequivocal evidence for distinct signaling roles for this family of protein kinases downstream of PI 3-K. One of the most obvious phenotypes that Akt isoforms regulate in a differential manner is breast cancer cell invasion migration and metastasis, whereby Akt2 enhances metastasis and Akt1 either does not, or actually functions as a metastasis suppressor. However, it is likely that other phenotypes associated with malignancy, including survival, growth and metabolic reprogramming are also regulated by specific Akt isoforms, and this information is predicted to emerge in the near future. Equally importantly will be the identification of the specific mechanisms that account for isoform selectivity, whether it be specific substrates exclusively phosphorylated by one Akt isoform, or specific intracellular localization, or differential binding partners, or a combination of these.
One critical hurdle to be overcome is the development of specific antibodies or biomarkers that can be used as surrogates for the activation of an individual isoform, in both cell lines and clinical specimens. Similarly, the complement of pan-Akt and isoform-specific substrates of Akt’s remains to be identified, and is likely to diverge considerably in different cell types and tissues. As discussed above, global phospho-proteomic mass spectrometry sequencing approaches are expected to provide this critical information in the near future. Answers to all these questions will provide valuable information on the specific functions of Akt proteins in cellular physiology and disease etiology. These are pressing issues, considering the numerous clinical trials targeting both PI 3-K and Akt for therapeutic benefit in a variety of human diseases, especially cancer, and considering the array of pathophysiologies that have been attributed to deregulated PI 3-K/Akt signaling.
Acknowledgments
I would like to thank all members of the Toker laboratory, past and present, for their contributions. Work in the laboratory is funded by the National Institutes of Health, the National Cancer Institute, the Department of Defense Breast Cancer Research Program and the Susan G. Komen Breast Cancer Foundation.
Footnotes
Conflict of Interest
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR. Discovery of PDK1, one of the missing links in insulin signal transduction. Colworth Medal Lecture. Biochem Soc Trans. 2001;29:1–14. doi: 10.1042/0300-5127:0290001. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer research. 2003;63:196–206. [PubMed] [Google Scholar]
- Bae SS, Cho H, Mu J, Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. The Journal of biological chemistry. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- Barnett SF, Defeo-Jones D, Fu S, Hancock PJ, Haskell KM, Jones RE, Kahana JA, Kral AM, Leander K, Lee LL, et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. The Biochemical journal. 2005;385:399–408. doi: 10.1042/BJ20041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J. Targeting the phosphoinositide-3 (PI3) kinase pathway in breast cancer. Oncologist. 2011;16(Suppl 1):12–19. doi: 10.1634/theoncologist.2011-S1-12. [DOI] [PubMed] [Google Scholar]
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villen J, Li J, Cohn MA, Cantley LC, Gygi SP. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Molecular cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Alimonti A, Pandolfi PP. PTEN level in tumor suppression: how much is too little? Cancer research. 2011;71:629–633. doi: 10.1158/0008-5472.CAN-10-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenni V, Bavelloni A, Beretti F, Tagliavini F, Manzoli L, Lattanzi G, Maraldi NM, Cocco L, Marmiroli S. Ankrd2/ARPP is a novel Akt2 specific substrate and regulates myogenic differentiation upon cellular exposure to H2O2. Molecular biology of the cell. 2011;22:2946–2956. doi: 10.1091/mbc.E10-11-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, O’Connor KL. Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene. 2005 doi: 10.1038/sj.onc.1208729. [DOI] [PubMed] [Google Scholar]
- Chen M, Pratt CP, Zeeman ME, Schultz N, Taylor BS, O’Neill A, Castillo-Martin M, Nowak DG, Naguib A, Grace DM, et al. Identification of PHLPP1 as a Tumor Suppressor Reveals the Role of Feedback Activation in PTEN-Mutant Prostate Cancer Progression. Cancer cell. 2011;20:173–186. doi: 10.1016/j.ccr.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes & development. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung LWT, Hennessy BT, Li J, Yu S, Myers AP, Djordjevic B, Lu Y, Stemke-Hale K, Dyer MD, Zhang F, et al. High Frequency of PIK3R1 and PIK3R2 Mutations in Endometrial Cancer Elucidates a Novel Mechanism for Regulation of PTEN Protein Stability. Cancer Discovery. 2011 doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Toker A. The Actin-Bundling Protein Palladin Is an Akt1-Specific Substrate that Regulates Breast Cancer Cell Migration. Molecular cell. 2010;38:333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001a;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. The Journal of biological chemistry. 2001b;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Cohen P, Knebel A. KESTREL: a powerful method for identifying the physiological substrates of protein kinases. The Biochemical journal. 2006;393:1–6. doi: 10.1042/BJ20051545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, Leander KR, McAvoy E, Robinson RG, Duggan ME, Lindsley CW, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol Cancer Ther. 2005;4:271–279. [PubMed] [Google Scholar]
- Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB, Muller WJ. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer research. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, et al. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Molecular and cellular biology. 2005;25:1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature reviews. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Murakami H, Asai N, Morone N, Watanabe T, Kawai K, Murakumo Y, Usukura J, Kaibuchi K, Takahashi M. Akt/PKB Regulates Actin Organization and Cell Motility via Girdin/APE. Dev Cell. 2005;9:389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Figueroa C, Tarras S, Taylor J, Vojtek AB. Akt2 negatively regulates assembly of the POSH-MLK-JNK signaling complex. The Journal of biological chemistry. 2003;278:47922–47927. doi: 10.1074/jbc.M307357200. [DOI] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nature cell biology. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Molecular cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer research. 2003;63:2172–2178. [PubMed] [Google Scholar]
