Abstract
This article describes the use of citric acid modified sugar beet pulp as new ion-exchanger sorbent for the removal of metal cations and colorants from thin juice. The results of batch adsorption runs concerning the effects of contact time, material dosage, temperature and pH drop were presented and discussed. Experimental data on the removal of metal cations showed that the sorption process was rapid and reached equilibrium in 60 min. Modified material in acidic form caused to a significant pH drop in thin juice, which could result with sucrose inversion. Uptake of metal cations increased with temperature whereas that of color decreased. Neutralised type modified product gave more satisfying results. After six successive contacts, 49.7%, 37.5% and 43.7% removals for Ca-Mg, K and color, respectively, were obtained by using neutralised form of modified sugar beet pulp.
Keywords: Sugar beet pulp, Modification, Cation removal, Color removal
Introduction
In sugar processing, it is aimed to produce high-purity juices by most commonly used method which is based upon the addition of lime and carbon dioxide. However, juices obtained after liming-carbonation processes still contain inorganic compounds which cause many problems in further processes. For example, calcium ions cause scales in evaporators and crystallizers and this will decrease the heat transfer. Consequently, increasing evaporation/crystallization time increases color formation and sugar destruction. Moreover, inorganic compounds remained in the juice after carbonation step can decrease the yield of sugar crystallization (named molassigenic compounds as its presence increase the quantity of solids in mother liquors). The potassium and sodium salts are strongly molasses-forming, the calcium salts are less strongly molasses-forming but are more strongly scale forming which has a very adverse effect on the power consumption and the evaporation. By these reasons, it is important to reduce the quantity of inorganic compounds in the juice before refining (Bento 2008; Asadi 2006).
During the last decade or so, a significant part of the US Beet Sugar Industry has introduced thin juice softening by ion-exchange in their operations (Rousset et al. 2003). Rousset et al. (2003) have reported that most of the US factories working on beet molasses separation also use ion-exchange softening of thin juice. It has been stated that ion-exchange softening provides many benefits such as true removal of calcium from juice and molasses, less evaporators scaling/cleaning, increased heat transfer coefficients etc. It has been noted that ion-exchanging processes already play important roles in the sugar industry, in both molasses desugarisation and decolorization (Bento 1998, 2008). Besides the use of commercial resins (Coca et al. 2008), some cheap ion exchanging materials have been tested for juice purification (Stevens 1989; L’Hermine and Lundquist 1999; Clarke 2001; Yu et al. 2001, 2002; Agudo et al. 2002).
On the other hand, color is one of the quality measures of sugar. Color is stemmed from melanins, melanoidins, caramels, alkaline degradation products of fructose and phenolics (Godshall 1999). Beet colorants tend to be produced during processing, mainly from alkaline degradation of invert and melanoidin formation (Godshall et al. 2002). In conventional process for the production of sugar from sugar beet, the removal of color is an important issue. Carbonation, to some extent, provides decolorization but thin juice obtained after this process still contains colorants that have an effect on the whiteness of sugar produced. In order to remove colored compounds from sugar juices, the techniques of sulfitation, carbon adsorption and ion exchanging have been practised (Asadi 2006). The removal of colorants from sugar juices is considered to be an important application of adsorption process using inexpensive sorption materials. Among the low cost adsorbents, activated carbons derived from various agricultural by-products (Ahmedna et al. 1997, 2000a, b; Pendyal et al. 1999; Mudoga et al. 2008), and inorganic materials such as bentonite, sepiolite, diatomite, montmorillonite, organic clays (Erdogan et al. 1996; Unal and Erdogan 1998; Jaruwong and Kiattikomol-Wibulswas 2003) have been investigated for sugar decolorization.
It has been shown that treating lignocellulosic materials with poly acids (oxalic, citric, tartaric, phosphoric, phytic acids i.e.) at mildly elevated temperatures enhance their sorption capacity for metal ions and basic dyes (Lehrfeld 1997; Wafwoyo et al. 1999; Marshall et al. 1999, 2000; Vaughan et al. 2001; Wong et al. 2003; Altundogan et al. 2007; Gong et al. 2006, 2007, 2008). It has been reported that an estimated production cost for citric acid-modified soybean hull is $1.17/kg versus typical retail costs $4–40/kg for commercial, petroleum-based cation exchange resins when purchased in bulk quantities (Marshall et al. 2001).
