Abstract
Adipose-derived adult stem cells (ASCs), bone marrow mesenchymal stem cells (bmMSCs), and human umbilical cord perivascular cells (HUCPVCs) tissue have been widely tested for regenerative applications, such as bone regeneration. Moreover, olfactory ensheathing cells (OECs) show promise in promoting spinal cord injury (SCI) regeneration. Our group recently proposed the use of a hybrid scaffold targeting both vertebral bone repair and SCI regeneration. According to this concept, both MSCs and OECs should be in close contact to be influenced by the factors that are involved in secretion. For this reason, here we studied the effects of the OEC secretome on the metabolic activity and proliferation of ASCs, bmMSCs, and HUCPVCs. The stem cells' secretome effects on metabolic activity and proliferation of the OECs were also considered. In co-cultures of OECs with ASCs, bmMSCs, or HUCPVCs, the metabolic activity/viability, proliferation, and total cell numbers were measured after 2 and 7 days of culture. The results demonstrated that the secretome of OECs has a positive effect on the metabolic activity and proliferation of MSCs from different origins, especially on ASCs. Furthermore, in general, the stem cells' secretome also had a positive effect on the OECs behavior, particularly when ASCs were in co-culture with OECs. These results suggest that the most suitable combination of cells to be used in our hybrid scaffold is the OECs with the ASCs. Finally, this work adds new knowledge to the cell therapy field, bringing new information about paracrine interactions between OECs and distinct mesenchymal stems.
Introduction
Transplantation of cells with regenerative capabilities holds great promise for the treatment of several diseases. However, the properties of the tissue into which the cells are to be transplanted, as well as the intrinsic properties of the transplanted cells will significantly influence the success of the therapy. In spinal cord injury (SCI), the host environment is particularly important. For instance, after SCI, an environment of necrosis, edema, inflammation, and degeneration emerges [1]. This unfavorable host environment will influence the ability of the transplanted cells to engraft, proliferate, differentiate, and, thus, to contribute to the repair of the damaged tissue. Poor engraftment and survival of transplanted cells within the injury site remains a major limitation for cell therapy. The survival of transplanted cells is an essential prerequisite for any successful cell transplantation approach. However, another fundamental requirement is the incorporation of the grafted cells into the body of the host. Therefore, some authors have been studying approaches to support engraftment and/or survival of implanted cells. For instance, Chacko et al. studied the effect of hypoxia pretreatment on cell expression of functional proteins that may increase their survival and engraftment after transplantation [2]. Hydrogels have also been used as vehicles for cell transplantation in order to improve survival [3–5]. For instance, Johnson et al. reported that fibrin scaffolds can enhance survival of neural stem/progenitors cells (NSPCs) in a sub-acute model of SCI [4]. Work from the Shoichet lab shows that a combination of cyclic-AMP, fibrin, and chitosan channels greatly enhances the survival of NSPCs after transplantation in SCI rats [5]. Our group recently proposed the use of a hybrid tubular scaffold that comprises a rigid layer (composed by a blend of starch with polycaprolactone - SPCL), surrounding the hydrogel gellan gum [6]. According to this concept, the SPCL tubular structure assures mechanical stability to the entire construct, namely by establishing a connection to the adjacent vertebral bone [7], while the gellan gum hydrogel is aimed as a cell encapsulation system to support axonal regeneration in the injured spinal cord. In order to improve bone repair, mesenchymal stem cells (MSCs), such as adipose-derived adult stem cells (ASCs), human umbilical cord perivascular cells (HUCPVCs), or bone marrow MSCs (bmMSCs), can be seeded on the SPCL layer. Previous studies have demonstrated that these cells have the capability to undergo osteogenic differentiation and secrete extracellular matrix (ECM) that is rich in calcium phosphates (ECM typically found in bone tissues) [8–10]. Alternatively, cells such as olfactory ensheathing cells (OECs), which are known to support and guide olfactory axons, secrete several neurotrophic factors, grow through the glial scar, and promote motor improvements of SCI rats [11–15], are suitable candidates to be encapsulated in the hydrogel phase aimed at fostering axonal regeneration. In this sense, our therapeutic approach puts in close contact both the MSCs and the OECs, allowing the secreted factors by these cells to diffuse and interact with each other (see schematic representation on Fig. 1). For this reason, in this work, we studied the interactions of the OECs secretome on the proliferation, metabolic activity, and differentiation of ASCs, HUCPVCs, and bmMSCs, as well as the effects of the stem cells' secretome on OECs behavior. The portmanteau secretome is not often used in literature, but it was defined as “the global group of secreted proteins into the extracellular space by a cell, tissue, organ, or organism, at any given time and condition through known and unknown secretory mechanisms” [16]. In this sense, we expect to find the best suitable secretome combination, between OECs and adult stem cells, to be then used in our hybrid scaffold.
FIG. 1.
