SUMMARY
A common thread among conserved lifespan regulators lies within intertwined roles in metabolism and energy homeostasis. We show that heterozygous mutations of adenosine monophosphate (AMP) biosynthetic enzymes extend Drosophila lifespan. The lifespan benefit of these mutations depends upon increased AMP to adenosine triphosphate (ATP) and adenosine diphosphate (ADP) to ATP ratios and adenosine monophosphate-activated protein kinase (AMPK). Transgenic expression of AMPK in adult fat body or adult muscle, key metabolic tissues, extended lifespan, while AMPK RNAi reduced lifespan. Supplementing adenine, a substrate for AMP biosynthesis, to the diet of long-lived AMP biosynthesis mutants reversed lifespan extension. Remarkably, this simple change in diet also blocked the pro-longevity effects of dietary restriction. These data establish AMP biosynthesis, adenosine nucleotide ratios, and AMPK as determinants of adult lifespan, provide a mechanistic link between cellular anabolism and energy sensing pathways, and indicate that dietary adenine manipulations might alter metabolism to influence animal lifespan.
INTRODUCTION
Recent dietary and genetic studies have begun to unravel the cascades that control longevity (Fontana et al., 2010; Libert et al., 2007; Piper and Bartke, 2008; Sinclair, 2005). What is becoming clearer is that metabolic tissues and processes are central components of lifespan regulation (Artal-Sanz and Tavernarakis, 2008; Houtkooper et al., 2010; Roberts and Rosenberg, 2006). Mechanistically, nutrient sensing pathways have been identified as a major class of conserved lifespan regulators. This group includes caloric restriction, insulin and insulin-like growth factor signaling, and target of rapamycin signaling (Antosh et al., 2011; Kapahi et al., 2004; Rogina and Helfand, 2004; Spindler, 2010; Zid et al., 2009).
A central aspect of cellular metabolism and energy homeostasis is the maintenance of adenosine derivatives (e.g., AMP, ADP, and ATP) at relatively constant levels (Hardie, 2003). ADP and ATP are formed from AMP (Hardie, 2003). AMP is generated by two parallel enzymatic processes, the de novo and the salvage AMP biosynthesis pathways (Rolfes, 2006). Interestingly, cellular adenosine derivative pools are also linked to organismal longevity. Recent work has uncovered a role for AMP biosynthesis in yeast chronologic lifespan (Matecic et al., 2010); however, the mechanism and relevance to multi-cellular organisms remains unclear. It seems plausible that the role of AMP in lifespan may be conserved; in C. elegans, AMP:ATP ratios appear predictive of lifespan, and increased AMPK activity, which can be mediated by concentrations of adenosine nucleotide derivatives, can delay worm aging (Apfeld et al., 2004; Curtis et al., 2006; Greer et al., 2007).
Due to the significant human interest in longevity and the possibility that drugs relevant to such an effect may also regulate metabolism, a variety of pharmacological approaches have been undertaken (Minor et al., 2010). These efforts have been hampered by the relative paucity of identified molecular pathways, with functionally druggable properties, that when altered can extend lifespan (Miller et al., 2011; Minor et al., 2011). To identify additional metabolic processes that regulate lifespan, we performed a metabolically targeted forward mutagenic screen and then analyzed the lifespan of the mutants. We found that enzymes that synthesize AMP regulate longevity. Notably, lifespan extension occurred when mutations of pathway components were present in the heterozygous state, indicating a dosage-sensitive relationship. Mechanistically, we found that long-lived heterozygous mutants had increased AMP:ATP and ADP:ATP ratios. Increased AMP:ATP or ADP:ATP ratios activate AMPK (Xiao et al., 2011). The long-lived heterozygous mutants had increased AMPK activity and inhibiting AMPK in a heterozygous mutant background blocked increased longevity. Notably, adult- and tissue-specific (fat body or muscle) transgenic expression of AMPK in a wild-type background increased AMPK activation and extended adult lifespan, while AMPK RNAi blunted AMPK activity and lifespan. Increased AMP:ATP and ADP:ATP ratios observed in long-lived mutants were restored by supplementing the diet of adult mutants with adenine. Dietary adenine feeding also rescued the extended longevity. Remarkably, adenine supplementation also blocked the lifespan benefit of dietary restriction. Thus, AMP biosynthetic enzymes are dosage-sensitive adult longevity regulators. In addition, the results indicate the importance of cellular energy homeostasis and AMPK signaling as conserved determinants of lifespan control that can be manipulated by specific diets.
RESULTS
Adenylosuccinate synthetase regulates lifespan
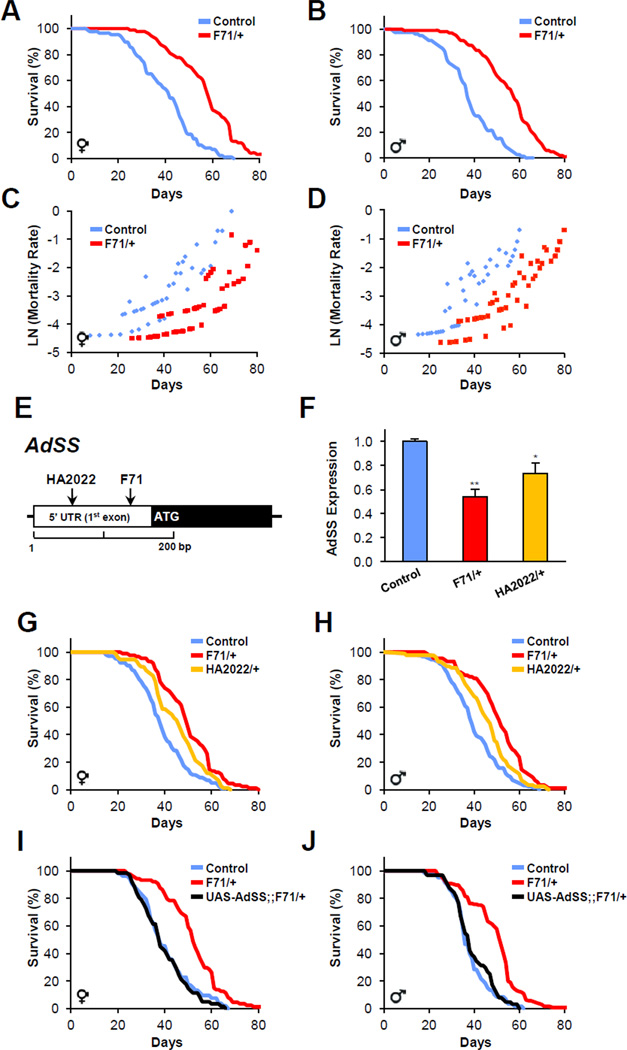
To identify metabolically relevant regulators of lifespan, we performed sequential screens, first an insertional enhancer trap designed to target mutations to metabolic tissues, and then longevity analyses (Suh et al., 2008). In brief, we conducted a minimal promoter GAL4/UAS-GFP reporter F1 enhancer trap screen to isolate lines with insertions in loci expressed in tissues intimately involved in metabolism, predominately in the Drosophila fat body, and also in oenocytes, anterior midgut, and muscle (Suh et al., 2008). This “metabolically–enriched” collection was then subjected to repeated longevity testing with a threshold of positive lifespan extension set to >130% of control strain lifespan, present in both male and female flies, at two temperatures, 25°C and 30°C. With this tiered methodology, we identified ten lines with significantly increased lifespan supporting the notion that metabolic-regulatory tissues are a rich source of genetic lifespan control (Suh et al., 2008). We focused on line F71 because it had one of the longest lifespans of the ten lines identified in our screen and because the F71 longevity benefit was present in the heterozygous state. To remove potential second site mutations, avoid background or modifier effects, and determine whether the increased lifespan was present in more than one background, we analyzed flies after backcrossing F71 >10 generations into a control w1118 strain. The lifespan of isogenic male and female heterozygous F71 mutants was approximately 20% longer than sibling controls and the mutants had reduced age-specific mortality (Figure 1A–1D). Homozygous F71 mutants were larval lethal.
Figure 1. Adenylosuccinate Synthetase regulates lifespan.
(A and B) Heterozygous F71 P-element insertion extends lifespan of female and male adult flies (log rank, P<0.00001 in either case).