Sugar beet pulp and its modified products have also been studied for both metals removal (Ozer et al. 1997a, b; Dronnet et al. 1997, 1998a, b; Aksu and Isoglu 2005, 2007; Altundogan et al. 2007) and dyes removal (Aksu and Isoglu 2006, 2007). In our recent study (Altundogan et al. 2007), it has been shown that sugar beet pulp treated by citric acid exhibits enhanced cation exchange capacity and copper uptake ability. These findings suggest that citric acid modified sugar beet pulp may be effective for cations removal from thin juice. From this standpoint, the cation removal from thin juice by using citric acid modified sugar beet pulp was explored in this study. For this purpose, modified materials obtained at the conditions given in the recent study by Altundogan et al. (2007) were used. A comparative study with citric acid treated sugar beet pulp and its neutralised form was carried out and several parameters such as contact time, material dose and temperature were investigated. In the course of study, decolorization effect of materials was also measured.
Materials and methods
Sugar beet pulp
Sugar beet pulp was collected from a local sugar factory (Elazig, Turkey) in the campaign 2005/2006. Sugar beet pulp was spread out on a polyethylene sheet and dried by air blowing. It was grinded and sieved to obtain the fraction between 16 and 30 mesh (0.6 mm < × <1.2 mm). The material was placed in a perforated ladle and washed with plenty of distilled water. Excessive moisture of pulps was removed by air blowing, and finally it was dried at 50°C for 24 h in an oven. This sample was named as sugar beet pulp (SBP).
Modification of sugar beet pulp
Modification of sugar beet pulp was carried out in three stages that first two stages were performed by the methods described in previous studies (Marshall et al. 1999; Altundogan et al. 2007). The last one is a neutralised form of the citric acid modified sugar beet pulp. Preparation methods and abbreviated names used for samples are given below:
100 grams of sugar beet pulp (SBP) was mixed with 2 L of 0.1 M NaOH solution in a 5 L polyethylene jar. The mixture was shaked at 200 rpm for 2 h at ambient temperature. The base-treated pulps were poured onto a perforated ladle and rinsed with distilled water. The pulps were added to 2 L of distilled water in jar and shaked at 200 rpm for 1 h at room temperature to remove excess base. The washed sugar beet pulps were poured onto the perforated ladle, rinsed and added to 2 L of fresh distilled water. This procedure was repeated until no pH variation in the wash water could be detected. This product is nominated as NaOH-treated sugar beet pulp (NTSBP). A portion of this product was dried at 50°C until constant weight. This was set aside for characterization tests. Another portion of NTSBP was exposed to air blowing for 6 h in order to remove excess humidity. Then, NTSBP was subjected to a citric acid esterification procedure the condition of which is an optimal of the study conducted by Marshall et al. 1999. For this purpose NTSBP was mixed with a 0.6 M citric acid in a ratio of 1.0 g material to 7.0 ml citric acid solution. NTSBP completely imbibed the citric acid solution within a couple of hour. The acid-NTSBP mixture was cooked for 12 h at 50°C. The cooked acid-NTSBP mixture was then finally heated at 120°C for 90 min. Modified product was added to hot distilled water at about 50°C in a water/solid ratio of 20 and shaked at 200 rpm for 1 h to remove unreacted acid. The mixture was poured onto the perforated ladle, rinsed and added to new hot distilled water. This procedure was repeated to ensure the complete removal of unreacted citric acid. The presence of citric acid in wash waters was tested by adding 0.1 M lead (II) nitrate solution. Washing was terminated when no turbidity from lead (II) citrate was observed. The modified pulp was coarsely dried by air blowing and then dried in an oven at 50°C until constant weight and final product is referred to as citric acid and NaOH -treated sugar beet pulp (CNTSBP). To obtain neutralised form of modified sugar beet pulp, a 50 g-portion of CNTSBP is placed in 5 L of 0.01 M NaOH solution. The mixture was shaken for 10 min at every 60 min for a total neutralisation period of 12 h. At the end of neutralisation treatment, the solution was removed by using a perforated ladle. Solid material was washed with distilled water until the pH of washing water is 7 ± 0.2. Washed material was dried at 50°C for 24 h.
Table 1 summarizes some properties of sugar beet pulp and its modified materials.
Table 1.