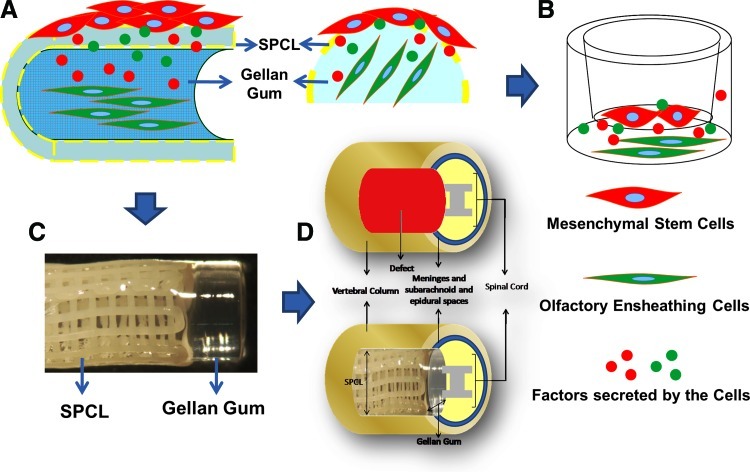
Rationality and schematic representation of the study. (A) Both adult stem cells (red cells seeded on the outer layer of the hybrid scaffolds) and olfactory ensheathing cells (OECs) (green cells encapsulated on the hydrogel) secreted factors that can affect the biological behavior of each other. (B) Transwell system used to study the effects of cell secretome. (C) Hybrid scaffold developed and previously characterized [5]. (D) Schematic representation of the scaffold implantation, with the SPCL layer at the vertebra bone level and the hydrogel at the nervous tissue level. Color images available online at www.liebertpub.com/scd
Materials and Methods
Cell culture
To evaluate the potential positive or negative effects of the cell secretome of OECs and adult stem cells, transwell co-culture experiments were performed. OECs were seeded (1×105 cells) on poly-D-lysine cover glass, and adult stem cells were seeded (4×104 cells) on the transwell. During the co-culture period, all conditions (co-cultured and control cells) were maintained in Dulbecco's modified Eagle medium supplemented with F12 (DMEM/F12, Gibco) with 10% of fetal bovine serum (FBS; Gibco) with Bovine Pituitary Extract (5 μg/mL; Gibco), Forskolin (2 μg/mL; Sigma), and 1% of antibiotic-antimycotic solution (Sigma) at 37°C and 5% CO2. Cell proliferation, metabolic activity, and differentiation were assessed after 2 and 7 days in culture. Cells were obtained and expanded as described next:
Olfactory ensheathing cells
Rat OECs were isolated as previously described [17]. Briefly, on olfactory bulb collection, all meninges were removed, and the tissue was digested with 0,125% Collagenase (Sigma) for 20 min at 37°C. The digested tissue was mechanically dissociated with a pipette and then filtrated through a 70 μm cell strainer (BD Falcon). After centrifugation at 1,000 rpm for 10 min, cells were resuspended and plated in uncoated wells for 18 h. Then, cells were plated again into new uncoated wells for 36 h; it is expected that most of the fibroblasts and astrocytes will attach in the first and second period, respectively. Finally, the cell suspensions were transferred to poly-D-lysine treated flasks and cultured in DMEM/F12 (Gibco) with 10% of FBS (Gibco) and 1% of antibiotic-antimycotic solution (Sigma) at 37°C and 5% CO2. OECs were enriched by medium supplementation with Bovine Pituitary Extract (5 μg/mL; Gibco) and Forskolin (2 μg/mL; Sigma).
Human umbilical cord perivascular cells
HUCPVCs were isolated according to the procedure originally described by Sarugaser et al. [18]. Pieces of cord were dissected by first removing the epithelium of the umbilical cord section along its length to expose the underlying Wharton's jelly. Each vessel, with its surrounding Wharton's Jelly matrix, was then pulled away, after which the ends of each dissected vessel were tied together with a suture, creating “loops” that were placed into a solution of 0.5–0.75 mg/mL collagenase (Sigma) with phosphate-buffered saline (PBS; Invitrogen/Gibco). After 18 h, the loops were removed from the suspension, which was then diluted with PBS to reduce the viscosity of the suspension and centrifuged. After the removal of the supernatant, cells were resuspended in culture media, α-MEM (Invitrogen/Gibco) supplemented with 10% FBS (Invitrogen/Gibco) and 1% antibiotic/antimycotic (Sigma), counted using a hemocytometer, and platted out in T75 flasks at a density of 4,000 cells/cm2. The culture medium was changed every 2/3 days. On confluence, cells were trypsinized and passaged to new T75 flasks. Using this isolation protocol, others have previously shown the isolation of a nonhematopoietic (CD45−, CD34−, SH2+, SH3+, Thy-1+, and CD44+) mesenchymal stem cell population capable of osteogenic, chondrogenic, and adipogenic differentiation [10,19].
Bone marrow-derived mesenchymal stem cells
BM-MSCs, acquired from Lonza, were cultured in α-MEM (Invitrogen/Gibco) supplemented with 10% FBS and a 1% antibiotic-antimycotic mixture. The culture medium was changed every 2/3 days. On confluence, cells were trypsinized and passaged to new T75 flasks. Cells were tested by Lonza for their ability to differentiate into osteogenic, chondrogenic, and adipogenic lineages. Moreover, cells are positive for CD105, CD166, CD29, and CD44 and negative for CD14, CD34, and CD45 [20].
