Abstract
As the thymus involutes with age, the maintenance of peripheral naive T cells in humans becomes strongly dependent on peripheral cell division. However, mechanisms that orchestrate homeostatic division remain unclear. In this study we present evidence that the frequency of naive CD4 T cells that express CD25 (IL-2 receptor α-chain) increases with age on subsets of both CD31+ and CD31− naive CD4 T cells. Analyses of TCR excision circles from sorted subsets indicate that CD25+ naive CD4 T cells have undergone more rounds of homeostatic proliferation than their CD25− counterparts in both the CD31+ and CD31− subsets, indicating that CD25 is a marker of naive CD4 T cells that have preferentially responded to survival signals from self-Ags or cytokines. CD25 expression on CD25− naive CD4 T cells can be induced by IL-7 in vitro in the absence of TCR activation. Although CD25+ naive T cells respond to lower concentrations of IL-2 as compared with their CD25− counterparts, IL-2 responsiveness is further increased in CD31− naive T cells by their expression of the signaling IL-2 receptor β-chain CD122, forming with common γ-chain functional high-affinity IL-2 receptors. CD25 plays a role during activation: CD25+ naive T cells stimulated in an APC-dependent manner were shown to produce increased levels of IL-2 as compared with their CD25− counterparts. This study establishes CD25+ naive CD4 T cells, which are further delineated by CD31 expression, as a major functionally distinct immune cell subset in humans that warrants further characterization in health and disease.
Introduction
Peripheral expansion of human naïve T cells is vital to maintain the naïve T cell pool, particularly after thymic involution. Naïve T cell expansion in the periphery preserves a diverse naïve TCR repertoire that is critical to provide immunity to foreign antigens and to maintain peripheral tolerance when the thymus, owing to progressive involution with increasing age, is no longer able to generate sufficient naïve TCR repertoire diversity. Recent quantitative studies of naïve CD4 T cell expansion provided evidence that, in contrast to mice, naïve T cells in healthy human adults are sustained almost exclusively by peripheral proliferation (1). Post-thymic naïve T cell expansion, which depends to various degrees on stimulation with cytokines such as IL-7 and interactions with antigen presenting cells, creates a heterogeneous pool of naïve T cells (2). Naïve CD4+ T cells can be sub-divided based on CD31 (PECAM-1) expression (3). CD31+ naïve CD4+ T cells have undergone minimal number of divisions after exiting the thymus while CD31− naïve T cells have undergone multiple rounds of division since emigrating from the thymus. During naïve CD4 T cell expansion, signals received through the TCR appear to drive CD31 downregulation, thereby forming the central naïve T cell subset (2, 4). Since naïve T cells are thought to downregulate the expression of CD31 after stimulation in the context of MHC class II molecules, their bona fide antigen inexperienced naïve T cell status has been questioned. Although the TCR signals that drive loss of CD31 expression on central naïve T cells are not strong enough to lead to naïve T cell activation and loss or acquisition of markers characterizing effector or memory cells i.e. loss of CD45RA and CCR7 and gain of CD45RO, the signals are sufficient to induce peripheral expansion, as manifested by loss of T cell receptor excision circles (TREC) and a reduction in the TCR repertoire of the expanding naïve CD4 T cell subset (2, 3).
CD25 has long been categorized as a T cell activation marker. As a consequence, the functional significance of homeostatic CD25 expression on unstimulated T cells has been largely ignored, except in the case of FOXP3+ regulatory CD4 T cells (Tregs) (5, 6). CD25 is the alpha chain of the high affinity trimeric IL-2 receptor; high levels of the high affinity IL-2 receptor on Tregs enables them to respond to low concentrations of IL-2 that are critical for Treg survival and the maintenance of their suppressive function. In addition to Tregs, a majority of resting memory CD4+ T cells express CD25 in a constitutive fashion, albeit at lower levels than Tregs (7) (Fig. 1A). We were, therefore, surprised to discover a subset of naïve CD4+ CD45RA+ T cells that expressed CD25 (7). This subpopulation, which increased in frequency with age reaching as much as 20% of naïve CD4+ by the 40 years of age. Here, we have confirmed and extended the evidence for the age-dependence of this expansion of CD25+ naïve T cells; their relation to loss of CD31 and TRECs; a role for IL-7; and the co-expression of the beta-chain of the IL-2 receptor to form functional, high affinity receptors on these naïve CD4+ T cells that correlates with their increased responsiveness to IL-2.
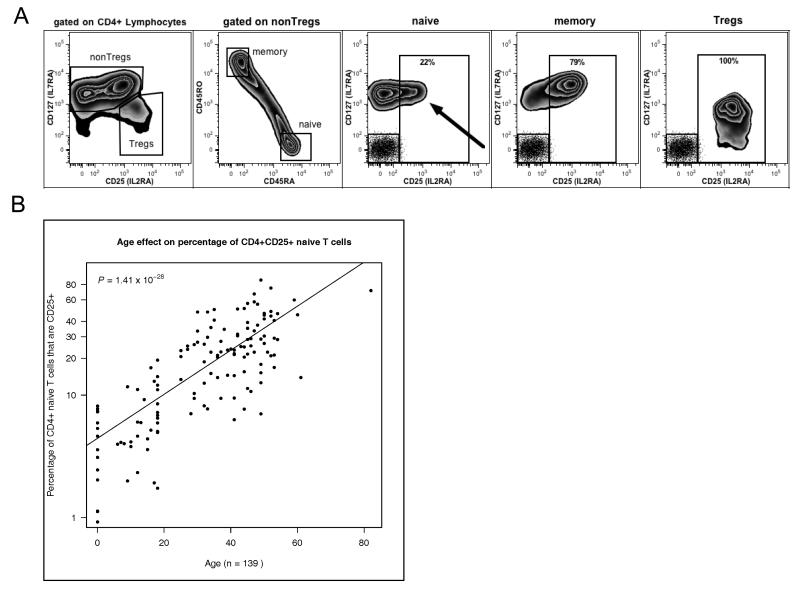
Fig. 1. Frequency of human CD25+ naïve CD4 T cells is determined by age.
(A) CD4+ naïve T cells were gated as CD4+ CD127+ CD25−/+ CD45RO− CD45RA+; CD4+ memory cells were gated as CD4+ CD127+ CD25−/+ CD45RO+ CD45RA−; and Tregs were gated as CD4+ CD127−/low CD25high. Dot plot inserts in the naive, memory and Treg plots represent isotype controls for both fluorochromes. (B) The proportion of naïve CD4 T cells that are CD25+ increases with age. (P=1.41×10 × 10-28, n=139).
Materials and Methods
Donors
Cambridge BioResource donors were collected with the prior approval of the National Health Service Cambridgeshire Research Ethics Committee, were selected as part of three studies: Genes and mechanisms of type 1 diabetes; Genotype/phenotype study of newly diagnosed children with type 1 diabetes and siblings; Investigating genes and phenotypes associated with type 1 diabetes. Diabetes—Genes, Autoimmunity, and prevention was approved by the National Research Ethics Committee London - Hampstead.
Genotyping
SNPs were genotyped using Custom TaqMan SNP Genotyping Assays or Taqman (Applied Biosystems) according to the manufacturers’ protocols.
Cytokines
rhIL-7 and rhIL-15 were obtained from R&D systems and diluted according to manufacturer’s instructions. rhIL-2 (Proleukin) was obtained from Novartis. Complete media for functional studies was X-VIVO 15 media (Lonza) supplemented with 5% heat-inactivated, filtered human AB serum (Sigma-Aldrich).
Whole blood and PBMC immunostaining
Blood samples were directly immunophenotyped within 5 hours following donation. Samples were blocked for 10 min with mouse IgG (20μg/ml), stained for 40 min at room temperature with appropriate antibodies (Supplemental Table 1) and then lysed with freshly prepared 1X BD FACS Lysing Solution (BD Biosciences). After lysis of red blood cells, samples were washed with BD CellWASH (BD Biosciences). Finally, the samples were fixed with freshly prepared 1X BD CellFIX (BD Biosciences). The samples were stored at 4 °C in the dark until analysis using a BD Fortessa flow cytometer. PBMC samples, prepared as previously described by Dendrou et al., (7) were blocked for 10min, stained for 1 hour at 4°C, washed twice and fixed as described for peripheral blood immunophenotyping except for intracellular staining when surface-stained cells after the wash-step were placed in FOXP3 Fix/perm buffer (eBioscience). The isotype control antibodies used were allophycocyanin-conjugated mouse IgG1 (BD Biosciences), Alexa Fluor 488–conjugated mouse IgG1 (BioLegend), FITC–conjugated mouse IgG2 (BioLegend), PE20 conjugated IgG1 and IgG2 (BD Bioscience, Biolegend, eBioscience) and PerCP-Cy5.5–conjugated mouse IgG1 and IgG2 (BioLegend). As described by Dendrou et al., CD25 detection sensitivity was increased by simultaneous application of two anti-CD25 monoclonal antibodies labelled with the same fluorochrome (clones 2A3 and M-A251, BD Biosciences). Antibody concentrations used were based on the manufacturer’s instructions as well as on optimization studies.