Some properties of sugar beet pulp and its modified products
| Property | SBP | NTSBP | CNTSBP | NCNTSBP |
|---|---|---|---|---|
| Bulk density (g/cm3) | 0.30 | 0.33 | 0.36 | 0.39 |
| pH | 5.1 | 8.1 | 4.0 | 7.2 |
| Solubility in water (%) | 4.4 | 3.1 | 0.96 | 0.86 |
| Solubility in acid (%) | 8.0 | 5.5 | 3.6 | 2.4 |
| Ash (%)(at 900°C) | 3.7 | 5.5 | 4.7 | 5.9 |
| Moisture | 6.1 | 7.8 | 6.8 | 6.5 |
| COD (mg-O2/l) | 177.0 | 48.1 | 75.3 | 81.6 |
| CEC (meq/g) | 0.93 | 1.5 | 3.4 | 3.6 |
| Water retention capacity (g/g) | 7.8 | 6.7 | 4.9 | 4.3 |
| Swelling capacity (cm3/g) | 6.6 | 4.3 | 3.9 | 3.2 |
| Copper sorption (meq/g) | 0.71 | 1.2 | 3.3 | 3.5 |
| Methylene blue sorption (mg/g) | 200.8 | 238.7 | 311.5 | 375.9 |
Each value is the mean of duplicate experiments
COD chemical oxygen demand; CEC cation exchange capacity; SBP raw sugar beet pulp; NTSBP NaOH-treated sugar beet pulp; CNTSBP citric acid and NaOH-treated sugar beet pulp; NCNTSBP neutralised form of CNTSBP
Thin juice
Thin juice was also provided from Elazig Sugar Factory in the campaign 2005/2006. It was kept in the refrigerator at 4°C during the experimental study. Some properties of thin juice sample are given in Table 2.
Table 2.
Characteristics of thin juice
| Property | Value |
|---|---|
| Dry matter | 13.5% |
| Sucrose | 12.2% |
| pH | 9.0 ± 0.05 |
| Color (ICUMSA Unit) | 2720 IU |
| Ca2+ + Mg2+ | 247 mg/l |
| Na+ | 440 mg/l |
| K+ | 1190 mg /l |
| Ash (at 900°C) | 3.7% |
Each value is the mean of duplicate experiments
ICUMSA International Commission for Uniform Methods of Sugar Analysis
Experimental procedure
Color of thin juice was measured (at pH 7 ± 0.2 and at 20°C) before each run. 200 ml samples were taken from the thin juice and heated to desired temperature. Appropriate amount of solid was added. The mixtures were shaken at 200 cpm. At the end of predetermined time, samples were taken and filtered through a blue band filter paper. The filtrate was cooled quickly to 20°C. The final pH was measured by using a Metler Delta 350 pH meter. A half of the sample was set aside in order to analyse the metal ions. In the other half of sample, the pH of the treated juice was adjusted to 7 ± 0.2 by using minimum amount of diluted HCl or NaOH solutions. Absorbance was measured at 420 nm in the pH adjusted juice as soon after sampling as possible by using Shimadzu UV-1200 spectrophotometer and the solid content was measured by using Atago 55-P2 refractometer. ICUMSA (International Commission for Uniform Methods of Sugar Analysis) color (as ICUMSA Unit) was calculated according to the following equation.
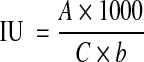 |
where A is absorbance of juice at pH 7 ± 0.2 (at 420 nm), b is light path (cm), C is dry substance content (g/cm3)(calculated by using brix-density tables for sugar solutions).
Decolorization percent of thin juice was calculated by using the following equation,
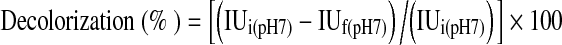 |
where IUi (pH 7) is calculated initial ICUMSA color of thin juice the pH of which was adjusted to 7 ± 0.2, IUf (pH 7) is calculated final ICUMSA color of the thin juice whose pH was adjusted to 7 ± 0.2.
The amount of removed metal ions was determined based on the soluble metal concentration measured before and after experiment. The amount of removal was obtained by the following expression.
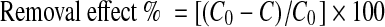 |
where C0 and C are the initial and residual concentrations (mg/l) of metals, respectively.
All the experiments were carried out in duplicates and the average values were used for further calculations.
Analytical methods
Methods used for characterization of sugar beet pulp and its derivatives are described in our previous study (Altundogan et al. 2007). Main characteristics of thin juice (color, dry matter, sucrose etc.) were determined according to ICUMSA (International Commission for Uniform Methods of Sugar Analysis) methods (ICUMSA 1978). Cation exchange capacity (CEC) was determined by a back titration method based on the saturation of the sample with standard acid solution (ASTM 1974). Potassium and sodium were analysed by using a flame photometer (Eppendorf, Serial no. 07765211). To eliminate the errors derived from matrix effect, the standard addition method (Skoog et al. 1996) was used instead of plotting a calibration curve. To apply the standard addition technique, accurately measured amounts of standard alkali metal (potassium or sodium) solutions were added to each of four equal aliquots of the unknown thin juice solutions. No alkali was added to one aliquot and amounts equivalent to approximately 50%, 100%, and 200% of the alkali in the unknown solution were added to the others. After the solutions were diluted to the same volume, the emission intensities were measured and the emission intensitiy-concentration curve extrapolated graphically to zero intensity. The absolute value of the concentration axes intercept was evaluated as the concentration of alkali in diluted unknown solution. Sum of calcium and magnesium in the thin juice samples was determined by EDTA (ethylenediaminetetraacetic acid) complexometric titration method (Kavas and Leblebici 2004) and the concentrations were given in calcium equivalent.