(C and D) Log mortality plots of F71 heterozygous females and males.
(E) Insertions of F71 and HA2022 in the 5’ untranslated region of AdSS.
(F) AdSS expression is reduced in F71 and HA2022 heterozygotes. Data presented ± s.e.m. (* P<0.05, ** P<0.01, student’s t test).
(G and H) Independently derived AdSS heterozygous insertions, F71 and HA2022, extend lifespan (log rank of any heterozygote compared to controls, P<0.008).
(I and J) Transgenic expression of wild type AdSS in the F71 heterozygous background restores lifespan to that of controls (log rank of F71 heterozygous to transgenic, P<0.00001; log rank of transgenic to controls, P = 0.58). Also see Table S1.
A variety of physical and behavioral traits have been linked to Drosophila lifespan benefit, for example: reduced feeding rates, smaller body size, inactivity, or decreased fertility, however none of these factors appeared to be associated with the lifespan benefit conferred by F71 heterozygosity (Figure S1A –S1D). The mutants had increased activity, which has recently been associated with the beneficial lifespan effects of caloric restriction (Cordts, 1996). We also examined courtship and mating, which are known to reduce female and male lifespan (Chapman et al., 1995; Cordts and Partridge, 1996). We found that F71 heterozygotes may have a slight trend toward increased mating frequency, possibly due to the reported effects of the red eye color induced by P-element insertion (Figure S1E) (He et al., 1996). We attempted to reduce potential confounds of mating by analyzing longevity in several settings designed to normalize courtship and mating frequencies. Female F71 heterozygotes maintained lifespan extension when exposed to both control and F71 heterozygous males (Figure S1F). F71 heterozygous males analyzed in the absence of female exposure were also long-lived (Figure S1G). We also assessed oxidative stress responses of controls and F71 heterozygotes. Unlike some long-lived animals (Broughton et al., 2005; Lin et al., 1998; Wang et al., 2003), F71 heterozygotes do not have altered resistance to oxidative stress (Figure S1H and S1I). Together, these results indicate that heterozygous F71 mutants were long-lived and healthy.
To identify the genomic location of the F71 insertion we performed 5’ and 3’ inverse PCR as well as plasmid rescue followed by DNA sequencing. Sequence alignment of the genomic DNA flanking the P-element identified a single insertion site in the third chromosome corresponding to cytogenetic band position 93A1. Drosophila genome database searches revealed that the F71 P-element inserted into the 5’ UTR of the gene CG17273, 25 base pairs upstream of the transcriptional start site (Figure 1E). Sequence similarity searches of the predicted polypeptide identified CG17273 as the Drosophila ortholog of Adenylosuccinate Synthetase (AdSS). To assess the generality of this gene’s effect on lifespan we acquired an additional, independently derived, P-element AdSS insertion, HA2022 (Figure 1E), and backcrossed it over 10 generations into w1118. We evaluated AdSS expression levels with qPCR of adult extracts and found F71 decreased levels approximately 50% while HA2022 diminished expression approximately 27% (Figure 1F). We then compared the lifespan of control, F71 and HA2022 flies and confirmed that females and males heterozygous for either AdSS insertion lived significantly longer than controls (Figure 1G and 1H). To determine if transgenic expression of AdSS rescued the F71 heterozygous mutant lifespan extension, we generated transgenic flies that contained full-length AdSS cDNA under the control of an upstream activating sequence (UAS). Taking advantage of the GAL4 enhancer trap module contained within the F71 P-element insertion, we determined whether wild-type AdSS transgensis, under the control of endogenous AdSS tissue enhancer elements, rescued the F71 heterozygous longevity phenotype simply by crossing the UAS-AdSS allele with F71 heterozygotes. The mortality curves of UAS-AdSS;;+/+, and UAS-AdSS;;F71/+ siblings showed that AdSS transgensis reversed the F71 heterozygous lifespan extension (Figure 1I and 1J). The accumulated data support the notion that AdSS regulates longevity.
AMP biosynthesis enzymes regulate lifespan
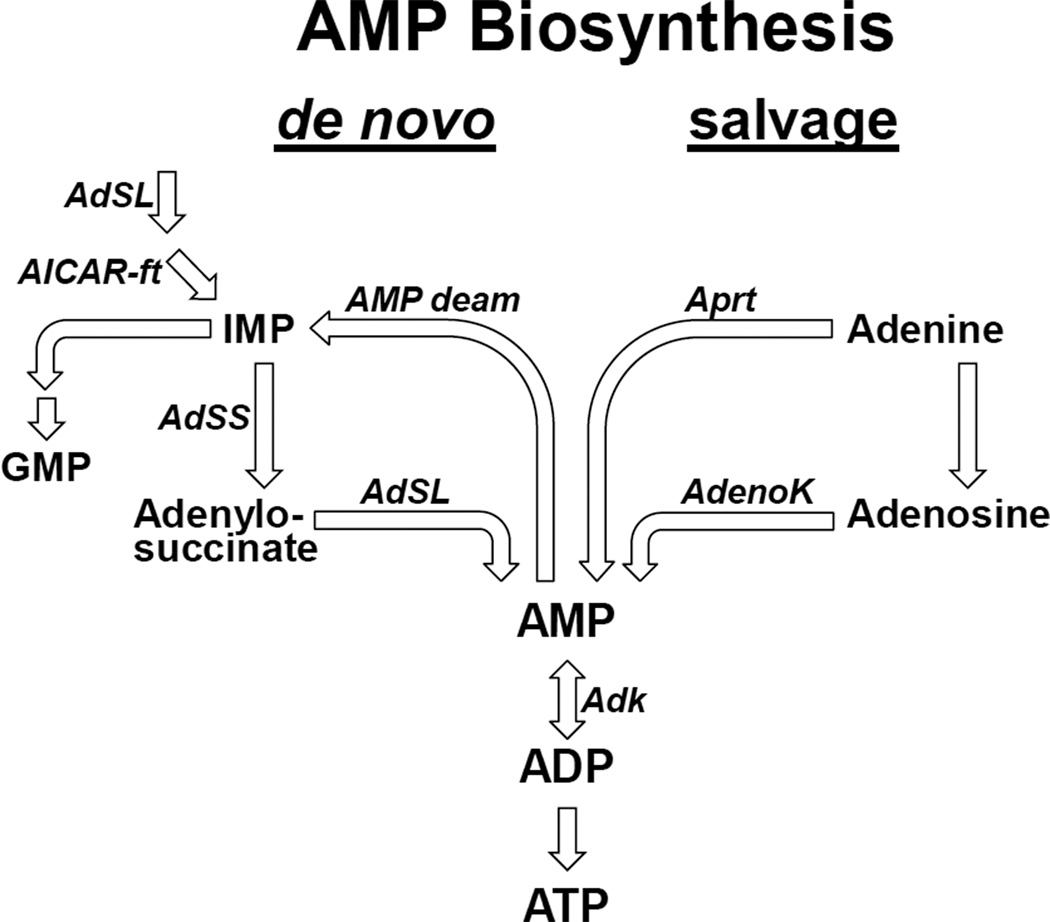
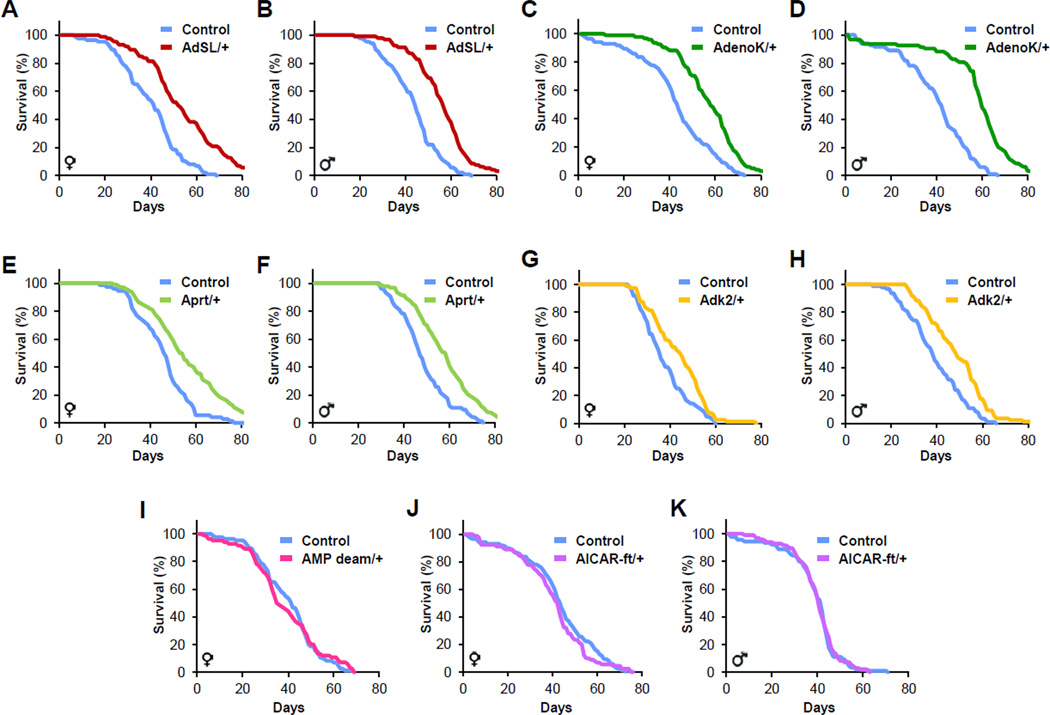
AdSS catalyses the first committed step of de novo AMP biosynthesis (Stayton et al., 1983) (Figure 2). We next evaluated the possibility that other components of de novo AMP biosynthesis might also control lifespan. To test this, we obtained a P-element insertion located within the Adenylosuccinate Lyase (AdSL) locus and again backcrossed into w1118. As observed with the AdSS alleles, male and female flies carrying heterozygous insertions in AdSL displayed extended longevity, and homozygous mutants died as larvae (Figure 3A and 3B). In addition to the de novo pathway, AMP can be synthesized via a salvage pathway (Figure 2). Therefore we acquired insertional mutations in two AMP salvage pathway components, Adenosine Kinase (AdenoK) and Adenine Phosphoribosyltransferase (Aprt), and backcrossed into w1118. Just as with the de novo pathway, males and females heterozygous for insertions in Adenosine Kinase or Aprt were long-lived (Figure 3C–3F).