Human adipose-derived adult stem cells
ASCs were isolated according to a protocol previously described by Dubois et al. [21]. All protocols were reviewed and approved by the Pennington Biomedical Research Center Institutional Research Boards (IRB) before the study. Liposuction aspirates from subcutaneous adipose tissue sites (abdomen, flank, and thighs) were obtained from female subjects undergoing elective plastic surgical procedures. Tissues were then digested in a 0.1% collagenase type I (Worthington Biochemical Corporation) prewarmed to 37°C for 60 min, after which they were centrifuged for 5 min at 300–500 g at room temperature. The supernatant, containing mature adipocytes, was aspirated. The pellet was identified as the stromal vascular fraction (SVF). The SVF were suspended and plated immediately in T225 flasks in Stromal Medium (DMEM/F 12 Ham's, 10% fetal bovine serum (Hyclone), 100 U penicillin/100 μg streptomycin/0.25 μg Fungizone) at a density of 0.156 mL of tissue digest/cm2 of surface area for expansion and culture. After reaching confluence, cells were passaged and kept in stromal medium. As previously shown, by using this isolation protocol, we are able to obtain cells capable of differentiating along multiple mesodermal lineage pathways. Moreover, the cell surface protein phenotype of ASCs resembles that of adult stem cells derived from bone marrow stroma [22].
Metabolic activity assay
Cell viability was measured using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega). This assay is based on the bioreduction of a tetrazolium compound (MTS), into a water-soluble brown formazan product. This conversion is accomplished by NADPH or NADH production by the dehydrogenase enzymes in metabolically active cells. Complete culture medium was replaced by standard DMEM/F12 medium containing MTS in a 5:1 ratio and incubated in a humidified atmosphere at 37°C and 5% CO2. After 3 h of incubation, the optic density for triplicates of each sample (n=3) was measured at 490 nm in a microplate reader.
Cell proliferation assessment
The cell proliferation rate was determined using the 5-bromo-2′-deoxyuridine assay (BrdU; Roche), which quantifies the BrdU incorporation during DNA synthesis in replicating cells. To perform the assay, BrdU was added to the cell cultures for 24 h and after that, the medium was removed. The cells were then fixed and their DNA was denatured with FixDenat (Roche) in a single step, after which the anti-BrdU peroxidase antibody (Roche) was added. The immune complexes were detected by the quantification of the substrate reaction product, measuring the optical density at 370 nm (reference filter set at 492 nm) in a multiplate reader (BioRad).
Immunocytochemistry and phalloidin/DAPI staining
The following primary antibodies were used for the immunocytochemical studies: monoclonal mouse anti-GFAP (Chemicon) for astrocytes; monoclonal mouse anti-O4 (R&D Systems) for oligodendrocytes; and polyclonal rabbit anti-p75 (Millipore) for OECs. For all immunohistochemical procedures, the appropriate controls were obtained by omission of the relevant primary antibody. Cells on the substrates were fixed with PBS solution containing 4% PFA for 20 min (on glass) or 1 h (on the hydrogel) at room temperature and then washed with PBS. After cell membrane permeation (except for p75 and O4 antibodies) and blocking by treating with 0.3% Triton X-100 (Sigma) and 10% of FBS solution at room temperature for 1 h, each specific primary antibody solution was added for 1 h. After washing with 0.5% of FBS in PBS, the samples were exposed to the specific secondary antibody (Invitrogen) for 1 h and then washed with 0.5% FBS. Finally, cell nuclei were counterstained with 1 μg/mL DAPI (Invitrogen) for 1 h.
For the phalloidin/DAPI staining, cells were fixed with 4% of paraformaldehyde for 30 min at room temperature and then treated with 0.3% Triton X-100. After PBS washing, 0.1 μg/mL of phalloidin (Sigma) was added to the cells during 30 min. Finally, cell nuclei were counterstained with DAPI (1 μg/mL; Invitrogen) for 10 min.
Statistical analysis
All statistical analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software). Differences among groups were assessed by 2-way ANOVA followed by a Bonferroni post-hoc test. A P-value of≤0.05 (95% confidence level) was set as the criteria for statistical significance. All data are presented as mean±standard deviation.