- Heron-Milhavet L, Franckhauser C, Rana V, Berthenet C, Fisher D, Hemmings BA, Fernandez A, Lamb NJ. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Molecular and cellular biology. 2006;26:8267–8280. doi: 10.1128/MCB.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- Hutchinson J, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Molecular and cellular biology. 2001;21:2203–2212. doi: 10.1128/MCB.21.6.2203-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer research. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Polytarchou C, Hatziapostolou M, Kottakis F, Maroulakou IG, Struhl K, Tsichlis PN. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. The Journal of cell biology. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju X, Katiyar S, Wang C, Liu M, Jiao X, Li S, Zhou J, Turner J, Lisanti MP, Russell RG, et al. Akt1 governs breast cancer progression in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim S, Koh H, Yoon SO, Chung AS, Cho KS, Chung J. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. Faseb J. 2001;15:1953–1962. doi: 10.1096/fj.01-0198com. [DOI] [PubMed] [Google Scholar]
- Li J, Simpson L, Takahashi M, Miliaresis C, Myers MP, Tonks N, Parsons R. The PTEN/MMAC1 tumor suppressor induces cell death that is rescued by the AKT/protein kinase B oncogene. Cancer research. 1998;58:5667–5672. [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer [see comments] Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, Bissell MJ. Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4134–4139. doi: 10.1073/pnas.0511342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. The Journal of biological chemistry. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Maiuri T, Ho J, Stambolic V. Regulation of adipocyte differentiation by distinct subcellular pools of protein kinase B (PKB/Akt) The Journal of biological chemistry. 2010;285:15038–15047. doi: 10.1074/jbc.M110.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. Hitting the target: emerging technologies in the search for kinase substrates. Sci STKE. 2002:PE49. doi: 10.1126/stke.2002.162.pe49. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Molecular cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer research. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Seminars in cell & developmental biology. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Moritz A, Li Y, Guo A, Villen J, Wang Y, MacNeill J, Kornhauser J, Sprott K, Zhou J, Possemato A, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Science signaling. 2010;3:ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Rotwein P. Akt promotes BMP2-mediated osteoblast differentiation and bone development. Journal of cell science. 2009;122:716–726. doi: 10.1242/jcs.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng Y, Ramm G, Lopez JA, James DE. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 2008;7:348–356. doi: 10.1016/j.cmet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. The Journal of biological chemistry. 2000;275:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- Oh WJ, Wu CC, Kim SJ, Facchinetti V, Julien LA, Finlan M, Roux PP, Su B, Jacinto E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. The EMBO journal. 2010;29:3939–3951. doi: 10.1038/emboj.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkenhaug K, Vanhaesebroeck B. New responsibilities for the PI3K regulatory subunit p85 alpha. Sci STKE. 2001:pe1. doi: 10.1126/stke.2001.65.pe1. [DOI] [PubMed] [Google Scholar]
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Rotwein P, Wilson EM. Distinct actions of Akt1 and Akt2 in skeletal muscle differentiation. Journal of cellular physiology. 2009;219:503–511. doi: 10.1002/jcp.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- Suizu F, Hiramuki Y, Okumura F, Matsuda M, Okumura AJ, Hirata N, Narita M, Kohno T, Yokota J, Bohgaki M, et al. The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated Akt. Dev Cell. 2009;17:800–810. doi: 10.1016/j.devcel.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer research. 2001;61:589–593. [PubMed] [Google Scholar]
- Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. Phosphoinositides and signal transduction. Cell Mol Life Sci. 2002;59:761–779. doi: 10.1007/s00018-002-8465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development. 2005;132:2943–2954. doi: 10.1242/dev.01864. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. The Biochemical journal. 2000;346(Pt 3):561–576. [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1488–1493. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Ouyang W, Lazorchak AS, Liu D, Shen HM, Su B. mTOR complex 2 targets Akt for proteasomal degradation via phosphorylation at the hydrophobic motif. The Journal of biological chemistry. 2011;286:14190–14198. doi: 10.1074/jbc.M111.219923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu GK, Kaunisto A, Chin YR, Toker A. NFAT Promotes Carcinoma Invasive Migration Through Glypican-6. The Biochemical journal. 2011 doi: 10.1042/BJ20110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu GK, Toker A. NFAT induces breast cancer cell invasion by promoting the induction of cyclooxygenase-2. The Journal of biological chemistry. 2006;281:12210–12217. doi: 10.1074/jbc.M600184200. [DOI] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Chin YR, Hansen CK, Toker A. Akt/Protein Kinase B and Glycogen Synthase Kinase-3{beta} Signaling Pathway Regulates Cell Migration through the NFAT1 Transcription Factor. Mol Cancer Res. 2009 doi: 10.1158/1541-7786.MCR-08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Molecular cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Yoshizaki T, Imamura T, Babendure JL, Lu JC, Sonoda N, Olefsky JM. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bbeta) substrate modulating GLUT4 vesicle translocation. Molecular and cellular biology. 2007;27:5172–5183. doi: 10.1128/MCB.02298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zha X, Tan Y, Hornbeck PV, Mastrangelo AJ, Alessi DR, Polakiewicz RD, Comb MJ. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. The Journal of biological chemistry. 2002;277:39379–39387. doi: 10.1074/jbc.M206399200. [DOI] [PubMed] [Google Scholar]
- Zhou GL, Tucker DF, Bae SS, Bhatheja K, Birnbaum MJ, Field J. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. The Journal of biological chemistry. 2006;281:36443–36453. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]