Figure 2. AMP biosynthesis pathways.
AMP biosynthesis occurs though two distinct pathways, de novo and salvage. Adenylosuccinate Synthetase (AdSS) catalyzes the first committed step in de novo AMP biosynthesis followed by the action of Adenylosuccinate Lyase (AdSL). AMP Deaminase (AMP deam) is the reverse of these two reactions, converting adenosine monophosphate (AMP) into inosine monophosphate (IMP). The salvage pathway converts adenine, via Adenine Phosphoribosyltransferase (Aprt), or adenosine, via Adenosine Kinase (AdenoK), to AMP. Adenylate Kinase (Adk) also produces AMP from ADP substrate molecules. Prior to committed AMP biosynthesis, AdSL and Aminoimidazolecarboxamide Formyltransferase (AICAR-ft) sequentially catalyze the final reactions of IMP synthesis. IMP is a substrate for the synthesis of both AMP and guanosine monophosphate (GMP).
Figure 3. AMP biosynthesis enzymes regulate lifespan.
(A and B) Heterozygous insertional mutation of AdSL extends lifespan of adult female and male flies (log rank, P<0.00001 in either case).
(C to F) Heterozygous insertions into AMP salvage pathway biosynthesis components (AdenoK and Aprt) extend female and male lifespan (log rank of any heterozygote compared to controls, P≤0.0002).
(G and H) Heterozygous insertion in Adk2 increases longevity (log rank, P<0.004 in either case).
(I) Heterozygous AMP deaminase insertion does not alter lifespan (log rank, P>0.8).
(J and K) Heterozygous insertion in AICAR-ft does not alter female or male lifespan (log rank, P>0.8 in either case). Also see Table S1.
In addition to the two AMP biosynthesis pathways, Adenylate Kinase (Adk) also generates AMP by catalyzing the conversion of two molecules of ADP into AMP and ATP. Three homologues of Adenylate Kinase are present in the fly genome and an insertion, which we backcrossed into w1118, into Adenylate Kinase 2 (Adk2) was available. Heterozygous Adk2 mutant males and females had increased lifespan (Figure 3G and 3H). We also tested an insertional mutation of AMP deaminase, which catalyzes the hydrolytic deamination of adenosine monophosphate into inosine monophosphate, the opposite direction of the longevity genes (Figure 2). Heterozygous insertional mutation of AMP deaminase had no effect on lifespan (Figure 3I). Of note, AMP deaminase is present on the X chromosome so only females are able to carry a heterozygous insertion.
AdSL is a bifunctional enzyme and participates in inosine monophosphate (IMP) synthesis prior to AMP specific production as well as later specifically in the AMP de novo biosynthesis pathway (Figure 2) (Toth and Yeates, 2000). Aminoimidazolecarboxamide formyltransferase (AICAR-ft) also acts to produce IMP, and prior to committed AMP biosynthesis, but, unlike AdSL, AICAR-ft does not function in the AMP specific biosynthesis pathways (Figure 2). IMP is a substrate for both AMP and guanosine monophosphate (GMP) production (Figure 2). To attempt to probe this bifurcation, that is whether the role of AdSL in longevity was due to IMP or AMP biosynthesis, we examined an AICAR-ft insertional mutant. Heterozygous insertional mutation of AICAR-ft had no effect on lifespan (Figure 3J and 3K). Of note, all heterozygous mutants examined had between 25% and 50% reduced transcript expression of their respective gene, similar to the reduction in long-lived AdSS mutants (Figure 1G and S2). Together these data indicate that heterozygous mutations in genes encoding enzymes committed to the synthesis of adenosine derivatives extend lifespan.
AMP biosynthesis mutations alter adenosine nucleotide ratios
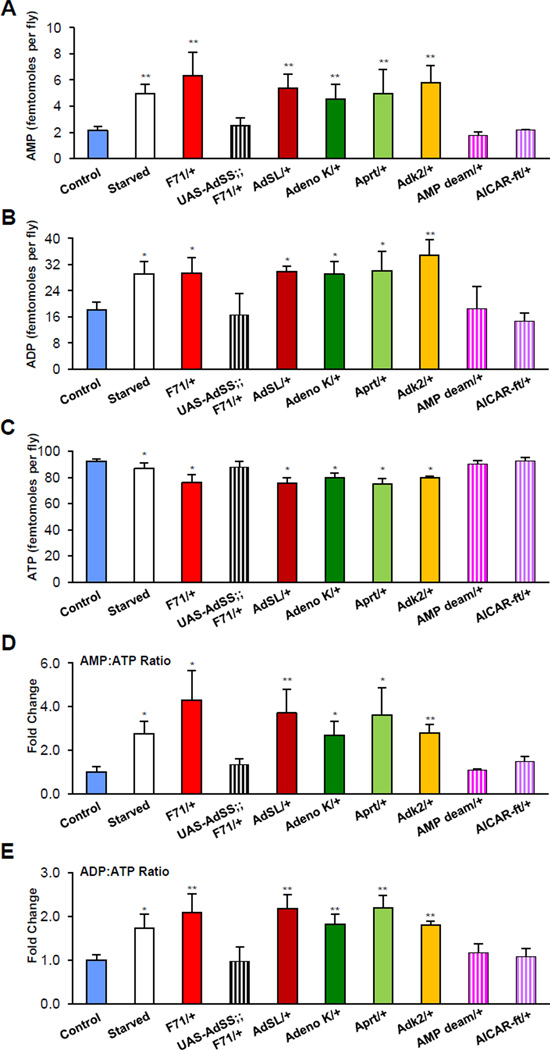
A plausible consequence of mutating components of adenosine nucleotide synthesis is the altered concentration or ratios of the products in vivo. Data in worms indicates that the ratios of these products (e.g., AMP:ATP) might be predictive of lifespan, for example, they increase in response to caloric restriction (Apfeld et al., 2004; Greer et al., 2007). To explore whether this effect might be conserved, we performed high-performance liquid chromatography (HPLC) on perchloric acid extracts of adult flies randomized to either normal feeding or 48 hours of food withdrawal (“starved”). We found that AMP and ADP concentrations were increased in the latter group, while ATP levels were reduced (Figure 4A–4C). Next we tested the possibility that the AMP biosynthetic pathways might also regulate AMP, ADP or ATP concentration. We found that heterozygous mutants that were long-lived had increased AMP and ADP, and reduced ATP concentrations, changes similar to those observed with food withdrawal; however, those with wild-type survival curves (e.g., AMP deaminase and AICAR-ft heterozygotes) had quantities equal to controls (Figure 4A–4C). Transgenic expression of AdSS in the F71 heterozygous mutant background, which rescued lifespan extension (Figure 1I and 1J), also restored AMP, ADP, and ATP levels (Figure 4A–4C). Of note, no significant alterations in total adenosine derivative pools were reached in any heterozygous mutant (data not shown); however, we cannot rule out a possible contribution of reduced total adenosine nucleotide derivatives that may escape detection limits.