Results
Metabolic activity assessment
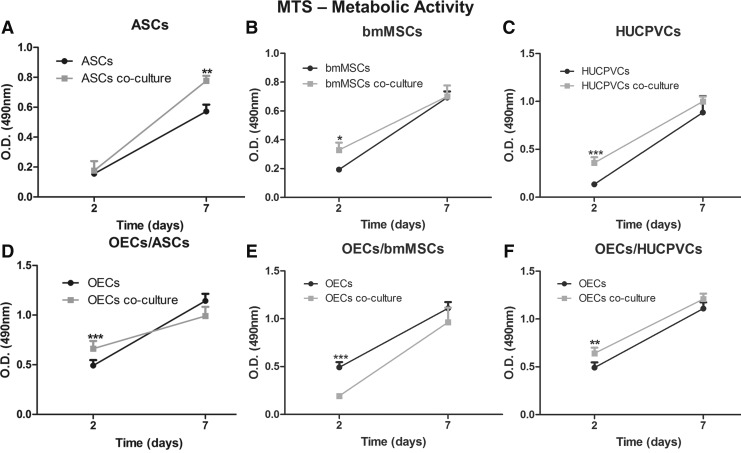
The MTS test revealed that the OECs secretome affects the metabolic activity of MSCs in distinct time-dependent manners. The mitochondrial activity of stem cells derived from the adipose tissue in co-culture with OECs was 1.4-fold higher (P<0.01) than when ASCs were cultured alone (Fig. 2A). However, this difference was only observed after 7 days of incubation. No differences were observed after 2 days of culture. On the contrary, for the stem cells derived from the bone marrow and from the umbilical cord, it was possible to observe a significantly higher metabolic activity when co-cultured with OECs after 2 days of incubation. The metabolic activity of co-cultured bmMSCs was 1.7-fold higher (P<0.05) than bmMSCs in monoculture (Fig. 2B), while the co-cultured HUCPVCs presented a 2.8-fold higher (P<0.001) activity than cells growing in monoculture (Fig. 2C) For these 2 cell populations, no differences were observed between controls and co-cultures after 7 days. Moreover, no detrimental effect of the OECs secretome on the metabolic activity of all adult stem cells used was observed.
FIG. 2.
Metabolic activity of OECs and adult stem cells culture in transwell systems and in monoculture. Metabolic activity of (A) adipose-derived adult stem cells (ASCs), (B) bone marrow mesenchymal stem cells (bmMSCs), and (C) human umbilical cord perivascular cells (HUCPVCs) in monoculture or in co-culture with OECs. Metabolic activity of OECs in monoculture or in co-culture with (D) ASCs, (E) bmMSCs, and (F) HUCPVCs. Values are shown as mean±standard deviation (n=3 samples of 2×104 adult stem cells and 1×105 OECs/sample, *P<0.05; **P<0.01; ***P<0.001).
The study of the stem cells' secretome effects on the metabolic activity of OECs revealed that, after 2 days of culture, each stem cell's secretome produced different outcomes on the OECs activity. The metabolic activity of the OECs was 1.3-fold higher (P<0.01) when co-cultured with ASCs (Fig. 2D) and HUCPVCs (Fig. 2F) than when in monoculture. In contrast, under the effects of the bmMSCs secretome, the OECs presented a 2.5-fold lower (P<0.001) metabolic activity than the control cells (Fig. 2E). All the effects observed after 2 days of culture, either positive or negative, were not observed after 7 days of culture, as the metabolic activity of OECs was similar in both cells in co-culture and controls.
Proliferation rate evaluation
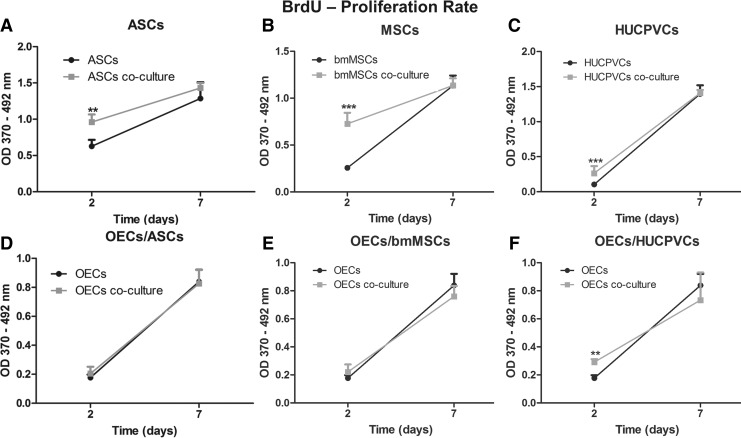
The BrdU incorporation during DNA synthesis in replicating cells was used to quantify the proliferation rate of the stem cells and OECs. Each of the stem cells was affected in a similar way by the OECs secretome. After 2 days of culture, all the distinct stem cells that were co-cultured together with the OECs presented significantly higher proliferation rates than the ones growing in monoculture. Co-cultured ASCs exhibited a 1.5-folder increase (P<0.01) with regard to the proliferation rate than the monoculture cells (Fig. 4A). In addition, co-cultured bmMSCs and HUCPVCs presented an approximately 2.5-fold increase (P<0.001) than monoculture cells (Fig. 4B, C). No differences in the proliferation rate of the distinct stem cells were detected after 7 days.
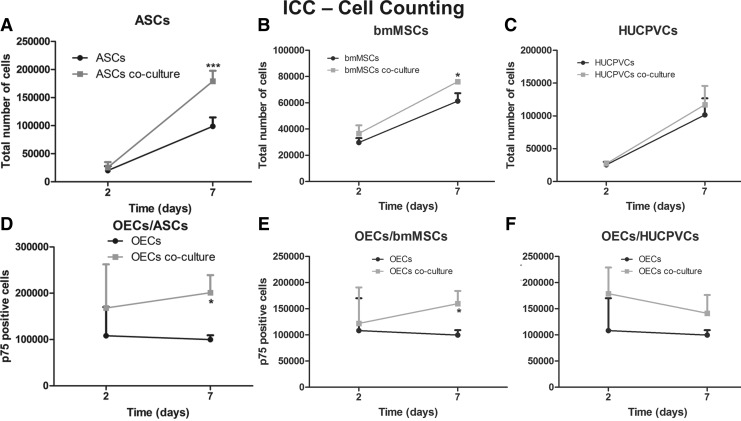
FIG. 4.