Intracellular detection of Ki-67 and FOXP3
Intracellular staining was performed on freshly surface-stained washed PBMCs that were placed in “FOXP3” fix/perm buffer (eBioscience) and further processed according to the manufacturer’s protocol (in order to achieve high resolution of the FOXP3 staining extensive washing steps were essential). The surface anti-human monoclonal abs used were Alexa Fluor 700–conjugated anti-CD4, allophycocyanin-conjugated anti-CD25 (clones M-A251 and 2A3; BD Biosciences), PE-CY7–conjugated anti-CD127 (eBioscience), eFluor605-conjugated anti-CD45RA (eBioscience). The anti-human monoclonal abs used for intracellular T-cell immunophenotyping were Pacific Blue-conjugated anti-FOXP3 (Biolegend), PerCP-Cy5.5-conjugated anti-KI-67 (BD Biosciences). Stained cells were washed, acquired, and analyzed using FlowJo (Treestar).
Flow cytometry detection of pSTAT5
For simultaneous detection of pSTAT5a, FOXP3, CD4, CD25, CD127, CD45RA and CD31, 500 μl of fresh blood was incubated with 500 μl X-Vivo medium with various concentrations of IL-2, IL-7, IL-15 for 10 min at 37°C. Phosphorylation was preserved by adding 30 ml warm BD Lyse/Fix buffer (BD Biosciences 558049). Cells were then washed with PBS containing 0.2% BSA and treated with 1 ml fresh ice-cold methanol for 20 min on ice (pSTAT5a-dimer disruption). After extensive washing with PBS containing 0.2% BSA, cells were stained for 1 hour at 4 °C in the dark. The following antibodies were used: Pacific Blue-conjugated anti-FOXP3 (BioLegend), A488-conjugated anti-pSTAT5(pY694) (BD Biosciences), Alexa Fluor 700–conjugated anti-CD4, allophycocyanin-conjugated anti-CD25 (clones MA251and 2A3; BD Biosciences), PE-CY7–conjugated anti-CD127 (eBioscience), eFluor605-conjugated anti-CD45RA (eBioscience) and PE-conjugated anti-CD31 (eBioscience). Stained cells were washed and analysed as above.
Flow cytometry and data analysis
Immunostained samples were analysed as previously described by Dendrou et al. (7, 8). Calibration Beads were used to calculate MEF values.
PBMC activation
PBMCs stimulated with 5 μg/ml of SEB (Sigma) for 4 h were cultured in 24-well flat-bottom plates as described previously by Dendrou et al. (7) except that PBMC were cultured overnight before the addition of SEB thereby allowing more robust IL-2 secretion. Naïve T cells responding to SEB were identified as CD69+ using Pacific Blue-conjugated anti-CD69 (Biolegend), APC-conjugated anti-CD25 (clones M-A251 and 2A3; BD Biosciences), FITC-conjugated anti CD31 (Ebioscience), Alexa Fluor 700–conjugated anti-CD4 (Biolegend) and PerCP-Cy5.5–conjugated anti-CD45RO (BioLegend). CD69+ naïve T cell subsets secreting IL-2 were identified using an IL-2 PE Secretion Assay (Miltenyi Biotec).
PBMC and CD4+ lymphocytes preparation
PBMC isolation, cryopreservation and thawing were performed as previously described (7). Briefly PBMC isolation was carried out using Lympholyte (CEDARLANE). PBMCs were cryopreserved in heat-inactivated, filtered human AB serum (Sigma-Aldrich) and 10% DMSO (Hybri-MAX, Sigma-Aldrich) at a final concentration of 10 × 106 /ml and were stored in liquid nitrogen. Cells were thawed in a 37 °C water bath for 2 min. PBMCs were subsequently washed by adding the cells to 10 ml of cold (4 °C) X-VIVO (Lonza) containing 10% AB serum per 10 × 106 cells, in a drop-wise fashion. PBMCs were then washed again with 10 ml of cold (4 °C), X-VIVO containing 1% AB serum per 10 × 106 cells. CD4+ T cells were purified using CD4+ RosetteSep (STEMCELL Technologies) according to manufacturer’s instructions.
Fluorescence activated cell sorting
CD4+ T cells were washed and immediately incubated with antibodies (Supplemental Table 1) for 40 minutes at 4 °C, washed and followed by flow cytometry sorting (BD ARIAIII).
RNA and DNA isolation
Following cell sorting cell subsets were checked for purity and placed in TRIZOL reagent (Life Technologies) at −80 °C. Total RNA was isolated using the phenol-chloroform method according to the manufacturer’s instructions. In order to isolate DNA sorted cell subsets were checked for purity and DNA was isolated using the DNA extraction reagent (Qiagen).
cDNA synthesis and microarray-gene expression analysis
Single-stranded cDNA was synthesised from 200 ng of total RNA using the Ambion WT Expression kit (Ambion) according to the manufacturer’s instructions. Labelled cDNA (GeneChip Terminal Labelling and Hybridization Kit, Affymetrix) was hybridized to a 96 Titan Affymetrix Human Gene 1.1 ST array. Data are deposited with ArrayExpress (http://www.ebi.ac.uk/arrayexpress/, accession number E-MTAB-1138).
TREC assay
TREC assay was performed as described previously (9). Briefly, a quantitative PCR assay was purchased from Sigma-Genosys for the single Joint TCR excision circle (sjTREC) that arises through an intermediate rearrangement in the TCRD/TCRA locus in developing TCRαβ+ T lymphocytes. An assay for the gene encoding albumin was used to normalise the data.
sjTREC.F TCGTGAGAACGGTGAATGAAG
sjTREC.R CCATGCTGACACCTCTGGTT
sjTREC.P FAM-CACGGTGATGCATAGGCACCTGC-TAMRA
Alb.F GCTGTCATCTCTTGTGGGCTGT
Alb.R ACTCATGGGAGCTGCTGGTTC
Alb.P FAM-CCTGTCATGCCCACACAAATCTCTCC-TAMRA
For each sample (24 ng DNA) was incubated in duplicate with both primers (700 nM), probe (150 nM) and 12.5 μl TaqMan mastermix (Applied Biosystems) on the 7900HT system (Applied Biosystems).
Statistical analysis of flow cytometry data versus age
We included 138 healthy individuals in the analysis in Fig. 1B. Statistical tests and model selections were implemented using the R software (http://www.R-project.org). Age and sex effects were tested by linear regression analysis, with the phenotype, percentage of CD25+ naïve T cells, logarithm transformed to base 10. Analysis of variance (ANOVA) and Chi-squared tests were used for model selections.
Statistical analysis of flow cytometry data interrogating T-cell subsets
Statistical analyses of the percentage of cells expressing Ki-67, CD31, IL-2 or pSTAT5 and the mean CD122 MEF were performed and presented using Prism 5 software (Graphpad.com). Comparisons between cell subsets were performed using a paired Student’s t-test. P < 0.05 was considered significant, error bars show the SD of the samples at each test condition.
Results
Frequency of CD25+ naïve T cells in humans increases with age
We carried out an immunophenotyping study using 139 donors, including samples from young children and umbilical cords. The percentage of CD4+ CD25+ naïve T cells (gating strategy Fig. 1A) was strongly associated with increasing age (P=1.41x10-28; Fig. 1B): over the age of 40 years greater than 20% of CD4+ naïve T cells are CD25+. CD25+ naïve CD4 T cells were also detected in cord blood, indicating that acquisition of CD25 expression by naïve CD4 T cells begins prior to birth (Supplemental Fig. 1A).