Figure 4. AMP biosynthesis enzymes affect adenosine nucleotide derivatives.
(A–C) Food deprivation (Starved) and those heterozygous mutations of AMP biosynthesis components that increase lifespan also increase AMP and ADP, and reduce ATP concentration compared to controls. AMP deaminase or AICAR-ft mutations do not affect lifespan or adenosine nucleotide concentration. Transgenic overexpression of wild type AdSS (black hatched bar) rescues the alterations observed in F71 heterozygotes. Data presented ± s.e.m. (* Pπ.05, ** P<0.01, student’s t test). Throughout the figure hatched bars indicate genetic alterations that do not affect lifespan.
(D and E) Food deprivation and heterozygous mutations of AMP biosynthesis components that increase lifespan increase AMP:ATP (D) and ADP:ATP (E) ratios compared to controls. AMP deaminase or AICAR-ft mutations do not affect lifespan, AMP:ATP or ADP:ATP ratios. Transgenic overexpression of wild type AdSS (black hatched bar) rescues the increased AMP:ATP and ADP:ATP ratios observed in F71 heterozygotes. Data presented ± s.e.m. (* P<0.05, ** P<0.01, student’s t test).
AMP:ATP and ADP:ATP ratios are key determinants of cellular energy status and are potent allosteric regulators of the evolutionarily conserved cellular energy sensor, Adenosine monophosphate-activated protein kinase (AMPK) (Xiao et al., 2011). To further understand the extent to which these ratios are influenced by the lifespan extending heterozygous AMP synthesis mutations, and to further normalize the HPLC data, we calculated the AMP:ATP and ADP:ATP ratios. We found that AMP:ATP ratios were approximately 3–4 fold and ADP:ATP approximately 2 fold higher in heterozygous AMP synthesis mutants with increased lifespan, similar to the ratios observed upon food withdrawal (Figure 4D and 4E). The ratios of the transgenic rescued flies and the flies with normal lifespan were not significantly altered (Figure 4D and 4E hatched bars).
AMP biosynthesis pathway mutants have increased AMPK activity and their longevity depends on AMPK
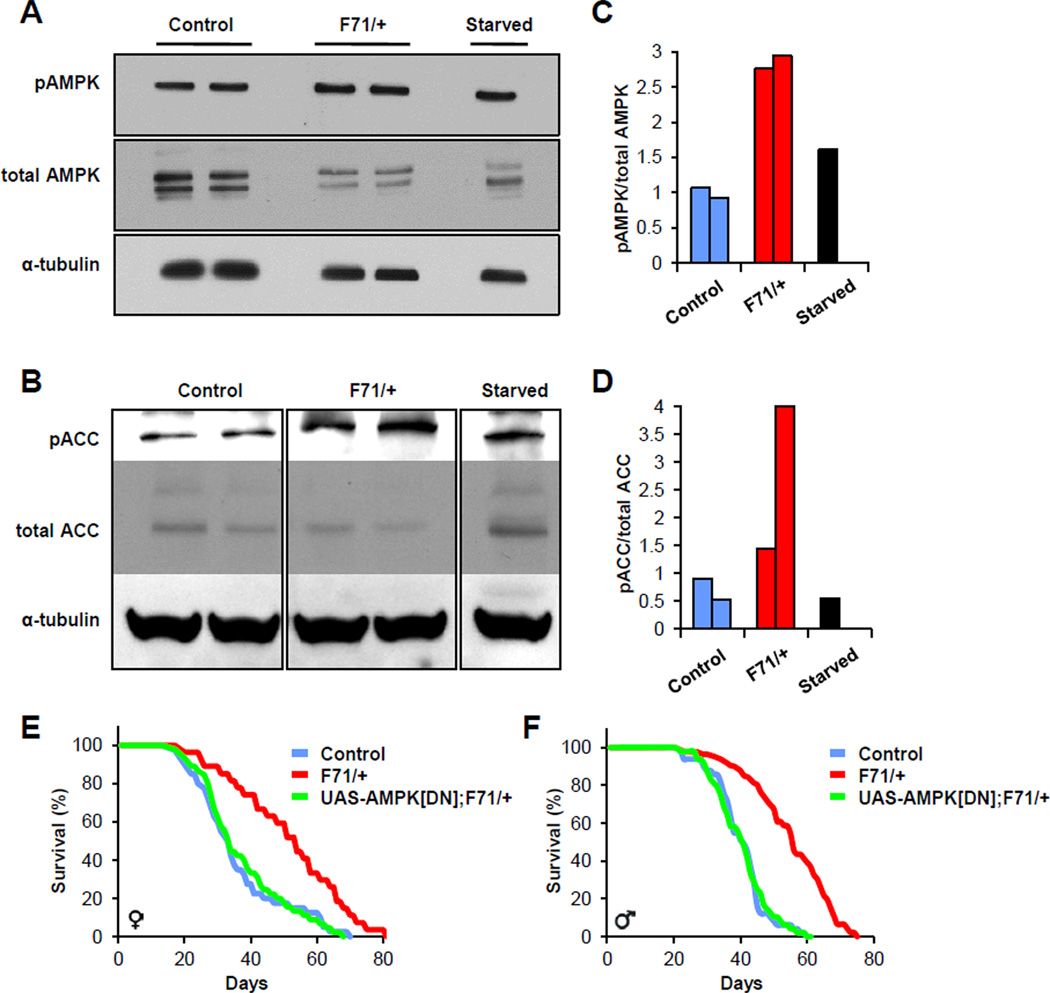
Increased AMP:ATP and ADP:ATP ratios, as observed in the AMP biosynthesis mutants, signal via AMPK to maintain cellular energy homeostasis (Pan and Hardie, 2002). In this manner, AMPK senses cellular energy to coordinate enzymes and transcription factors shifting metabolic processes between energy utilization and energy conservation/generation (Carling, 2004; Hardie, 2007; Kahn et al., 2005). The increased ratios of AMP:ATP and ADP:ATP observed in the AMP biosynthetic pathway longevity mutants indicate that AMPK activity might be increased in the heterozygous mutants. To test this, we harvested adult control and F71 heterozygous mutant flies, and probed AMPK activity, reflected by the phosphorylation state of AMPK and its downstream target acetyl-CoA carboxylase (ACC); extracts of flies that were deprived of food for 48 hours served as a positive control. We found that levels of AMPK and ACC phosphorylation were increased in the mutants, supporting the idea that AMPK was activated (Figure 5A–D).
Figure 5. AMPK is activated in the heterozygous mutants and is required for their longevity benefit.
(A and B) Food deprivation (Starved) and F71 heterozygous mutation increases AMPK and ACC phosphorylation. Each panel presented is from analysis of a single blot.
(C and D) ImageJ quantification of increased AMPK and ACC phosphorylation, respectively.
(E and F) Dominant negative AMPK (AMPKDN) rescues F71 heterozygous lifespan extension (log rank, P>0.5). Also see Table S1.
To test whether AMPK activity was necessary for the lifespan benefit of heterozygous AMP biosynthesis mutants we used the GAL4 present in the F71 insertion to drive expression of a UAS-AMPK dominant negative allele (UAS-AMPKDN) in AdSS heterozygous mutants. We found that expression of dominant negative AMPK eliminated the lifespan benefit seen in male and female F71 heterozygotes (Figure 5E and 5F). These data indicate that AMPK is activated in the heterozygous AMP biosynthesis mutants and that the lifespan extension of the heterozygotes depends upon AMPK. Further, the observation that dominant negative AMPK suppressed the longevity of F71 heterozygous mutants when driven by the GAL4 present in the F71 locus suggests that the effects of AMP synthesis mutation on lifespan determination may act cell autonomously.