Immunocytochemistry counting of OECs and adult stem cells culture in transwell systems (gray line) and in monoculture (black line). Total number of (A) ASCs, (B) bmMSCs, and (C) HUCPVCs in monoculture or in co-culture with OECs. Total number of p75-positive cells (OECs) in monoculture or in co-culture with (D) ASCs, (E) bmMSCs, and (F) HUCPVCs. Values are shown as mean±standard deviation (n=3 samples of 2×104 adult stem cells and 1×105 OECs/sample, *P<0.05; ***P<0.001).
The study of the stem cells' secretome effect on the proliferation rate of OECs showed that just the secretome of HUCPVCs significantly influenced the OECs proliferation. Co-cultured OECs presented a 1.7-fold increase (P<0.01) of BrdU incorporation than monocultured OECs (Fig. 3F). This difference was observed only at the day 2 of co-culture, as no differences were observed at day 7. OECs growing under the influence of the ASCs or bmMSCs secretome did not present significant differences when compared with OECs in monoculture both after 2 and 7 days (Fig. 3D, E). Finally, results show that both the stem cells and OECs secretomes do not negatively influence the proliferation rate of each other.
FIG. 3.
Proliferation rate of OECs and adult stem cells culture in transwell systems and in monoculture. Proliferation rate of (A) ASCs, (B) bmMSCs, and (C) HUCPVCs in monoculture or in co-culture with OECs. Proliferation rate of OECs in monoculture or in co-culture with (D) ASCs, (E) bmMSCs, and (F) HUCPVCs. Values are shown as mean±standard deviation (n=3 samples of 2×104 adult stem cells and 1×105 OECs/sample, **P<0.01; ***P<0.001).
Total number of cells
After 2 and 7 days in co-culture, the stem cells and the OECs were subjected to an immunocytochemistry protocol in relation to the cell density and the stem cell differentiation. The proliferation studies revealed that the total number of ASCs and bmMSCs evolved from similar levels at day 2 to significantly higher cell densities at day 7, only when cells were under the influence of the OEC secretome. The number of co-cultured ASCs was 1.8-fold higher (P<0.001) than the monocultured ASCs (Fig. 4A). Moreover, bmMSCs conditioned by the OEC secretome were 1.2-fold more (P<0.05) than bmMSCs in monoculture (Fig. 4B). In contrast, it was possible to observe that the factors produced by the OECs did not significantly affect the density of HUCPVCs (Fig. 4C).
The factors produced by the stem cells revealed to have a positive effect on the OECs. The number of p75-positive cells (marker for OECs) was similar after 2 days of culture; however, after 7 days, the density of p75-positive OECs was significantly higher when these cells were co-cultured with ASCSs or bmMSCs than in monoculture. The number of OECs conditioned by the ASCs' secretome was 2.0-fold higher (P<0.05) than OECs growing solo (Fig. 4D). In addition, OECs conditioned by bmMSCs' secretome was 1.6-fold higher than controls (Fig. 4E). This positive effect was not observed under the HUCPVCs secretome influence; in this circumstance, OECs did not significantly increase in number when compared with controls (Fig. 4F).
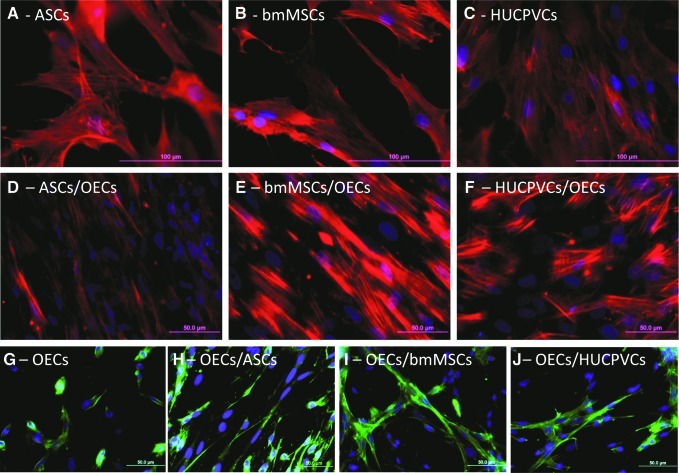
Finally, immunocytochemistry studies revealed that the factors secreted by the OECs did not induce the differentiation of the adult stem cells in OECs. The adult stem cells either in monoculture or in co-culture did not express the p75, S100, or GFAP biomarker, as no positive staining (green color) was observed in Fig. 5A–F. Nevertheless, the phalloidin/DAPI staining confirmed the results described earlier. The factors produced by the OECs significantly promoted proliferation of the ASCSs (Fig. 5D) and bmMSCs (Fig. 5E) but not the HUCPCVs (Fig. 5F). In addition, immunocytochemistry revealed that the factors secreted by ASCs and bmMSCs increased the density of OECs (Fig. 5G–J).