CD25+ CD45RA+ CD4+ T cells: confirmation of the naïve phenotype
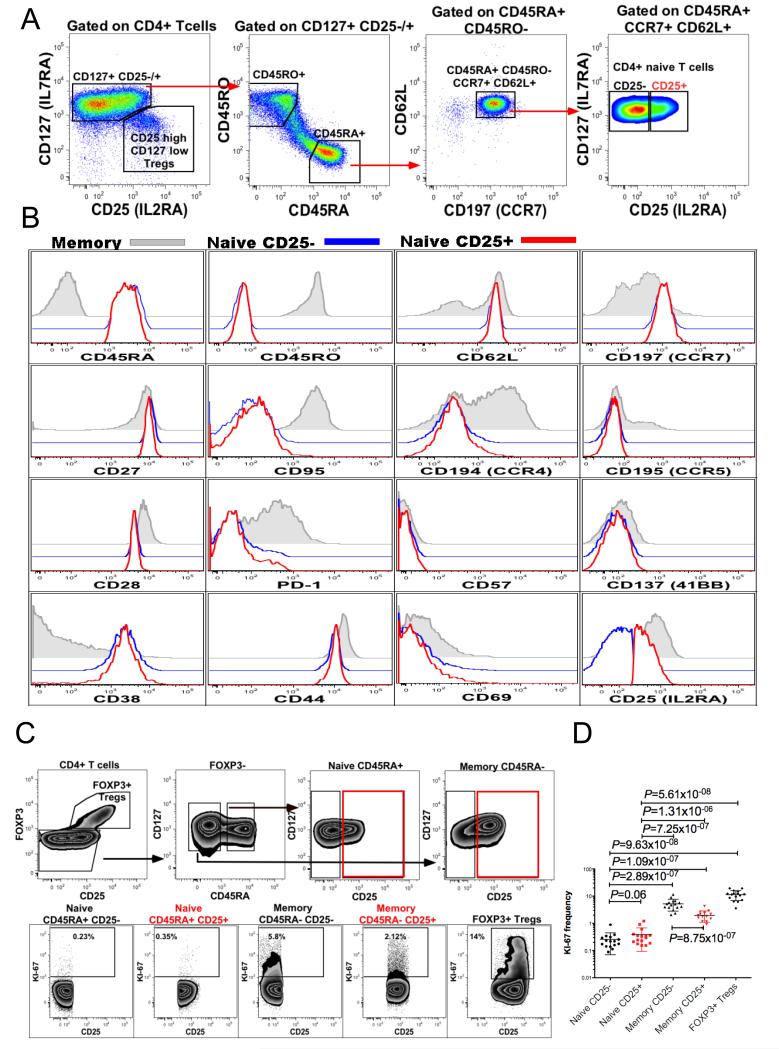
Given the high prevalence of CD25+ naïve CD4+ T cells we wanted to obtain further evidence that they are in fact naïve. CD25+ naïve CD4+ T cells were immunophenotyped in detail using established markers that differentiate naïve from memory CD4 T cells and characterise their activation, differentiation or exhaustion status: CD27, CD28, CD45RA, CD45RO, CD38, CD57, CD44, CD62L, CD69, CD95, CD137, CD194, CD195, CD197 and PD-1. Expression of these molecules did not discriminate CD25+ naïve CD4+ T cells from their CD25− counterparts (Fig. 2A-B). CD25+ and CD25− naïve CD4 T cells expressed equal amounts of CCR7, CD62L and CD27, indicating that CD25+ naïve T cells do not belong to a memory subset that has reverted to CD45RA expression (10-14). We also detected a rare subset of memory cells with stem cell-like properties that display in general a naïve phenotype but express higher levels of CD95 and CD122 as compared to naïve T cells (15). These cells are also characterised by heterogeneous CD25 expression (Supplemental Fig. 2A-C).
Fig. 2. Immunophenotyping of CD25+ and CD25− naïve CD4+ T cells.
(A) Gating strategy used to define the CD25+ and CD25− subsets of human peripheral blood CD4+, CD45RA+, CD45RO−, CCR7+, CD62L+ naïve T cells (representative of 20 donors). (B) Representative examples of flow cytometric analyses of CD25+ and CD25− naïve CD4+ T cell subsets: overlaid histograms show the expression of the indicated molecule in three CD4+ T cell subsets defined as memory CD45RA− CD45RO+ (grey solid histogram), naïve CD45RA+ CD45RO− CD62L+ CD25− (blue histogram) and naïve CD45RA+ CD45RO− CD62L+ CD25+ (red histogram), representative example of between 5 and 20 donors. (C) Gating strategy to assess the proportion of cells in cycle (Ki-67+) within various CD4+ T cell subsets: CD25−/+ CD45RA+ FOXP3− naïve CD4 T cells, CD25−/+ CD45RA− FOXP3− memory CD4 T cells and CD25high Tregs. (D) Combined analysis of Ki-67+ T cell frequency in five CD4 T cell subsets in 16 donors.
Homeostatic proliferation of naïve T cells averages around 0.4% measured using an antibody against the proliferation marker Ki-67 and distinguishes the naïve CD4+ T-cell subset from CD4+ memory and regulatory T cells that proliferate at higher rates (16, 17). In order to investigate a potential relationship between CD25 expression and naïve T-cell proliferation, naïve T cells were stained for Ki-67. The analysis of Ki-67+ T cells indicated that CD25+ naïve T cell proliferation was not significantly different from the proliferation rate of CD25− naïve T cells (P=0.06) (Fig. 2C-D). It is important to note, however, that the percentage of a cell population that is currently in cycle as detected by Ki-67 does not assess the proliferative history of the cells.
CD25+ naïve T cells display naïve T cell transcription profile
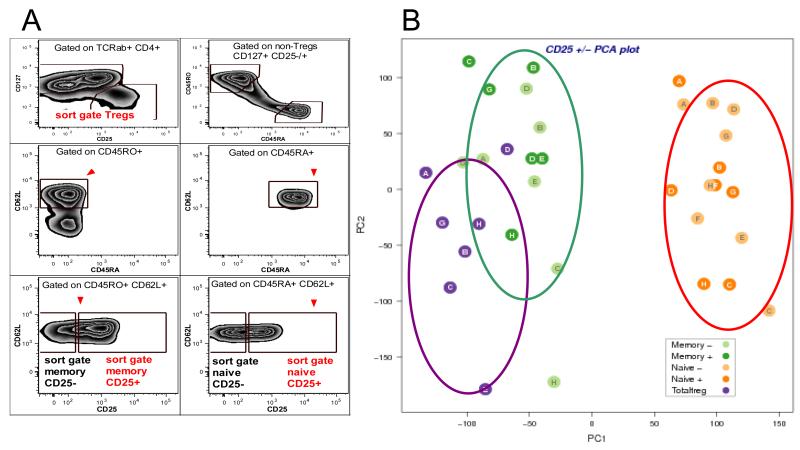
Since neither an obvious phenotypic difference nor a difference in ex-vivo proliferation was observed between CD25+ and CD25− naïve CD4+ T cells, we next compared the gene expression of sorted CD4 T cell subsets. From each of seven individual donors, we sorted five CD4 T cell populations (Fig. 3A, Supplemental Fig. 3C): CD25+ and CD25− naïve T cells, CD25+ and CD25− memory T cells and Tregs, and gene expression was compared by microarray. A principal component analysis of the five studied populations demonstrated that the CD25+ naïve CD4 T cell subset could not be distinguished from the CD25− naïve subset but was clearly distinct from both of the memory and the Treg subsets (Fig. 3B). Specific analysis of Affymetrix HumanGene 1.1 ST Probesets mapping to IL2RA demonstrated that CD25+ naïve T cells contain on average 5 fold more mRNA encoding IL2RA than their CD25− counterparts (Supplemental Fig. 3A-B), validating the approach.
Fig. 3. Principal component analysis of CD4+ CD25+ naïve T cells confirm their naïve gene expression profile.
(A) CD4+ T cells purified from peripheral blood were labelled with antibodies to TCRαβ, CD4, CD127, CD25, CD45RA, CD45RO, CD62L and five subsets of CD4+ T cells were sorted as follows: naïve CD25− (TCRαβ+, CD4+, CD127+, CD45RA+, CD62L+, CD45RO−, CD25−), naïve CD25+ (TCRαβ+, CD4+, CD127+, CD45RA+, CD62L+, CD45RO−, CD25+), memory CD25− (TCRαβ+, CD4+, CD127+, CD45RA−, CD62L+, CD45RO+, CD25−), memory CD25+ (TCRαβ+, CD4+, CD127+, CD45RA−, CD62L+, CD45RO+, CD25+) and Tregs (TCRαβ+, CD4+, CD127−/low, CD25high). (B) Principal component analysis (PCA) of five CD4+ T cell subsets purified from 7 donors delineated by a 96-sample titan Affymetrix Human Gene 1.1 ST array. The Human Gene 1.1 ST Array Plate interrogates more than 28,000 well annotated genes with more than 750,000 distinct probes. The array provides deep exon coverage and contains a median of 26 probes per gene. PCA analysis indicated that the CD25− and CD25+ naïve T cell subsets cluster together and their expression profiles are both different from both memory T cells and Tregs.