Transgenic activation of AMPK in the adult fat body or muscle increases lifespan
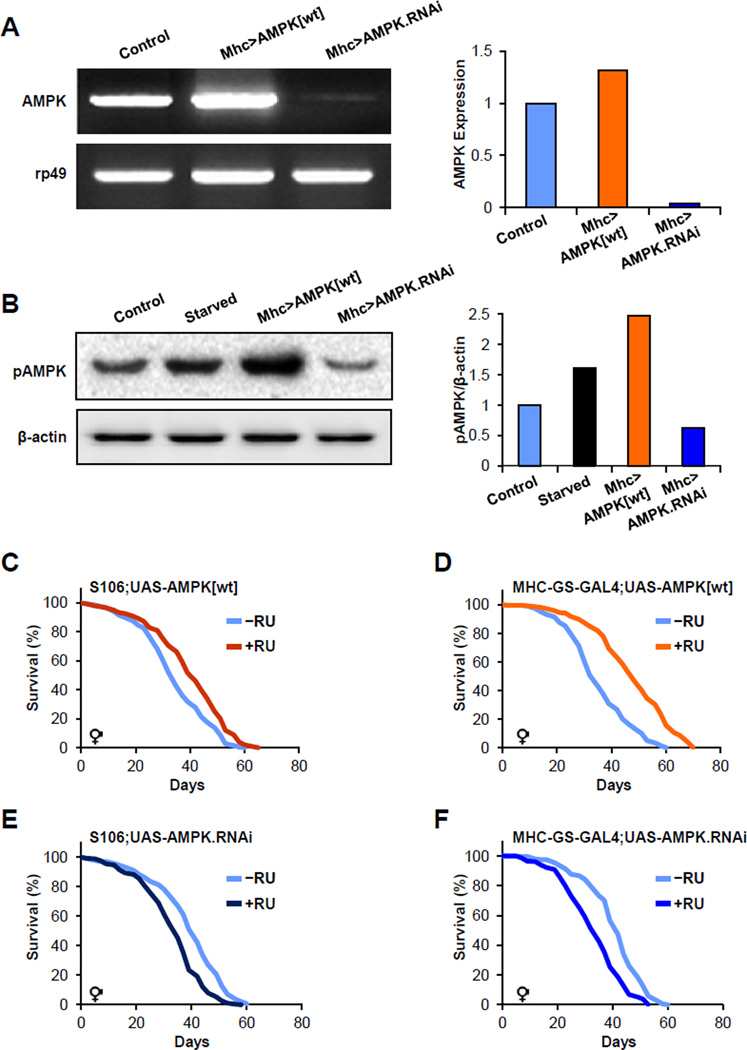
We attempted to further dissect the role of AMPK in lifespan control by increasing or decreasing its levels through transgenesis. Based upon the tissue and cell autonomous necessity of AMPK in the lifespan extension of the F71 mutant we first evaluated the tissues in which the F71 mutation, and hence AMPK, might act to regulate lifespan. For this, we exploited the F71-GAL4 insertion to drive a UAS-eGFP allele and then examined GFP fluorescence. We detected strong GFP expression in the larval and adult fat body, muscle and some in brain (Figure S3); tissues that are key regulators of fly, and mammalian, metabolism and longevity. These results also provided a subset of tissues in which to test the hypothesis that AMPK might regulate lifespan. We also attempted to explore the notion that AMPK might govern adult longevity, as dietary restriction is often tested in adults. So we combined a UAS-AMPKwt or a UAS-AMPK RNAi (AMPK-RNAi) allele with two different RU486-inducible GeneSwitch GAL4 lines: fat body (S106 GAL4) or muscle (myosin heavy chain GeneSwitch GAL4, MHC-GS-GAL4). Then, we randomized adults containing the appropriate genetic arrangements to vehicle or RU486. Of note, AMPK transgenesis increased AMPK expression levels and AMPK activity while AMPK RNAi had the opposite effects (Figure 6A and 6B). We next examined survival and found that adult-specific expression of AMPK in the fat body or in muscle increased Drosophila lifespan (Figure 6C and 6D); conversely fat body and muscle specific AMPK RNAi reduced lifespan (Figure 6E and 6F). These data indicate that AMPK plays a conserved role in lifespan determination in a tissue- and adult-specific manner. They also support a model in which heterozygous AMP biosynthesis mutations alter adenine nucleotide ratios, which in turn activate AMPK, and then lead to increased longevity.
Figure 6. Tissue specific AMPK function controls adult lifespan determination.
(A) AMPK or AMPK RNAi transgenesis alters AMPK transcript levels in the expected manner (right, ImageJ quantification).
(B) Tissue specific overexpression of AMPK increases AMPK phosphorylation, while AMPK RNAi reduces AMPK phosphorylation (right, ImageJ quantification).
(C) Adult fat body expression of a wild-type AMPK (UAS-AMPKwt) via the S106 RU486-inducible GAL4 driver (S106) extends lifespan (log rank, P<0.00001).
(D) Muscle expression, using the myosin heavy chain GeneSwitch GAL4 driver (MHC-GS-GAL4), of AMPKwt during adulthood extends lifespan (log rank, P<0.00001).
(E) Adult fat body expression via S106 of an RNAi allele targeting AMPK reduces lifespan (log rank, P<0.00001).
(F) Adult muscle expression, via MHC-GS-GAL4, of an RNAi allele targeting AMPK reduces lifespan (log rank, P<0.00001). Also see Table S1.
Adenine supplementation restores adenosine nucleotide ratios and rescues longevity conferred by AMP synthesis mutation or dietary restriction
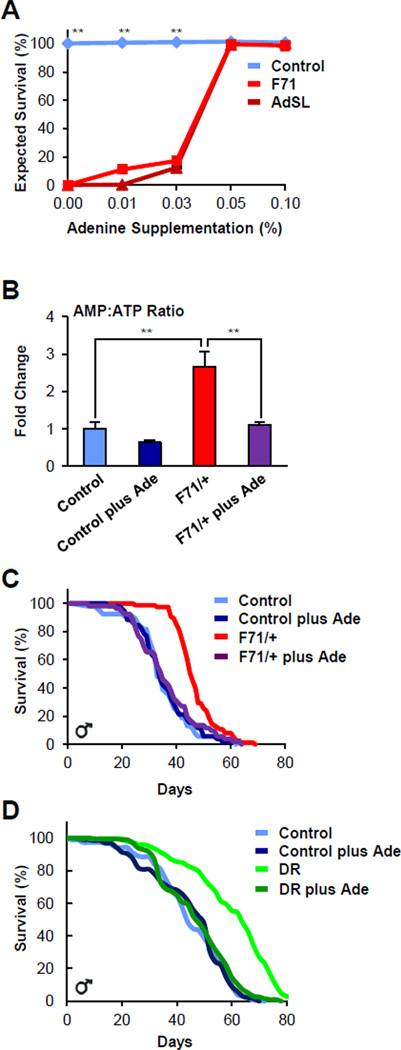
Since AMP biosynthesis can occur through the modification of adenine via the salvage pathway (Figure 2), we hypothesized that dietary adenine supplementation might affect adenosine derivative pools. To identify an appropriate concentration of adenine, we exploited the larval lethality of mutant AdSS and AdSL homozygosity to determine whether adenine supplementation rescued the mortality. We found that adding 0.05% and 0.1% (w/v) adenine to the diet produced Mendelian ratios of both AdSS and AdSL homozygous pupae (Figure 7A). Unfortunately, the resulting pupae remained pharate, perhaps due to the lack of continued adenine consumption during metamorphosis, which precluded adult lifespan analysis of the rescued larvae. To attempt to circumvent this issue, and also to assess potential adult-specific lifespan extension, we supplemented the diet of adult control and F71 heterozygotes with 0.05% (w/v) adenine, a concentration that rescued larval lethality. Adenine supplementation restored the ratio of AMP:ATP and ADP:ATP in F71 heterozygotes to that of unsupplemented controls (Figure 7B, not shown). Notably, adenine addition rescued heterozygous lifespan extension (Figure 7C). Adenine feeding had no effect on control lifespan, indicating that the supplementation at this dose was not significantly toxic (Figure 7C).