FIG. 5.
Images of fluorescence microscopy of adult stem cells and OECs either in monoculture or in transwell cultures. (A–F) The cytoplasm of adult stem cells was stained with the anti-F-actin dye phalloidin and nuclei counterstained with DAPI. (G–J) The OECs were identified with immunocytochemistry against p75 protein. Color images available online at www.liebertpub.com/scd
Discussion
The use of cells with the capacity to repair or substitute a damaged tissue holds great promise for the regenerative medicine field. Due to their sui generis characteristics, such as the ability to differentiate and replace damaged cells, to secrete trophic factors, and to be easily obtained from human patients, adult stem cells are potential candidates for clinical treatments. In context of SCI, OECs hold particular promise for promoting regeneration. OECs are responsible for the continuous growth of new axons into the central nervous system (CNS) tissue that takes place in the adult olfactory system [11]. Moreover, several authors previously demonstrated that OECs have the ability to promote regeneration of CNS axons both in vitro and in vivo [14,23–25]. However, most of the data obtained until now suggest that no single cell therapy will be sufficient to overcome all the biological complications caused by SCI. For this reason, in the work presented here, we study the possible positive or detrimental effects of the secretome of OECs and adult stem cells derived from the adipose, bone marrow, and umbilical cord tissue.
The results presented here show that the secretome of OECs exerts a positive effect on the metabolic activity and proliferation of MSCs from different origins (All results are summarized in Table 1). However, it is possible to observe that this effect is higher for the ASCs than for the other stem cells, namely in proliferation. Moreover, the stem cells secretome also have beneficial effects in the metabolic activity and proliferation/survival of OECs. Again, the stem cells from adipose origin have a stronger impact on the OECs behavior than the other stem cells. In this sense, the most adequate cell type to be combined with the OECs in future studies would be the ASCs. It is important to point out that in future experiments, after the cell seeding (the stem cells on the SPCL layer, and the OECs on the hydrogel) and after the scaffold implantation in SCI animal models, the secreted factors produced by the stem cells will most likely influence the OECs behavior as well as the nervous cells from the injured tissue where the OECs are implanted. In this sense, the MSCs secretome can also be advantageous for the injured spinal cord site, promoting the growth and regeneration of damaged tissue. Previous studies demonstrated that the secretome of the stem cells used here have a beneficial effect on nervous cells. For instance, Ribeiro et al. previously reported that the secretome of bmMSCs increased the cell viability of hippocampal cultures as well as the number of neurons in culture [26]. Moreover, similar results were obtained using the conditioned medium of HUCPVCs and ASCs in neuronal and glial cultures [27,28]. To the best of our knowledge, the results presented here show for the first time the secretome interactions between OECs and ASCs, HUCPVCs, and bmMSCs on the metabolic activity and proliferation of the cells.
Table 1.
Summary of the Secretome Effects Between Olfactory Ensheathing Cells and Adult Stem Cells
| ASCs↔OECs | MSCs↔OECs | PVCs↔OECs | ||||
|---|---|---|---|---|---|---|
| Metabolic activity | ↑ | ↑ | ↑↑ | ↓↓ | ↑↑ | ↑ |
| Proliferation rate | ↑↑ | — | ↑↑ | — | ↑↑ | ↑↑ |
| Cell number | ↑ | ↑↑ | ↑ | ↑ | — | — |
| Culture purity | n/a | ↑↑ | n/a | ↑↑ | n/a | ↑↑ |
One arrow symbolizes a difference of 1.01- to 1.49-fold, and two arrows symbolize a difference of 1.5-fold or higher on the parameter tested. The direction of the arrow (up or down) means positive or negative effects, respectively. Lines mean not significantly different. n/a means not applicable.
The explanation behind the positive effects of the OECs secretome on the stem cells behavior may be related with the ability of OECs to produce several neurotrophic factors. It was previously reported that OECs are capable of producing nerve growth factor, brain-derived neurotrophic factor (BDNF), and glial-derived neurotrophic factor (GDNF) [13]. The higher metabolic activity and proliferation rate presented by the stem cells may be a consequence of the action of these factors. Moreover, these results are also in accordance with a previous work in which we demonstrated that the OEC secretome is able to promote the proliferation of neural stem cells in either 2D or 3D culture [29].
The stem cells used here are also able to secrete several factors. For instance, Rehman et al. previously reported that ASCSs produce basic fibroblast growth factor, vascular endothelial growth factor (VEGF), and transforming growth factor beta [30]. In addition, previous studies also demonstrated that bmMSCs and HUCPVCs are capable of secreting factors such as BDNF, GDNF, VEGF, and granulocyte-colony stimulating factor [31,32]. These factors secreted by the stem cells may explain why the OECs in co-culture presented a higher number of p75-positive cells.
It was only possible to find a single report using a similar strategy to the one that we used in this work. Wang et al. co-cultured OECs and ASCs and found out that in this condition, ASCs were able to differentiate into OECs-like cells [33]. These results contrast with the ones presented here, where no differentiation was detected for all the stem cells used, including the ASCs. The possible explanations for this difference may reside on the substrate where the ASCs were growing. In Wang's work, the authors seeded the ASCs on a collagen scaffold, as they believe that the 3D culture is an important factor in the ASCs differentiation. We believe that not only the 3D culture, but also the collagen itself may have influence on the ASCs differentiation.