Functional consequences of CD25 expression by naïve T cells
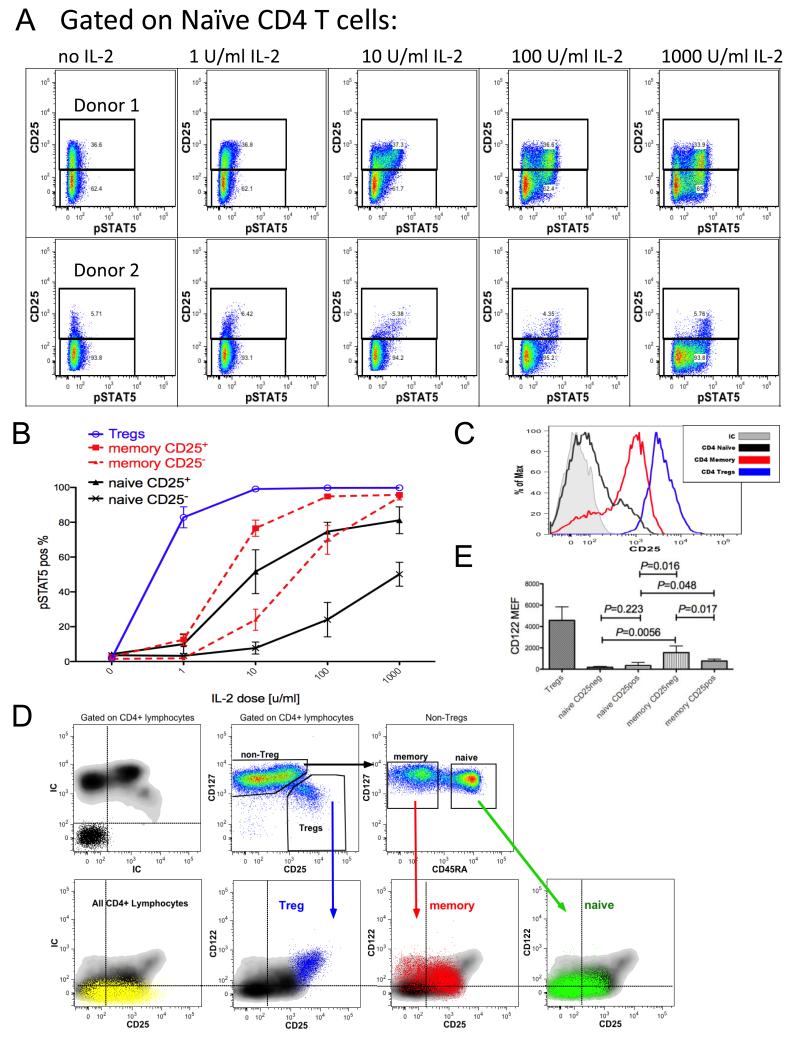
To assess the functional significance of CD25 expression on naïve CD4 T cells, peripheral blood was incubated with IL-2 in vitro and the phosphorylation of STAT5a was monitored by flow cytometry (Fig. 4A-B). The percentage of pSTAT5-positive CD4+ CD45RA+ FOXP3− naïve T cells increased with the dose of IL-2. At 1 U/ml of IL-2, STAT5 was preferentially phosphorylated in naïve CD4 T cells expressing the highest level of the α chain (CD25) of the IL-2 receptor, which is consistent with the α chain’s ability to confer a high affinity status to the receptor. A large proportion of all CD25+ naïve CD4 T cells had increased pSTAT5 levels at a 10 U/ml dose of IL-2 while only a minimal response was observed with CD25− naïve T CD4 cells (P = 0.042). The majority of both CD25+ and CD25− naïve CD4 T cells phosphorylated STAT5 in response to 1000 U/ml of IL-2 although fewer of the CD25− naïve CD4 T cells responded at this relatively high dose of IL-2 (P = 0.041). Notably, the CD25+ naïve CD4 T cells had an IL-2 dose response more comparable to that of CD25+ memory CD4 T cells than to their CD25− naïve CD4 T cell counterparts. Similarly, CD25− memory CD4 T cells resembled CD25− naïve CD4 T cells more than CD25+ memory CD4 T cells at the 1 and 10 U/ml IL-2 doses. This similarity disappeared at 100 and 1000 U/ml where a higher proportion of the CD25− memory cells phosphorylated STAT5 as compared to the CD25− naïve cells (P = 0.008 and 0.0006, respectively) (Fig. 4B). We hypothesized that differential expression of CD122 (the β chain of the IL-2R which is encoded by IL2RB) by naïve and memory CD4 T cell subsets stratified by CD25 expression (Fig. 4C) could account for the observed IL-2 sensitivity differences. Indeed, CD122 cell surface expression analysis revealed that CD25+ memory T cells express 2-fold more CD122 than CD25+ naïve T cells (mean molecules of equivalent fluorescence (MEF) of 767 and 342 respectively, P = 0.048) and CD25− memory T cells express 8-fold more surface CD122 than CD25− naïve T cells (mean MEF of 1558 and 184 respectively, P = 0.0056) (Figs. 4D-E). The relatively high level of CD122 expression on CD25− memory CD4 T cells likely accounts for the fact that nearly all of the cells phosphorylated STAT5 at 1000 U/ml IL-2.
Fig. 4. CD25 and CD122 expression modulates IL-2 responsiveness on CD4+ T cell subsets.
(A) Peripheral blood lymphocytes were stimulated with various doses of IL-2 for 10 min followed by pSTAT5 staining. Flow cytometry analysis of CD4+ CD127+ CD45RA+ FOXP3− naive T cells from two representative donors defined by a high (top panel) and low frequency (lower panel) of naïve T cells expressing CD25. (B) Dose-dependent induction of pSTAT5 among CD4+ CD25+ vs CD25− subsets of both naïve and memory T cells as well as FOXP3+ Tregs (representative example of 20 donors; analysis performed on 4 donors). (C) Representative flow cytometry histograms of CD25 expression among CD4+ naïve, memory and regulatory T cell subsets. (D) Parallel flow cytometry analysis of CD25 and CD122 expression on the CD4+ naïve, memory and regulatory T cell subsets (representative of 20 donors, appropriate CD4 T cell- subsets are overlaid on the total CD4 density plots). (E) Analysis of CD122 MEF from five representative donors of 20 tested indicates that the β chain of the IL-2 receptor is expressed on naïve T cells, however, its expression is increased on memory T cells and is the highest on the Treg subset. Note that memory cells that express the highest level of cell-surface CD122 belong to the CD25− memory T cell subset.
CD25 is expressed on CD31+ and CD31− naïve CD4 T cells
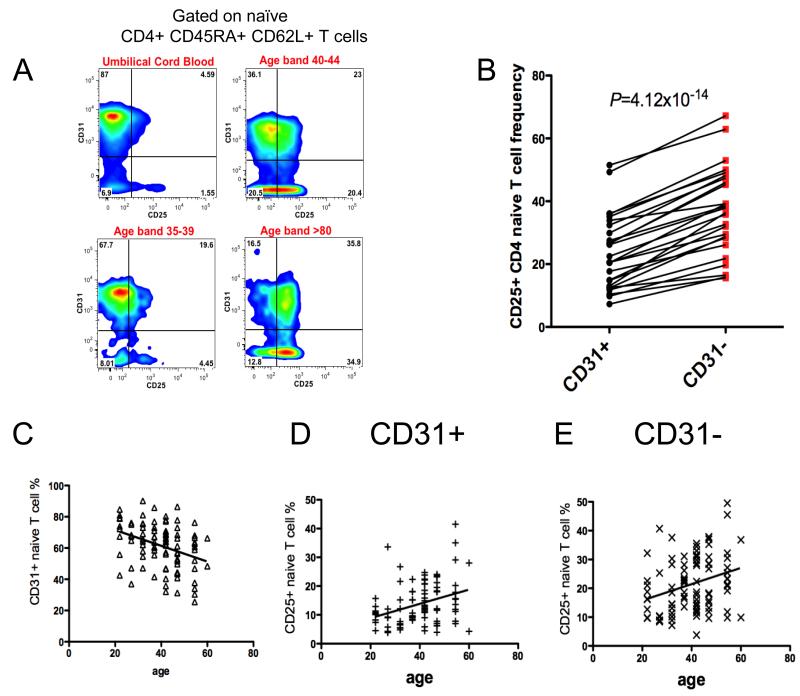
The association of the frequency of CD25+ naïve CD4 T cells with age led us to investigate its relationship to another molecule expressed on naïve CD4 T cells that decreases with age: CD31. CD31 is thought to mark naïve CD4 T cells that have undergone a minimal number of divisions since leaving the thymus; naïve cells lacking CD31 expression have undergone homeostatic proliferation and are characterized by a less diverse TCR repertoire, leading to the hypothesis that CD31− cells have responded to peptide/MHC complexes (2, 4). We, therefore, hypothesized that expanded naïve CD31− cells should express CD25 while CD31+ cells should lack CD25 expression. However, dual parameter analysis of CD31 and CD25 on naïve CD4 T cells demonstrated that CD25 is expressed on both CD31+ and CD31− naïve CD4+ T cells (Fig. 5A, representative example of four donors of 128 tested).
Fig. 5. CD25 is expressed in age dependent manner on both CD31+ and CD31− naïve T cell subsets.
(A) Flow cytometry analysis of CD25 expression on CD31+ and CD31− naïve T cell subsets demonstrated that although CD25 is expressed on a subset of CD31+ naïve T cells, there is a 1.6 fold increase in the frequency of CD25+ cells among central CD31− naïve T cell subset, four representative examples of the peripheral blood immunophenotyping from 128 donors. (B) Summary of the flow cytometry based analysis of the CD25 expression of CD31+ and CD31− naïve T cells from 26 donors (19 females and 7 males, age: 25-49). (C) Flow cytometry examination of 92 PBMCs samples (52 females, 40 males) confirmed age dependent decrease in the CD31+ naïve T cell frequency. (D-E) Flow cytometry analysis of 92 PBMCs samples, grouped in 5 year age bands, revealed age dependent acquisition of CD25 expression among both subsets of CD31+ and CD31− naïve T cells. Age effect on CD25 expression among CD31+ and CD31− naïve CD4 T cells was tested by linear regression analysis, with the phenotype, percentage of CD25+ naïve T cells, logarithm transformed to base 10.