Figure 7. Dietary adenine supplementation blocks adult lifespan extension.
(A) Dietary adenine supplementation [0.05% or 0.1% (w/v)] restores expected Mendelian ratios of homozygous AdSS and AdSL larval mutant lethality. Data presented ± s.e.m. (* P<0.05, ** P<0.01, student’s t test).
(B) Adding 0.05% (w/v) adenine to the diet of adult F71 heterozygotes restores AMP:ATP ratios to control levels. Data presented ± s.e.m. (* P<0.05, ** P<0.01, student’s t test).
(C) Supplementing the diet of adult F71 heterozygotes with 0.05% (w/v) adenine rescues lifespan extension, but does not alter control longevity (log rank, P>0.3).
(D) Dietary adenine supplementation blocks the lifespan benefit of dietary restriction (DR) (log rank of DR compared to all other curves, P<0.00001; log rank comparing all other curves, P>0.1). Also see Table S1.
The reversal of lifespan extension of F71 heterozygotes by dietary adenine suggested that other lifespan-extending interventions, particularly those acting through AMPK activation, might also be influenced by adenine supplementation. In C. elegans, AMPK is necessary for some caloric restriction protocols (Apfeld et al., 2004; Greer and Brunet, 2009; Greer et al., 2007; Mair et al., 2011). It seemed plausible that some of the life-extending effects of caloric restriction (CR) might be due to reduced adenine consumption. So we next tested the effect of adding adenine to the diet of calorie restricted long-lived flies. For this, we randomized adult wild-type flies to normal conditions or dietary restriction (DR), and then subdivided each of these groups to with or without 0.05% (w/v) adenine supplementation. We found that the dietary adenine supplementation reduced the longevity benefit of dietary restriction (Figure 7D). Thus, a single dietary component can regulate adult lifespan, in both long-lived AMP biosynthesis mutants and long-lived calorically restricted flies.
DISCUSSION
Lifespan is controlled by a complex interaction of genetics and diet (Fontana et al., 2010; Houtkooper et al., 2010; Libert et al., 2007; Piper and Bartke, 2008; Sinclair, 2005). Work over the past few decades has identified caloric restriction and a few molecular mechanisms as conserved routes to lifespan extension (McGuire et al., 1996; Meredith et al., 1996). The proposed benefit of increased lifespan is obvious; most humans would like to live longer. Longevity protocols not only increase lifespan, but they can also increase health at old age and can delay the onset and morbidity of disease (Berrington et al., 1996; Partridge et al., 1996a; Sampalis et al., 1996). That is, they increase lifespan and “healthspan” (Suh et al., 2008). This indicates that therapeutic strategies to initiate pro-longevity pathways might be an important goal, and could, for example, increase well being while reducing health care costs and the need for health care access, which are currently major topics of concern. Both dietary and pharmaceutical strategies are plausible methods to affect such benefits. However, the unpleasantness of markedly reduced caloric intake, a variety of side effects, and various barriers to clinical trials have precluded widespread use of either modality. Potential approaches to ameliorate some of these issues include: directed dietary manipulations, demonstration of pathway effectiveness across evolutionary distance, adult specificity, and characterization of pathway components with adequate druggability. The latter can include enzymes, especially those with dosage-sensitive effects.
To uncover potentially novel mechanisms of lifespan determination, we generated and screened a collection of metabolically targeted insertional mutants for lifespan extension. We found that biochemical pathways that control AMP biosynthesis regulate longevity and that lifespan benefit was observed in heterozygous mutants. The mechanism of lifespan extension involves increased AMP:ATP and ADP:ATP ratios. Although the increased ratios may seem counterintuitive to the mutations, feedback regulation, impinging mechanisms, and pool maintenance could be underlying. Previous studies in worms and yeast have been key drivers identifying conserved pathways of lifespan extension, such as sirtuin, TOR, and insulin signaling, that appear functional in mammals (Greenberg et al., 1996; Harrison et al., 2009; Partridge, 1996). Studies in these two organisms also amplify the role of AMP biosynthesis and altered AMP:ATP and ADP:ATP ratios in longevity. A yeast expression profiling screen identified many potential genes important in lifespan including purine import and biosynthesis (Matecic et al., 2010). In worms, AMP:ATP and ADP:ATP ratios are elevated in some longevity mutants, in response to caloric restriction, and may be predictive of lifespan (Apfeld et al., 2004; Curtis et al., 2006). Thus our studies indicate conservation across significant evolutionary distances.
AMPK is a major, and conserved, sensor and regulator of intracellular energy homeostasis (Pan and Hardie, 2002). Increased ratios of AMP:ATP and ADP:ATP, such as those present in the AMP biosynthesis pathway mutants, activate AMPK. In turn, AMPK orchestrates a host of responses and downstream targets, including various enzymes and transcription factors, to allow cells to respond to metabolic challenges, thereby transforming the metabolic state from energy consuming to energy storing and generating (Carling, 2004; Hardie, 2007; Kahn et al., 2005). Thus, the increased nucleotide ratios observed in the AMP biosynthesis longevity mutants, led to several testable hypotheses: 1) Was AMPK activated in AMP biosynthesis heterozygous mutants, 2) Might AMPK regulate longevity 3) Might dietary manipulation of reaction substrates, in our case adenine, reverse the lifespan extension of AMP biosynthesis heterozygous mutants, 4) Might adenine or other small molecules that are part of AMP biosynthesis (substrates or products), which are plentiful in the diet, play a role in the longevity associated with caloric restriction.
We performed a series of studies to attempt to dissect possible roles of AMPK in lifespan control. First, we examined the notion that AMPK was activated in long-lived mutant flies with elevated AMP:ATP and ADP:ATP ratios. We probed AMPK activity by examining phosphorylation levels of relevant substrates using Western blots, comparing extracts of adult control and heterozygous mutants. Based upon the phosphorylation status of AMPK and its downstream target ACC, AMPK was activated in the adult long-lived mutants. We then attempted to extend these findings probing the idea that AMPK activity might be more than just a marker of the altered ratios, and perhaps was even required for the lifespan benefit observed in the mutant flies. To test this, we reduced AMPK activity by expressing a dominant negative form of AMPK and in the tissues in which the mutation was expressed, exploiting the UAS-GAL4 system, inherent to our initial insertional screen. This maneuver reversed the lifespan benefit, supporting the concept that AMP biosynthesis pathway mutant longevity depends on AMPK.
The observations that nucleotide ratios and AMPK activity were elevated in adult long-lived mutants and also in flies under starved conditions, a crude surrogate of acute caloric restriction, and that AMPK activity appeared required for their lifespan extension raised the possibility that AMPK might play a more general role in adult lifespan control. To attempt to examine this notion, we transgenically expressed wild-type AMPK and AMPK RNAi using the inducible Gene Switch system, which provided both temporal and spatial control. As AMPK can function in a cell autonomous fashion and because the UAS-AMPKDN;F71/+ necessity tests indicated AMPK was required in the tissues affected by the F71 insertion, we examined the expression of the insertion. We found strong levels in larval and adult fat body and muscle. So we focused our experimentation to these tissues and attempted to increase or decrease AMPK by combining UAS-AMPKWT and UAS-AMPK RNAi –transgenes with fat body and muscle RU-486 inducible GAL4 alleles. Notably, adult specific transgenic expression of AMPK within tissues strongly expressing AMP biosynthesis pathway components, fat body and muscle, extended Drosophila lifespan and AMPK RNAi had the opposite effect.
To further dissect the role of AMP biosynthesis in longevity, we attempted to alter the nucleotide ratios through manipulation of the nucleotide concentration in the diet. As adenine is a substrate for the AMP biosynthesis salvage pathway, it seemed a logical choice as a means to restore the ratios in the de novo pathway mutants. Consistent with this notion, adenine rescued the larval lethality of the de novo mutants (e.g., AdSS and AdSL). Using a dose that rescued larval lethality, we began to probe the ratios in adult AdSS mutant flies after adenine feeding. We found that adult dietary adenine supplementation restored the adenosine nucleotide ratio to that of control flies. Remarkably, the same dose rescued lifespan extension, further supporting the possibility that the altered ratios and the lifespan extension were mechanistically linked.