Amemori et al. previously demonstrated that the co-transplantation of OECs and bmMSCs does not have synergistic effects after SCI in the rat compression model [34]. Interestingly, the only negative effect observed here was between the OECs and bmMSCs, namely the secretome of the bone marrow-derived stem cells negatively affected the metabolic activity of the OECs. This may be one of the reasons for the absence of synergistic effects.
Conclusions
In summary, the results described here add new knowledge in the cell therapy field about the secretome effects between OECs and MSCs from adipose, bone marrow, and umbilical cord origins. The results demonstrated that the most suitable combination of cells to be used in our therapeutic concept is the OECs with the ASCs. It should be noticed, however, that in this work we are combining rat secretome to act in human targets. Therefore, in order to fully evaluate its translation potential to clinical applications, further work will focus on the study of the secretome interactions between MSCs and OECs derived from human sources.
Acknowledgments
The authors would like to acknowledge the Portuguese Foundation for Science and Technology (grant no. PTDC/SAU-BMA/114059/2009; pre-doctoral fellowship to Nuno Silva - SFRH/BD/40684/2007; Ciência 2007 Program–A.J. Salgado); the Foundation Calouste de Gulbenkian for funds attributed to A.J. Salgado under the scope of the the Gulbenkian Program to Support Research in the Life Sciences;
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Choo AM. Liu J. Dvorak M. Tetzlaff W. Oxland TR. Secondary pathology following contusion, dislocation, and distraction spinal cord injuries. Exp Neuro. 2008;212:490–506. doi: 10.1016/j.expneurol.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Chacko SM. Ahmed S. Selvendiran K. Kuppusamy ML. Khan M. Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J of Physiol Cell Physiol. 2010;299:C1562–C1570. doi: 10.1152/ajpcell.00221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tate CC. Shear DA. Tate MC. Archer DR. Stein DG. LaPlaca MC. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J Tissue Eng Regen Med. 2009;3:208–217. doi: 10.1002/term.154. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PJ. Tatara A. McCreedy DA. Shiu A. Sakiyama-Elbert SE. Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI. Soft Matter. 2010;6:5127–5137. doi: 10.1039/c0sm00173b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H. Zahir T. Tator CH. Shoichet MS. Effects of dibutyryl cyclic-AMP on survival and neuronal differentiation of neural stem/progenitor cells transplanted into spinal cord injured rats. PLoS One. 2011;6:e21744. doi: 10.1371/journal.pone.0021744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva NA. Salgado AJ. Sousa RA. Oliveira JT. Pedro AJ. Leite-Almeida H. Cerqueira R. Almeida A. Mastronardi F, et al. Development and characterization of a novel hybrid tissue engineering based scaffold for spinal cord injury repair. Tissue Eng Part A. 2010;16:45–54. doi: 10.1089/ten.TEA.2008.0559. [DOI] [PubMed] [Google Scholar]
- 7.Silva NA. Sousa RA. Oliveira JT. Fraga JS. Fontes M. Cerqueira R. Leite-Almeida H. Almeida A. Sousa N. Reis RL. Salgado AJ. Benefits of spine stabilization with biodegradable scaffolds in spinal cord injured rats. Tissue Eng Part C Methods. 2012 doi: 10.1089/ten.TEC.2012.0264. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rada T. Santos TC. Marques AP. Correlo VM. Frias AM. Castro AG. Neves NM. Gomes ME. Reis RL. Osteogenic differentiation of two distinct subpopulations of human adipose-derived stem cells: an in vitro and in vivo study. J Tissue Eng Regen Med. 2012;6:1–11. doi: 10.1002/term.388. [DOI] [PubMed] [Google Scholar]
- 9.Martins A. Duarte ARC. Faria S. Marques AP. Reis RL. Neves NM. Osteogenic induction of hBMSCs by electrospun scaffolds with dexamethasone release functionality. Biomaterials. 2010;31:5875–5885. doi: 10.1016/j.biomaterials.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Baksh D. Yao R. Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 11.Doucette R. Glial influences on axonal growth in the primary olfactory system. Glia. 1990;3:433–449. doi: 10.1002/glia.440030602. [DOI] [PubMed] [Google Scholar]
- 12.Ramón-Cueto A. Avila J. Olfactory ensheathing glia: properties and function. Brain Res Bull. 1998;46:175–187. doi: 10.1016/s0361-9230(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 13.Woodhall E. West AK. Chuah MI. Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Brain Res Mol Brain Res. 2001;88:203–213. doi: 10.1016/s0169-328x(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 14.Li Y. Field PM. Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 15.Verdú E. García-Alías G. Forés J. López-Vales R. Navarro X. Olfactory ensheathing cells transplanted in lesioned spinal cord prevent loss of spinal cord parenchyma and promote functional recovery. Glia. 2003;42:275–286. doi: 10.1002/glia.10217. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal GK. Jwa N-S. Lebrun M-H. Job D. Rakwal R. Plant secretome: unlocking secrets of the secreted proteins. Proteomics. 2010;10:799–827. doi: 10.1002/pmic.200900514. [DOI] [PubMed] [Google Scholar]