Nevertheless, as compared with CD31+ naïve CD4+ T cells, the frequency of CD25+ cells in the CD31− naïve subset is increased 1.6-fold (mean CD25+ frequency of 23% and 37% for the CD31+ and CD31− subsets, respectively, P=4.1x10-14) (Fig. 5B). The same relationship of CD31 and CD25 expression amongst naïve CD4 T cells was observed within umbilical cord blood (Fig. 5A, Supplemental Fig. 1B). Previous studies reporting that the percentage of naïve CD4 T cells expressing CD31 decreases with age (1) were confirmed (P=0.0005) using previously frozen PBMCs from 92 Cambridge BioResource donors (Fig. 5C). Consistent with our observation that both the CD31+ and CD31− subsets express CD25 (Fig. 5A-B) and that there is an age-dependent increase in the percentage of CD25+ cells in the naïve CD4 T cell subset (Fig. 1B), we observed an age-dependent increase among the 92 donors in the percentage of CD25+ cells for both subsets of naïve cells: CD31+ (P=0.0072) and CD31− (P=0.0019) (Fig. 5D-E).
CD25 expression identifies homeostatically expanded naïve CD4 T cells within the CD31+ subset
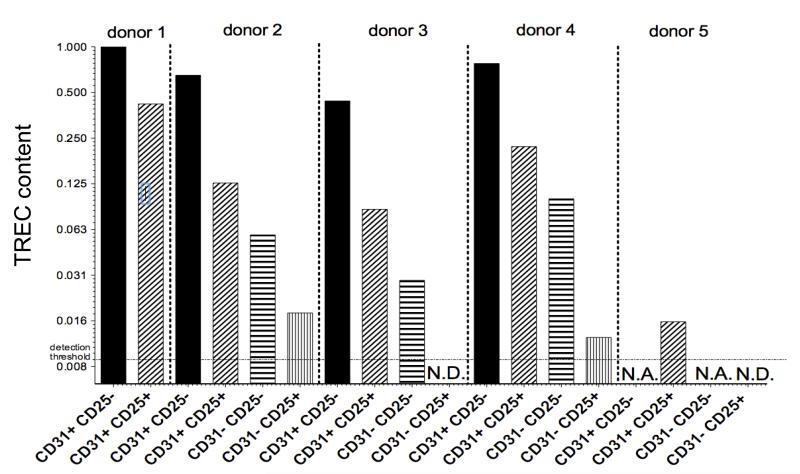
It has been reported that TRECs are reduced among CD31+ naïve T cells with age (1, 18), demonstrating that, as for CD31− subset, CD31+ naïve CD4 T cells are in part produced by peripheral division. However, no phenotypic marker has been described to identify CD31+ cells that had undergone homeostatic proliferation. To test the hypothesis that CD25 expression identifies naïve CD31+ CD4 T cells that have divided more extensively than their CD25− CD31+ counterparts, we sorted naïve CD4 T cells based on CD31 and CD25 expression and quantified the TREC content (Fig. 6). Importantly, when comparing sorted CD31+ CD25+ and CD31+ CD25− naïve CD4 T cells obtained from the same donor, the difference in TREC levels can be accounted for by on average fewer than three cell divisions (2.37 to 5.18-fold TREC reduction) in the CD31+ CD25+ population. TREC values in the most naïve CD4 T cell subset (CD25− CD31+) varied only 2.27-fold amongst the donors whereas the TREC values varied 4.98-fold in the CD31+ CD25+ cells, suggesting that the age-dependent decrease in TREC content observed by others in CD31+ naïve CD4 T cells (1, 18) is predominantly accounted for by the CD31+ CD25+ subpopulation. Despite the fact that CD31+ CD25+ naïve CD4 T cells have diluted their TREC content more than CD31+ CD25− cells, within an individual’s naïve CD4 T cell populations, CD31+ CD25+ naïve CD4 T cells still represent a population that has expanded less than either subset of CD31− naïve CD4 T cells (Fig. 6). Finally, in the three donors in whom sufficient numbers of both CD31− subsets could be isolated for TREC-analysis, the CD25+ CD31− naïve CD4 T cell subset was consistently enriched for cells having undergone the highest number of divisions (Fig. 6).
Fig. 6. CD25 expression marks expanded naïve T cells in CD31+ subset.
Analysis of TREC content among sorted naïve T cells divided by CD25 and CD31 expression. Prepurified CD4+ T cells from 5 different donors (donors 1-4 age band 30-49, donor 5 age band: 82-84) were stained for TCRαβ, CD4, CD127, CD25, CD45RA, CD31, CD62L and subsequently 4 naïve T cell subsets were sorted as follows: CD31+ CD25-, CD31+ CD25+, CD31− CD25-, CD31− CD25+. (N.D., not detected, TREC levels below detection threshold; N.A., not analyzed, insufficient yield of DNA from sorted cells).
IL-7 induces CD25 on CD31+ and CD31− CD25− naïve T cells
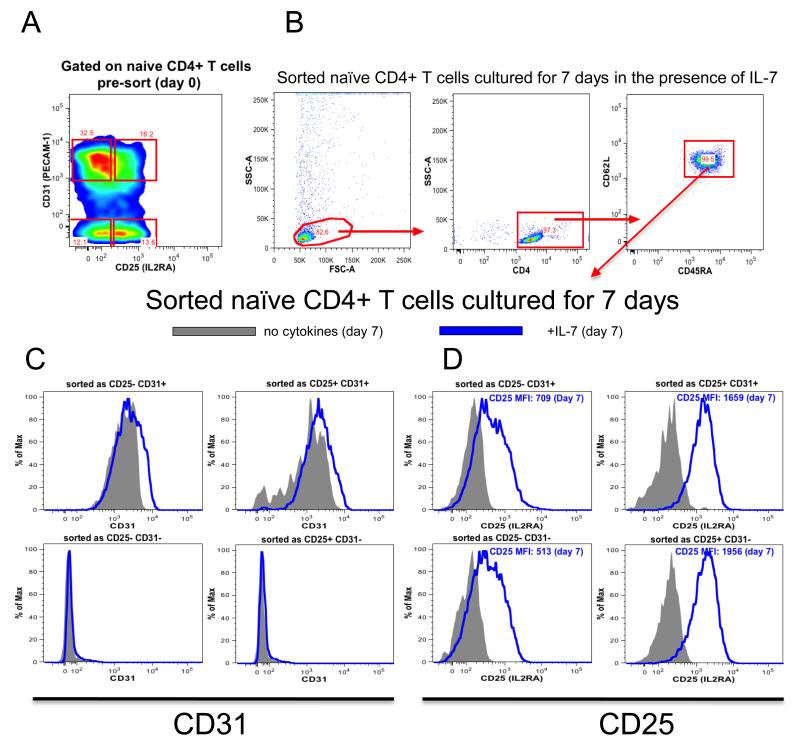
Azevedo et al. (19) have shown that IL-7 sustains CD31 expression on naïve CD31+ CD4 T cells without inducing substantial proliferation limited only to CD31+ subset. We tested the hypothesis that IL-7 also influences the expression of CD25 by culturing the four sorted naïve CD4 T cell subsets (Fig. 7A-B) in the presence or absence of IL-7 and assessing CD31 and CD25 expression seven days later (Fig. 7C-D). In agreement with the previous study (19), sorted CD31+ naïve CD4 T cells remained CD31+ with (10 ng/ml) or without the addition of IL-7, while CD31− naïve CD4 T cells remained CD31− (Fig. 7C). Notably, a majority of both CD31+ and CD31− naïve CD4 T cells sorted as CD25− and cultured for seven days expressed CD25 in an IL-7-dependent manner (Fig. 7D).
Fig. 7. IL-7 induces CD25 expression on naïve CD4 T cells in a TCR independent manner.
(A) TCRαβ+, CD4+, CD127+, CD45RA+, CD62L+ naïve T cells were divided into four subsets on the basis of CD25 and CD31 expression followed by FACS as: CD31+ CD25−, CD31+ CD25+, CD31− CD25+, CD31− CD25−. (B) Sorted cells were placed in culture for 7 days in the presence (10ng/ml) or absence of IL7, representative example of four experiments showing the gating strategy for analysing sorted naïve T cell subsets cultured for 7 days in the presence of IL-7. Flow cytometry analysis of CD31 (C) and CD25 (D) expression of four sorted naïve T cell subsets cultured for 7 days in the presence (blue histogram) or absence (grey histogram) of IL7 (representative example of 3 independent experiments).