The results that adenine dietary manipulation in mutant adults was sufficient to control lifespan harkened to the well-established relationship between dietary restriction and longevity. Further, we had found, similar to data in worms, that food restriction altered the ratios of AMP:ATP and in exactly the opposite direction to that of adenine supplementation. So we examined the effects of adenine addition to calorie restricted flies. Remarkably, supplementing 0.05% adenine in the food effectively reversed the longevity benefit associated with dietary restriction. These data raise the possibility that decreased levels of adenine derivatives from either dietary consumption or endogenous synthesis may account for some of the lifespan benefits conferred by caloric restriction.
Our observations in flies support the role of AMP biosynthesis, AMP:ATP and ADP:ATP ratios, and AMPK as lifespan regulators that can function across broad evolutionary distances. These roles appear dosage-sensitive, cell autonomous, and functional in adults based upon dietary adenine supplementation and genetic manipulation. These results suggest that interventions reducing adenine conversion to nucleotides might be plausible methods to extend lifespan. It is also possible that reducing dietary adenine, which may be less arduous than caloric restriction, might be a new approach. In addition, the dosage sensitivity and enzymatic nature of de novo and salvage AMP biosynthesis, and the conserved aspects of adenosine nucleotide derivatives and lifespan extension, indicate that these pathways are potential targets amenable to small molecule manipulation and worth continued exploration. AMP biosynthesis pathway mutants require AMPK for lifespan extension and thus the AMPK pathway represents yet another set of targets that might be relevant. AMPK activation is known to produce beneficial effects in disease states, e.g. increase glucose uptake in people with Type II diabetes, and clinically approved AMPK agonists are currently prescribed. Our data indicate that AMPK activation produces lifespan extension in a conserved manner. Furthermore, we demonstrate that AMPK activation within specific tissues during adulthood is sufficient for longevity. Taken together our data support potential dietary and therapeutic interventions that may extend lifespan as well as improve healthspan.
Experimental Procedures
Fly Stocks and Culture
Flies were reared under uncrowded conditions in standard cornmeal dextrose-agar-yeast media with yeast granules unless otherwise noted. All lines involved in the enhancer trap screen are as described (Suh et al., 2008). The location of the P-insertion site for F71 was determined by inverse PCR and plasmid rescue. The additional AdSS insertional line, HA2022 (P{RS5}CG17273[5-HA-2022]), was obtained from the Drosophila Genetic Resource Center. AdSL (PBac{w[+mC]=PB}CG3590[c02781]/TM6B,Tb1), AdenoK (P{w[+mC] y[+mDint2]=EPgy2}CG11255[EY07694]), Aprt (ru[1] Aprt[5] h[1]), AMP deam (P{w[+mC]=EPg}CG32626[HP10734]) lines were obtained from the Bloomington Stock Center. Adk2, (P{EP)Adk2[EP2149]) was obtained from the Exelixis Collection at Harvard Medical School. All lines were backcrossed at least 10 generations into w1118, which was used as the control unless otherwise noted. MHC-GS-GAL4 (muscle gene switch) was generously provided by T. Osterwalder (Osterwalder et al., 2001). The fat body inducible GAL4 driver was obtained from Bloomington Stock Center (Roman et al., 2001). The UAS-AMPKwt allele was a gift from J. Chung (Lee et al., 2007). UAS-AMPK-RNAi (v106200) was obtained from the Vienna Drosophila RNAi Center.
Transgenic Flies
Full-length AdSS cDNA was PCR amplified and cloned into pUAST from the cDNA clone RE23826 to generate pUAST-AdSS. Transgenic lines harboring pUAST-AdSS were generated using P-element-mediated germline transformation as described previously (Chen and McKearin, 2003; Rubin and Spradling, 1982). Primer sequences used for PCR amplification and sequence verification are available upon request. The dominant negative AMPK allele, UAS-AMPKDN, (P{w[+mC]=UAS-SNF1A.K57A}2), was previously described (Johnson et al., 2010) and acquired from the Bloomington Stock Center. Controls for all experiments involving transgenic flies were UAS allele alone.
Lifespan Assays
Flies that emerged within a 2 day period were pooled and aged for an additional 3 days. Approximately 80 males and 80 females were placed in demography cages in duplicate or triplicate cohorts per trial. Assays were performed at room temperature (22–23°C) as described previously (Hwangbo et al., 2004) and mortality scored daily when changing to fresh food vials. For the inducible experiments, the GAL4 (GeneSwitch, GS) was activated in 1–2 day old adult male or female flies by continuous exposure to medium containing 200 µM RU486 (Mifepristone, Sigma). For these experiments, flies on a diet containing only vehicle were used as controls and fresh medium was provided every 2 days.
RNA Extraction, cDNA Synthesis and Real-time PCR
Total RNA from adult males was extracted using TRIzol (Invitrogen), DNase I-treated, and reverse-transcribed with random hexamers, gene expression was analyzed using the ABI 7500 Real-Time PCR System. Data were normalized over endogenous ribosomal protein 49 expression. All real-time primer sequences were validated for specificity and efficiency prior to use. Sequences are available upon request.
Nucleotide Measurements
Adult flies were isolated in sex-specific groups of 40, washed, and placed on ice. Ice-cold 8% (v/v) HClO4 was added and samples immediately subjected to repeat sonication. The solution was then neutralized with 1 N K2CO3 and centrifuged. The supernatant was passed through a 0.2-µm filter, and subjected to reversed phase chromatography using a Targa C18 250 × 4.6 mm 5-µm column as described (Stocchi et al., 1985). Nucleotides were detected at 254 nm, and peak areas were measured using 32 Karat software. Nucleotide identities were confirmed by co-migration with known standards. Food deprived flies (Starved) were given water only for 48 hours prior to measurement. Adenine supplemented (plus Ade) flies were given either 0.05% w/v adenine supplemented or control diet (see below) prior to analysis.
Western Blot Analyses
Whole fly lysates boiled in SDS sample buffer [58.3 mM Tris-HCl (pH 6.8), 6% glycerol, 0.002% bromophenolblue, 1.7% SDS, and 0.1M DTT] were resolved by SDS–PAGE and transferred to nitrocellulose membranes. Membranes were blocked and probed using primary antibodies to phospho-AMPK (Cell Signaling Technology), phospho-DmACC (Kinasource), total AMPK (Abcam), total ACC (Pierce) and alpha-tubulin (Cell Signaling Technology). After wash and incubation with secondary antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch) signals were detected with ECL prime chemiluminescent detection reagent (Amersham).
Adenine Supplementation
Standard diet contained approximately 64.2 g cornmeal (MP Biomedicals), 16.0 g dry active yeast (Fleischmann’s), 12.4 g agar (MoorAgar), 85.5 mL molasses (Grandma’s), 8.5 ml Tegosept (240 g l−1 in ethanol; Genesee), and 2.4 ml Propionic Acid (Fisher) per liter. 10X adenine hemisulfate (Sigma #A9126) stock solutions were prepared fresh in ddH2O with 30% (w/v) autolysed yeast powder (Genesee) and added in order to avoid additional yeast supplementation. Stock solution was then added to 55 °C molten standard diet to generate proper 1X concentration. An equal amount of autolysed yeast solution without adenine added to standard diet was used as control. For larval lethality experiments, progeny from w1118;;TM6B,Tb1/+, F71/TM6B,Tb1, or AdSL/TM6B,Tb1 adults were cultured in bottles containing adenine supplemented diet. Percent homozygous pupae was calculated based on the absence of the TM6B,Tb1 balancer chromosome. Expected survival percentage was calculated after normalization to the expected homozygous Mendelian ratio of 1:3. For lifespan assays 0.05% adenine supplemented food prepared fresh twice throughout the experiment, and stored at 4 °C.
Dietary Restriction
Control diet contained approximately 160 g (16%) yeast, 110 g sucrose, 52 g cornmeal, 8 g agar, 1.8 ml Tegosept, and 5 ml Propionic Acid per liter. Restricted diet maintained control composition save reduction to 4% yeast. Adenine supplementation was achieved through addition of a stock solution to 0.05%. Three replicates were performed.