- 17.Silva NA. Sousa RA. Pires AO. Sousa N. Salgado AJ. Reis RL. Interactions between Schwann and olfactory ensheathing cells with a starch/polycaprolactone scaffold aimed at spinal cord injury repair. J Biomed Mater Res A. 2012;100:470–476. doi: 10.1002/jbm.a.33289. [DOI] [PubMed] [Google Scholar]
- 18.Sarugaser R. Ennis J. Stanford WL. Davies JE. Isolation, propagation, and characterization of human umbilical cord perivascular cells (HUCPVCs) In: Audet J, editor; Stanford WL, editor. Methods in Molecular Biology. Humana Press, Inc.; New York: 2009. pp. 269–279. [DOI] [PubMed] [Google Scholar]
- 19.Sarugaser R. Hanoun L. Keating A. Stanford WL. Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4:e6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonza. hMSC Human Mesenchymal Stem Cells. www.lonza.com/products-services/bio-research/primary-and-stem-cells/adult-stem-cells-and-media/human-mesenchymal-stem-cells-media/hmsc-human-mesenchymal-stem-cells.aspx www.lonza.com/products-services/bio-research/primary-and-stem-cells/adult-stem-cells-and-media/human-mesenchymal-stem-cells-media/hmsc-human-mesenchymal-stem-cells.aspx
- 21.Dubois SG. Floyd EZ. Zvonic S. Kilroy G. Wu X. Carling S. Halvorsen YDC. Ravussin E. Gimble JM. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. In: Prockop DJ, editor; Phinney DG, editor; Bunnell BA, editor. Methods in Molecular Biology. Humana Press, Inc.; New York: 2008. pp. 69–79. [DOI] [PubMed] [Google Scholar]
- 22.Gronthos S. Franklin DM. Leddy HA. Robey PG. Storms RW. Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 23.Jiao Y. Novozhilova E. Karlén A. Muhr J. Olivius P. Olfactory ensheathing cells promote neurite outgrowth from co-cultured brain stem slice. Exp Neurol. 2011;229:65–71. doi: 10.1016/j.expneurol.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Chuah MI. Hale DM. West AK. Interaction of olfactory ensheathing cells with other cell types in vitro and after transplantation: glial scars and inflammation. Exp Neurol. 2011;229:46–53. doi: 10.1016/j.expneurol.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Ramón-Cueto A. Cordero MI. Santos-Benito FF. Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro CA. Salgado AJ. Fraga JS. Silva NA. Reis RL. Sousa N. The secretome of bone marrow mesenchymal stem cells-conditioned media varies with time and drives a distinct effect on mature neurons and glial cells (primary cultures) J Tissue Eng Regen Med. 2011;5:668–672. doi: 10.1002/term.365. [DOI] [PubMed] [Google Scholar]
- 27.Salgado AJ. Fraga JS. Mesquita AR. Neves NM. Reis RL. Sousa N. Role of human umbilical cord mesenchymal progenitors conditioned media in neuronal/glial cell densities, viability, and proliferation. Stem Cells Dev. 2009;19:1067–1074. doi: 10.1089/scd.2009.0279. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro CA. Fraga JS. Grãos M. Neves N. Reis R. Gimble J. Sousa N. Salgado A. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Ther. 2012;3:18. doi: 10.1186/scrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva NA. Cooke MJ. Tam RY. Sousa N. Salgado AJ. Reis RL. Shoichet MS. The effects of peptide modified gellan gum and olfactory ensheathing glia cells on neural stem/progenitor cell fate. Biomaterials. 2012;33:6345–6354. doi: 10.1016/j.biomaterials.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 30.Rehman J. Traktuev D. Li J. Merfeld-Clauss S. Temm-Grove CJ. Bovenkerk JE. Pell CL. Johnstone BH. Considine RV. March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 31.Koh S-H. Kim KS. Choi MR. Jung KH. Park KS. Chai YG. Roh W. Hwang SJ. Ko H-J, et al. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233–248. doi: 10.1016/j.brainres.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho MM. Teixeira FG. Reis RL. Sousa N. Salgado AJ. Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Curr Stem Cell Res Ther. 2011;6:221–228. doi: 10.2174/157488811796575332. [DOI] [PubMed] [Google Scholar]
- 33.Wang B. Han J. Gao Y. Xiao Z. Chen B. Wang X. Zhao W. Dai J. The differentiation of rat adipose-derived stem cells into OEC-like cells on collagen scaffolds by co-culturing with OECs. Neurosci Lett. 2007;421:191–196. doi: 10.1016/j.neulet.2007.04.081. [DOI] [PubMed] [Google Scholar]
- 34.Amemori T. Jendelová P. Růžičková K. Arboleda D. Syková E. Co-transplantation of olfactory ensheathing glia and mesenchymal stromal cells does not have synergistic effects after spinal cord injury in the rat. Cytotherapy. 2010;12:212–225. doi: 10.3109/14653240903440103. [DOI] [PubMed] [Google Scholar]