Differential IL-2 responsiveness by the four naïve CD4 T cell subsets
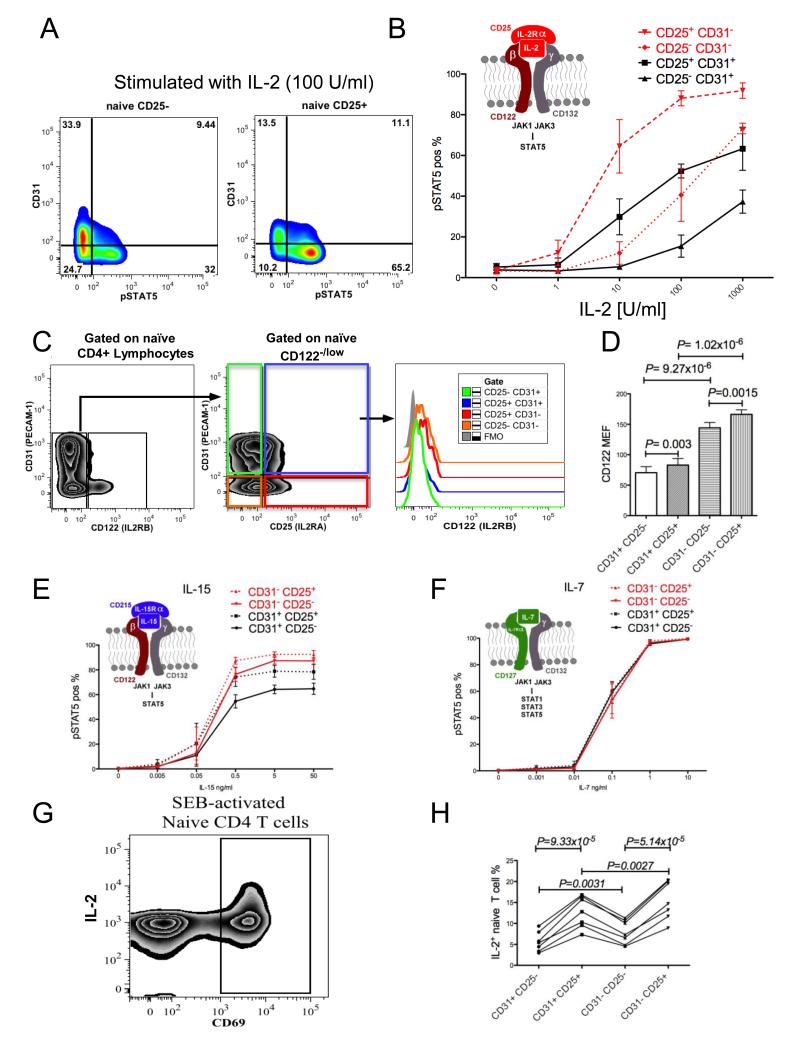
Although a portion of both CD31+ and CD31− naïve T cell subsets express CD25, the potential of the CD25+ CD31+ and CD25+ CD31− subsets to respond to IL-2 may vary due to their distinct immune histories, as defined by CD31 expression differences. In order to test this hypothesis, we stimulated blood samples with IL-2 and monitored pSTAT5a phosphorylation in naïve T cells by flow cytometry. Fig. 8A shows that CD25+ naïve T cells phosphorylated increased amounts of STAT5 in response to 100 U/ml of IL-2 compared to their CD25− counterparts and this IL-2 responsiveness was further increased in the CD31− subset. We than compared STAT5a phosphorylation in response to increasing doses of IL-2 between the CD25− and CD25+ subsets of CD31+ and CD31− naïve CD4 T cells. CD25+ cells showed increased levels of pSTAT5a as compared to CD25− cells in both CD31 subsets (for the CD31+ fraction P= 0.009, 0.00002 and 0.0098 for 10, 100 and 1000 U/ml IL-2, respectively, and for the CD31− fraction P= 0.0024, 0.0065 and 0.0029 for 10, 100 and 1000 U/ml IL-2, respectively, Fig. 8B). Interestingly, further analysis of STAT5a phosphorylation indicated that the CD31+ naïve CD4 T cell fraction that expresses CD25 is less responsive to IL-2 than the CD25+ fraction of CD31− naïve T cells (P= 0.0014, 0.0002 and 0.0038 for 10, 10 0 and 1000 U/ml IL-2, respectively (Fig. 8B). The difference in responsiveness between the CD25+ CD31+ and CD25+ CD31− subsets was not attributable primarily to a difference in CD25 expression (mean CD25 MEFs for the CD25+ CD31+ and CD25+ CD31− subsets were 180 +/− 32 SD, and 228 +/− 46, respectively), but rather to the difference in the expression levels of the β chain of the IL-2 receptor (CD122; mean MEF= 70 and 166 for the CD25+ CD31+ and CD25+ CD31− subsets, respectively, Fig. 8C-D).
Fig. 8. Expression of high and low affinity IL-2 receptors as well as IL-2 responsiveness is differentially regulated on CD31+ and CD31− naïve T cells.
(A) Representative example of pSTAT5 vs CD31 from CD25− and CD25+ naïve CD4+ CD127+ CD45RA+ FOXP3− T cells after 10 min stimulation with 100 U/ml of IL-2 (n=4). (B) Combined, dose dependent IL-2 responsiveness measured by flow cytometry analysis of pSTAT5 in CD4+ naïve T cells that were divided into four subsets on the basis of their CD25 and CD31 expression (n=4). (C) Representative flow cytometry analysis of CD122 expression in four different CD4+ naïve T cell subsets defined on the basis of the CD25 and CD31 expression (CD45RA+ CD122high cells were excluded from the analysis; see Supplemental Fig. 2). (D) Combined analysis of CD122 MEF determined that the CD31− central naïve T cells express increased levels of CD122 (n=5). CD122high cells were excluded from the analysis see (C) and Supplemental Fig. 2. CD4 T cells present within the peripheral blood were independently stimulated with increasing doses of IL-15 or IL-7 and cytokine responsiveness was measured by a flow cytometric pSTAT5 assay. Analysis of pSTAT5 present in the peripheral blood CD4+ CD127+ CD45RA+ FOXP3− naïve T cells that were divided into the four subsets on the basis of their CD25 and CD31 expression revealed that CD31− naïve T cells subsets show increased responsiveness to IL-15 (E) as compared to CD31+ naïve T cells. In contrast to IL-15, IL-7-stimulated cells (F) showed no difference in their IL-7 responsiveness (n=3). Graphical models of the cytokine:cytokine receptor complex are presented above the appropriate pSTAT5 analysis. (G) Representative example of SEB-activated CD45RO− naïve T cells; CD4 T cells responding to SEB were gated as CD69+. (H) CD69+ naïve CD4 T cells were gated further based on CD25 and CD31 expression and subsequently analysed for differences in IL-2 secretion (n=7). The four naïve subsets from each donor are connected by a line.
Similar to the CD25+ subsets, the CD25− fraction of CD31− naïve CD4 T cells stimulated with IL-2 responded to a greater extent than the CD25− fraction of CD31+ naïve T cells (P= 0.0072 and P=0.0003 for 100 and 1000 U/ml, respectively, Fig. 8B). Consistent with the previous results, higher IL-2 responsiveness in the CD31− CD25− subset corresponded to a higher expression of CD122 as compared to the CD31+ CD25− fraction (Fig. 8C-D).
Because the difference in IL-2 responsiveness by CD31+ and CD31− naïve CD4 T cells appeared to be attributable to CD122 levels, we next examined the pSTAT5a response of the four naïve CD4 T cell subsets to IL-15 since the IL-15 receptor shares both the β (CD122) and γ (CD132) chains with the IL-2 receptor (Fig. 8E). As predicted by the pattern of β chain expression (Fig. 8D), the responses by the CD25− and CD25+ CD31+ cell subsets were lower than their CD31− counterparts (P=0.0025 and P=0.0559 respectively for 0.5 ng/ml IL-15, P= 0.0015 and P= 0.0378, respectively, for 5 ng/ml IL-15 and P=0.035 and P= 0.0063, respectively, for 50 ng/ml IL-15, Fig. 8E). As a further control to demonstrate that all naïve cells, regardless of CD31 and CD25 expression, are capable of phosphorylating STAT5a, we incubated blood with IL-7. The response to IL-7 is CD122-independent but requires the common γ chain, CD132, and IL-7Rα. All four naïve CD4 T cell subsets produced identical responses (Fig. 8F).