Statistical Analyses
Log-rank tests were performed for survival curves. Differences between experimental and control groups were assessed using the student’s unpaired t test. Statistical significance was determined at a value of p < 0.05 or p < 0.01 and is denoted with a single or double asterisk, respectively. Error bars represent standard error of the mean (s.e.m.).
Supplementary Material
HIGHLIGHTS.
Heterozygous AMP biosynthesis mutations act via AMPK to extend lifespan.
Tissue and adult specific transgenic expression of AMPK extends lifespan.
Dietary adenine reverses the lifespan extension of adult AMP biosynthesis mutants.
Addition of dietary adenine blocks the longevity effects of dietary restriction.
Acknowledgements
We are grateful to Drs. Margaret Phillips and Elliot Ross, and their respective labs, specifically Chelsea Pratt, Suong Nguyen, and Jimmy Woodson, for use of equipment and helpful discussions. We thank the numerous stock centers and colleagues for fly stocks. We also thank members of the Graff lab for support, reagents, and insights. This study was supported by the National Institute of Health and the National Institute of Diabetes and Digestive and Kidney Disease grants (R01 DK066556, R01 DK064261 and R01 DK088220), Inha University and The National Research Foundation of Korea (NRF, 2011–0030133), and the KRIBB Research Initiative Program. J.M.G., D.S. J.M.S, K.S.L., K.Y., J.S.K. and K.J.M conceived, designed and interpreted the experiments. D.S., J.M.S., J.S., K.S.L., K.Y., J.S.K. and K.J.M. performed the experiments. J.M.G., D.S., and J.M.S wrote the paper. J.M.G. is a co-founder and shareholder of Reata Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antosh M, Whitaker R, Kroll A, Hosier S, Chang C, Bauer J, Cooper L, Neretti N, Helfand SL. Comparative transcriptional pathway bioinformatic analysis of dietary restriction, Sir2, p53 and resveratrol life span extension in Drosophila. Cell Cycle. 2011;10:904–911. doi: 10.4161/cc.10.6.14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, O'Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artal-Sanz M, Tavernarakis N. Mechanisms of aging and energy metabolism in Caenorhabditis elegans. IUBMB Life. 2008;60:315–322. doi: 10.1002/iub.66. [DOI] [PubMed] [Google Scholar]
- Berrington A, Partridge S, Bates C, Ridgway E. Community-sampling of blood in suspected meningococcal infection. Lancet. 1996;348:1103–1104. doi: 10.1016/s0140-6736(05)64452-2. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- Cordts R, Partridge L. Courtship reduces longevity of male Drosophila melanogaster. Animal Behaviour. 1996;52:269–278. [Google Scholar]
- Cordts RaP, Linda Courtship reduces longevity of male Drosophila melanogaster. Animal Behaviour. 1996;52:269–278. [Google Scholar]
- Curtis R, O'Connor G, DiStefano PS. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell. 2006;5:119–126. doi: 10.1111/j.1474-9726.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DP, Vadheim CM, Marcy SM, Partridge S, Jing J, Chiu CY, Greene T, Margolis HS, Ward JI. Safety and immunogenicity of a recombinant hepatitis B vaccine administered to infants at 2, 4 and 6 months of age. The Kaiser-UCLA Vaccine Study Group. Vaccine. 1996;14:811–816. doi: 10.1016/0264-410x(95)00228-s. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He NG, Singhal SS, Chaubey M, Awasthi S, Zimniak P, Partridge CA, Awasthi YC. Purification and characterization of a 4-hydroxynonenal metabolizing glutathione S-transferase isozyme from bovine pulmonary microvessel endothelial cells. Biochim Biophys Acta. 1996;1291:182–188. doi: 10.1016/s0304-4165(96)00064-5. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Williams RW, Auwerx J. Metabolic networks of longevity. Cell. 2010;142:9–14. doi: 10.1016/j.cell.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Kazgan N, Bretz CA, Forsberg LJ, Hector CE, Worthen RJ, Onyenwoke R, Brenman JE. Altered metabolism and persistent starvation behaviors caused by reduced AMPK function in Drosophila. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kwon OY, Lee JH, Kwon K, Min KJ, Jung SA, Kim AK, You KH, Tatar M, Yu k. Drosophila short neuropeptide F signalling regulates growth by ERK-mediated insulin signalling. Nat Cell Biol. 2008;10:468–475. doi: 10.1038/ncb1710. [DOI] [PubMed] [Google Scholar]
- Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matecic M, Smith DL, Pan X, Maqani N, Bekiranov S, Boeke JD, Smith JS. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1000921. e1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin versus paclitaxel and cisplatin: a phase III randomized trial in patients with suboptimal stage III/IV ovarian cancer (from the Gynecologic Oncology Group) Semin Oncol. 1996;23:40–47. [PubMed] [Google Scholar]
- Meredith RF, Partridge EE, Alvarez RD, Khazaeli MB, Plott G, Russell CD, Wheeler RH, Liu T, Grizzle WE, Schlom J, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with lutetium-177-CC49. J Nucl Med. 1996;37:1491–1496. [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol A Biol Sci Med Sci. 2010;65:695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin Y-K, Canto C, Scheibye-Knudsen M, et al. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1 doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DA, Hardie DG. A homologue of AMP-activated protein kinase in Drosophila melanogaster is sensitive to AMP and is activated by ATP depletion. Biochem J. 2002;367:179–186. doi: 10.1042/BJ20020703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge EE, Phillips JL, Menck HR. The National Cancer Data Base report on ovarian cancer treatment in United States hospitals. Cancer. 1996a;78:2236–2246. doi: 10.1002/(sici)1097-0142(19961115)78:10<2236::aid-cncr28>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Partridge F, Richardson W, Kearns P, Wilcox R, Majumdar G. Marked bone marrow eosinophilia at the time of relapse of acute myeloblastic leukaemia in association with the appearance of translocation t(12;20)(q24;q11) Leuk Lymphoma. 1996b;22:181–182. doi: 10.3109/10428199609051747. [DOI] [PubMed] [Google Scholar]
- Partridge PS. Learning from McDonald's.Making healthcare into a franchise. Profiles Healthc Mark. 1996;12:49–50. [PubMed] [Google Scholar]
- Piper MD, Bartke A. Diet and aging. Cell Metab. 2008;8:99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev. 2006;86:651–667. doi: 10.1152/physrev.00019.2005. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes RJ. Regulation of purine nucleotide biosynthesis: in yeast and beyond. Biochem Soc Trans. 2006;34:786–790. doi: 10.1042/BST0340786. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sampalis JS, Medsger TA, Jr, Fries JF, Yeadon C, Senecal JL, Myhal D, Harth M, Gutkowski A, Carette S, Beaudet F, et al. Risk factors for adult Still's disease. J Rheumatol. 1996;23:2049–2054. [PubMed] [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010;9:324–353. doi: 10.1016/j.arr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Stayton MM, Rudolph FB, Fromm HJ. Regulation, genetics, and properties of adenylosuccinate synthetase: a review. Curr Top Cell Regul. 1983;22:103–141. doi: 10.1016/b978-0-12-152822-5.50008-7. [DOI] [PubMed] [Google Scholar]
- Stocchi V, Cucchiarini L, Magnani M, Chiarantini L, Palma P, Crescentini G. Simultaneous extraction and reverse-phase high-performance liquid chromatographic determination of adenine and pyridine nucleotides in human red blood cells. Anal Biochem. 1985;146:118–124. doi: 10.1016/0003-2697(85)90405-1. [DOI] [PubMed] [Google Scholar]
- Suh JM, Stenesen D, Peters JM, Inoue A, Cade A, Graff JM. An RGS-containing sorting nexin controls Drosophila lifespan. PLoS One. 2008;3:e2152. doi: 10.1371/journal.pone.0002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth EA, Yeates TO. The structure of adenylosuccinate lyase, an enzyme with dual activity in the de novo purine biosynthetic pathway. Structure. 2000;8:163–174. doi: 10.1016/s0969-2126(00)00092-7. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes F, Quartey EL, Kiguwa S, Partridge M. Expression of TNF and the 55-kDa TNF receptor in epidermis, oral mucosa, lichen planus and squamous cell carcinoma. Oral Dis. 1996;2:25–31. doi: 10.1111/j.1601-0825.1996.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.