Differential IL-2 production by the four naïve CD4 T cell subsets upon APC-dependent activation
Having demonstrated that four naïve T cell subsets defined by CD25 and CD31 expression display different IL-2 responsiveness (Fig. 9), we sought evidence for a functional role of CD25 during naïve CD4 T cell activation. Based on the previous study by Wuest et al. (20) who demonstrated a role for CD25 during early APC-T cell interactions, we hypothesized that CD25 on naïve CD4 T cells would increase the likelihood that a peptide—MHC complex presented by immunostimulatory APC causes cell activation and IL-2 production. PBMCs were stimulated for 4 hours with Staphylococcal enterotoxin B (SEB), which is an APC-dependent response (21), and CD69+ naïve T cells were analysed for IL-2 production (Fig. 8G). In agreement with our initial hypothesis, we have observed increased IL-2 production amongst CD25+ naïve T cells as compared to CD25− naïve T cells in both the CD31+ and CD31−subsets (P = 9.33x10-5 and P = 5.14x10-5 respectively, Fig. 8H). In addition we have observed increased IL-2 production amongst CD31− naïve T cells as compared to CD31+ naïve T cells in both the CD25+ and CD25− subsets (P=0.0027 and P=0.0031 respectively, Fig. 8H).
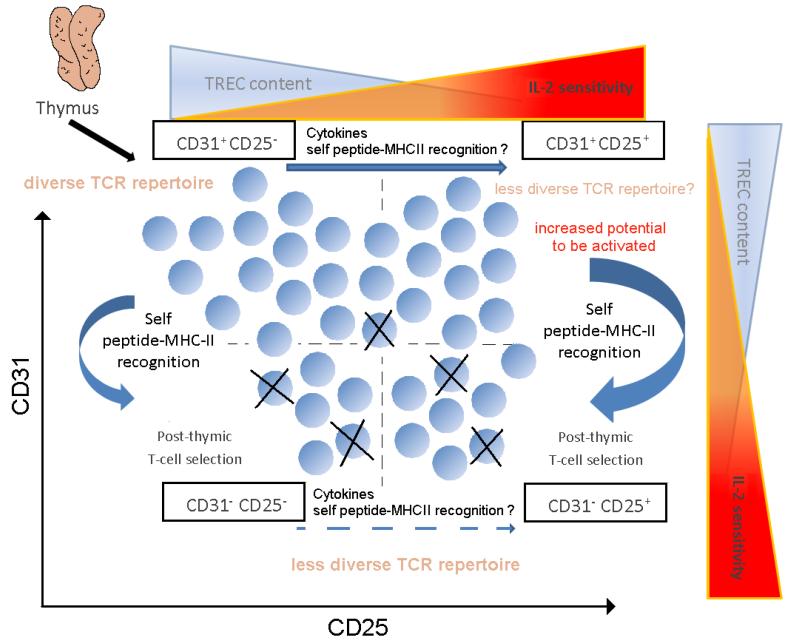
Figure 9. Model of post-thymic naïve T cell expansion.
Discussion
The production of thymically-derived human naïve CD4 T cells decreases with age due to the involution of the thymus. As thymic involution progresses, the maintenance of an adequate number of naïve CD4 T cells having a sufficiently broad TCR repertoire in the periphery becomes increasingly dependent on the homeostatic expansion of naïve CD4 T cells that exited the thymus years or decades earlier (1). In the current study we have obtained evidence supporting the hypothesis that CD25 expression identifies naïve CD4 T cells that have undergone homeostatic expansion in vivo. Together with CD31, a previously reported marker differentiating naïve CD4 T cells with a limited proliferative history outside the thymus (2), CD25 expression defines four subsets of naïve CD4 T cells: 1) CD31+ CD25−, 2) CD31+ CD25+, 3) CD31− CD25−, and 4) CD31− CD25+ (Fig. 9). Analysis of IL-2 responsiveness indicated that the four populations of naïve CD4 T cells differ in their responsiveness to IL-2 consistent with each subset’s CD25 and CD122 levels. Notably, even though CD31− CD25− cells do not express CD25 constitutively, increased CD122 co-expression to form the high affinity trimeric receptor with γ chain on this subset of naïve cells allows responsiveness to low doses of IL-2 (Fig. 8B). The previously uncharacterised increased sensitivity and responsiveness to IL-2 in naïve CD4 T cells that have undergone post-thymic rounds of division is likely to play a modulatory role in these cells during their further homeostatic proliferation and in their encounters with antigen.
The previous description of an approximately 2-fold dilution of TRECs in CD31+ naïve CD4 T cells isolated from donors aged 20 to 60 years old provided evidence for homeostatic proliferation within the CD31+ subset (1). However, no markers were defined identifying CD31+ cells that had divided (1, 18). In this study, TREC analyses of sorted subsets indicated that the CD31+ CD25+ naïve CD4 T cell subset was enriched with cells that have undergone more rounds of peripheral division than their CD31+ CD25− counterparts, thereby making CD25 a cell-surface marker that can be used to identify and study this expanded population. Thus, much of the approximately 2-fold dilution of TRECs in CD31+ naïve CD4 T cells that occurs in adulthood is likely accounted for by the increase of CD25+ cells in the CD31+ population, which on average represent 10 to 20% of this subset (Fig. 5D). As noted in the previous age-dependent TREC analyses of CD31+ cells, there is a large variation in the percentage of naïve CD31+ cells that are CD25+ within individuals of the same age which likely reflects genetic and environmental influences on the regulation of homeostatic proliferation. Although the CD31+ CD25− subset contains the highest number of TRECs amongst the four naïve populations that we tested (Fig. 6), it remains likely that the CD31+ CD25− subset still contains some cells that have divided after exiting the thymus.
It is clear that CD31− naïve cells that lack constitutive CD25 expression have diluted their TREC content more than their CD31+ CD25− counterparts (Fig. 6). Thus, cell division by naïve CD4 cells does not necessarily lead to stable CD25 expression. One explanation for this apparent paradox is that the signals that stimulate the loss of CD31 and the several rounds of proliferation that ensue do not lead to the stable expression of CD25. It has been proposed previously that CD31 downregulation marks cells that have been stimulated in the context of self-peptide MHC complexes resulting in a reduced TCR repertoire, a finding that has important implications since CD31− cells constitute a progressively larger proportion of the naïve CD4 T cell pool with age (3).
Since CD31− CD25+ naïve CD4 T cells have the greatest dilution of their TREC content, it is logical to hypothesize that this subset represents the “least naïve” of naïve CD4 T cells. However, because of our limited understanding about the selection processes acting on CD31+ CD25− cells, it is not possible to understand which naïve cell subset or subsets are the direct precursors of CD31−CD25+ naïve CD4 T cells. Indeed, it is possible that all three non-CD31−CD25+ subsets have direct progenitors for this subset that has expanded to the greatest extent. Deep sequencing of the TCR repertoire of the four subsets could help determine the origin of the CD31−CD25+ subset. Of particular interest is the question of whether the TCR diversity among CD31−CD25+ cells is more similar to that within CD31− CD25− or CD31+ CD25+ cells.
The expression of CD25 on naïve CD4 T cells could increase the likelihood of their long term survival into old age, mediated by increased survival signals transmitted through the IL-2 receptor. To test this possibility, we have compared the survival of CD25+ versus CD25− naïve cells with and without IL-2 during extended in vitro cultures but we have not obtained evidence that IL-2 alters naïve T cell survival even when CD25 is expressed (data not shown). However, we have demonstrated that the presence of functional, high-affinity IL-2 receptors on naïve CD4 T cells is likely to influence immune responses during early activation events since upon APC-dependent stimulation with SEB CD25+ naïve CD4 T cells produce more IL-2 as compared to their CD25− counterparts (Fig. 8H). The importance of IL-2 signalling during the early phases of activating allo- and antigen-specific T cells has been highlighted by the observations that CD25 co-polarizes with the TCR at the immunological synapse (22) and that immunostimulatory DCs express CD25 and secrete IL-2 toward the DC-T cell interface (23-26). The observation that daclizumab (anti-CD25), a potent immunosuppressive agent in humans, is a poor inhibitor of polyclonal T cell proliferation but a potent inhibitor of antigen-specific responses is consistent with the hypothesis that IL-2 signalling is a key early event in T cell activation stimulated by peptide MHC complexes (20). From the CD25 expression data presented in this study, we suggest that naïve CD25+ CD4 T cells having constitutive expression of CD25 will be less dependent on IL-2 trans-presentation by DC.
The expression of CD25 on naïve T cells could also directly alter the immune response by influencing the differentiation of naïve cells towards effector phenotypes: STAT5 signalling has been shown to regulate naïve T cell differentiation towards effector T cell subsets including iTregs (5), Th17- (27, 28) Th2- (29) and Tfh-cells (30, 31). Thus, the high affinity IL-2 receptors on naïve T cells that are exposed to foreign or self antigens could make a substantial contribution to the numbers and quality of the effector cells that are generated.
The dynamics of naïve T cell expansion could influence susceptibility and development of autoimmune diseases such as multiple sclerosis (32-37). In this context it is interesting to note that single nucleotide polymorphisms in IL2RA associated with multiple sclerosis and type 1 diabetes also control the frequency of CD25+ naïve T cells (7, 38). Polymorphisms in the IL7R have also been associated with the risk of multiple sclerosis (39).
In summary, the upregulation of CD25 (and CD122) on naïve CD4 T cell subsets in humans not only marks cells that have undergone more divisions in vivo but also provides a more heterogeneous naïve CD4 T cell pool based on differing IL-2 sensitivities and differing TCR diversities. The ability of each of the four naïve T cell subsets defined in this study to contribute precursors to the even more heterogeneous groups of differentiated effector and memory T cells as well as their clinical significance will be investigated in future studies.
Supplementary Material
Acknowledgments
We thank all donors for taking part in this study and Simon McCallum and Andreas Schwarzer for FACS. We gratefully acknowledge the Cambridge BioResource Scientific Advisory Board and Management Committee for support and the National Institute for Health Research Cambridge Biomedical Research Centre for funding. We thank Gwynneth L. Bell, Maureen Wiesner, Kelly Beer, and Jennifer Denesha for sample collection and processing and Matt Woodburn and Tony Attwood for contributing to sample management. We are grateful to staff at participating Diabetes—Genes, Autoimmunity, and Prevention hospital sites, including the Wellcome Trust Clinical Research Facility, Addenbrooke’s Clinical Research Centre, Cambridge, for help in conducting the study. We are also grateful to Dan Rainbow for help with manuscript editing.
Abbreviations
- APC
Antigen presenting cell
- DC
Dendritic cell
- IL-2
Interleukin 2
- IL2RA
Interleukin 2 receptor α gene
- IL2RB
Interleukin 2 receptor β gene
- MEF
Molecules of equivalent fluorescence
- MS
Multiple sclerosis
- SEB
Staphylococcal enterotoxin B
- TCR
T-cell receptor
- TRECs
T-cell receptor excision circles
- Treg
Regulatory T cell
Footnotes
This work was supported by Wellcome Trust Grant 096388, Juvenile Diabetes Research Foundation International Grant 9-2011-253, the National Institute for Health Research Cambridge Biomedical Research Centre, the Juvenile Diabetes Research Foundation International, Centre for Diabetes—Genes, Autoimmunity, and Prevention Grant 4-2007-1003, and by European Union Seventh Framework Programme Grant FP7/2007-2013 under Grant Agreement 241447 (Natural Immunomodulators as Novel Immunotherapies for Type 1 Diabetes). The Cambridge Institute for Medical Research is in receipt of Wellcome Trust Strategic Award 100140.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Mowes B, Julke K, Romagnani C, Thiel A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35:1987–1994. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- 3.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31- human naive CD4+ T-cell subsets. Blood. 2009;113:769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 4.Demeure CE, Byun DG, Yang LP, Vezzio N, Delespesse G. CD31 (PECAM-1) is a differentiation antigen lost during human CD4 T-cell maturation into Th1 or Th2 effector cells. Immunology. 1996;88:110–115. doi: 10.1046/j.1365-2567.1996.d01-652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dendrou CA, Plagnol V, Fung E, Yang JH, Downes K, Cooper JD, Nutland S, Coleman G, Himsworth M, Hardy M, Burren O, Healy B, Walker NM, Koch K, Ouwehand WH, Bradley JR, Wareham NJ, Todd JA, Wicker LS. Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat Genet. 2009;41:1011–1015. doi: 10.1038/ng.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dendrou CA, Fung E, Esposito L, Todd JA, Wicker LS, Plagnol V. Fluorescence intensity normalisation: correcting for time effects in large-scale flow cytometric analysis. Adv Bioinformatics. 2009:476106. doi: 10.1155/2009/476106. Epub 2009 Nov 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zubakov D, Liu F, van Zelm MC, Vermeulen J, Oostra BA, van Duijn CM, Driessen GJ, van Dongen JJ, Kayser M, Langerak AW. Estimating human age from T-cell DNA rearrangements. Curr Biol. 2010;20:R970–971. doi: 10.1016/j.cub.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Henson SM, Riddell NE, Akbar AN. Properties of end-stage human T cells defined by CD45RA re-expression. Curr Opin Immunol. 2012;24:476–481. doi: 10.1016/j.coi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN. Reversible senescence in human CD4+CD45RA+CD27- memory T cells. J Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- 13.De Rosa SC, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–248. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- 14.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 15.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, Vukmanovic-Stejic M. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 17.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–475. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, Phair J, Jamieson BD. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azevedo RI, Soares MV, Barata JT, Tendeiro R, Serra-Caetano A, Victorino RM, Sousa AE. IL-7 sustains CD31 expression in human naive CD4+ T cells and preferentially expands the CD31+ subset in a PI3K-dependent manner. Blood. 2009;113:2999–3007. doi: 10.1182/blood-2008-07-166223. [DOI] [PubMed] [Google Scholar]
- 20.Wuest SC, Edwan JH, Martin JF, Han S, Perry JS, Cartagena CM, Matsuura E, Maric D, Waldmann TA, Bielekova B. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med. 2011;17:604–609. doi: 10.1038/nm.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittelbrunn M, Molina A, Escribese MM, Yanez-Mo M, Escudero E, Ursa A, Tejedor R, Mampaso F, Sanchez-Madrid F. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci U S A. 2004;101:11058–11063. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 23.Mnasria K, Lagaraine C, Velge-Roussel F, Lebranchu Y, Baron C. Anti-CD25 antibodies decrease the ability of human dendritic cells to prime allogeneic CD4 T cells. Transplant Proc. 2009;41:695–697. doi: 10.1016/j.transproceed.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Mnasria K, Lagaraine C, Velge-Roussel F, Oueslati R, Lebranchu Y, Baron C. Anti-CD25 antibodies affect cytokine synthesis pattern of human dendritic cells and decrease their ability to prime allogeneic CD4+ T cells. J Leukoc Biol. 2008;84:460–467. doi: 10.1189/jlb.1007712. [DOI] [PubMed] [Google Scholar]
- 25.Granucci F, Zanoni I, Feau S, Ricciardi-Castagnoli P. Dendritic cell regulation of immune responses: a new role for interleukin 2 at the intersection of innate and adaptive immunity. EMBO J. 2003;22:2546–2551. doi: 10.1093/emboj/cdg261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velten FW, Rambow F, Metharom P, Goerdt S. Enhanced T-cell activation and T-cell-dependent IL-2 production by CD83+, CD25high, CD43high human monocyte-derived dendritic cells. Mol Immunol. 2007;44:1544–1550. doi: 10.1016/j.molimm.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 27.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Y. Kanno J, O’Shea J, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea J J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, Huang H, Wen R, Wang J, Li HS, Watowich SS, Qi H, Dong C, Wang D. STAT5 negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haegert DG, Hackenbroch JD, Duszczyszyn D, Fitz-Gerald L, Zastepa E, Mason H, Lapierre Y, Antel J, Bar-Or A. Reduced thymic output and peripheral naive CD4 T-cell alterations in primary progressive multiple sclerosis (PPMS) J Neuroimmunol. 2011;233:233–239. doi: 10.1016/j.jneuroim.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Haegert DG. Multiple sclerosis: a disorder of altered T-cell homeostasis. Mult Scler Int. 2011:461304. doi: 10.1155/2011/461304. 2011. Epub 2011 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duszczyszyn DA, Williams JL, Mason H, Lapierre Y, Antel J, Haegert DG. Thymic involution and proliferative T-cell responses in multiple sclerosis. J Neuroimmunol. 2010;221:73–80. doi: 10.1016/j.jneuroim.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Broux B, Hellings N, Venken K, Rummens JL, Hensen K, Van Wijmeersch B, Stinissen P. Haplotype 4 of the multiple sclerosis-associated interleukin-7 receptor alpha gene influences the frequency of recent thymic emigrants. Genes Immun. 2010;11:326–333. doi: 10.1038/gene.2009.106. [DOI] [PubMed] [Google Scholar]
- 36.Hug A, Korporal M, Schroder I, Haas J, Glatz K, Storch-Hagenlocher B, Wildemann B. Thymic export function and T cell homeostasis in patients with relapsing remitting multiple sclerosis. J Immunol. 2003;171:432–437. doi: 10.4049/jimmunol.171.1.432. [DOI] [PubMed] [Google Scholar]
- 37.Haas J, Fritzsching B, Trubswetter P, Korporal M, Milkova L, Fritz B, Vobis D, Krammer PH, Suri-Payer E, Wildemann B. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179:1322–1330. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]
- 38.International Multiple Sclerosis Genetics Consortium (IMSGC) Refining genetic associations in multiple sclerosis. Lancet Neurol. 2008;7:567–569. doi: 10.1016/S1474-4422(08)70122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D’Alfonso S, Blackburn H, Martinelli Boneschi F, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BA, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PI, Debouverie M, D’Hooghe M B, Dixon K, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SF, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung HP, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppa V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero IL, Mihalova T, Montalban X, Mottershead J, Myhr KM, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Ruckert IM, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PM, Smestad C, Sorensen PS, Sondergaard HB, Stankovich J, Strange RC, Sulonen AM, Sundqvist E, Syvanen AC, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CN, Wichmann HE, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.