Results and discussion
The modified products derived from sugar beet pulp exhibit the enhanced cation exchange capacity, in contrast, depleted solubility, chemical oxygen demand (COD), swelling capacity and water retention capacity. Similar results have been obtained in our recent study (Altundogan et al. 2007) and values obtained after modification treatments have been attributed to stabilisation in the structure of sugar beet pulp. Enhanced methylene blue and copper sorption abilities confirm the increasing values in cation exchange capacity (CEC).
As seen from Table 1, successively subjecting the sugar beet pulp to base treatment, citric acid treatment and then neutralisation treatment gradually increase its cation uptake ability. The positive effect of the NaOH treatment on the cation exchange capacity of sugar beet pulp (SBP) could be mechanistically explained by hydrolyzing esterified carboxylate groups of pectic substances, which can lead an increase number of carboxylate functional groups. This treatment may also liberate primary alcohol groups esterified in cellulose molecules. In addition, treatment with dilute NaOH solutions at mild conditions may provide stability to the material by removing soluble substances with low molecular weight. Decreased chemical oxygen demand (COD) and solubility values confirm this idea. As a result, it can be noted that NaOH treatment increases both the cation uptake ability of pectic fraction and the number of primary alcohol groups which are appropriate sites in cellulosic fraction to be esterified with citric acid (Altundogan et al. 2007).
In the citric acid treatment stage, cellulose having primary alcohol groups could bind carboxyl groups. This esterification may further enhance the cation uptake ability of sugar beet pulp. Findings show that citric acid modification applied after the base treatment is much more effective for enhancing cation exchange capacity, which is 3.4 meq/g compared to the value of 1.5 meq/g (Table 1).
Neutralised form of citric acid modified pulp (NCNTSBP) exhibits a little more enhanced cation exchange capacity compared to that of citric acid modified pulp (CNTSBP). This may be stemmed from the fact that the higher dissociation ability of sodium form compared to that of hydrogen form of esterified material.
As a preliminary study, materials were tested by contacting with thin juice at dosage of 5 g/l for a period of 60 min at 20°C. Results are given in Table 3. As seen, cations and color removal efficiencies are not significant for raw sugar beet pulp (SBP) and NaOH treated sugar beet pulp (NTSBP) samples. Citric acid modified sugar beet pulp (CNTSBP) and its neutralised form (NCNTSBP) gave more efficient results. Cation exchange capacity values given in Table 1 confirm the efficiency order. From this observation, it was decided to use CNTSBP and NCNTSBP samples in further studies.
Table 3.
Results of preliminary study (Experimental conditions: 1 g material, 200 ml thin juice, initial pH, 9.0 ± 0.05; contact time, 60 min.; shaking rate, 200 rpm; temperature, 20°C)
| Material | pHaf | Cation removal (%) | Initial color of thin juice (ICUMSA Unit) | Color removal (%) | ||
|---|---|---|---|---|---|---|
| Na | K | Ca-Mgb | ||||
| SBP | 7.3 | 5.7 | 5.8 | 7.0 | 2720 | 7.6 |
| NTSBP | 7.6 | nd | 11.0 | 12.9 | 2742 | 6.1 |
| CNTSBP | 5.5 | 18.4 | 17.4 | 25.5 | 2770 | 27.7 |
| NCNTSBP | 8.0 | nd | 21.8 | 32.3 | 2784 | 34.9 |
Each value is the mean of duplicate experiments
aFinal pH, bin the base of Ca
ICUMSA International Commission for Uniform Methods of Sugar Analysis; SBP raw sugar beet pulp; NTSBP NaOH-treated sugar beet pulp; CNTSBP citric acid and NaOH-treated sugar beet pulp; NCNTSBP neutralised form of CNTSBP
To study the effect of dosage and contact time on cations and color removal from thin juice, the experiments were carried out with the sorbent mass varying from 2.5 to 20 g/l and contact time varying 5 to 120 min at 20°C. Ca-Mg, K and color removal percentages and pH measurements at the end of periods are shown all together in Fig. 1. For NCNTSBP, Na concentrations in final solutions were not determined. Therefore, Na removal was not evaluated for this group of studies.
Fig. 1.
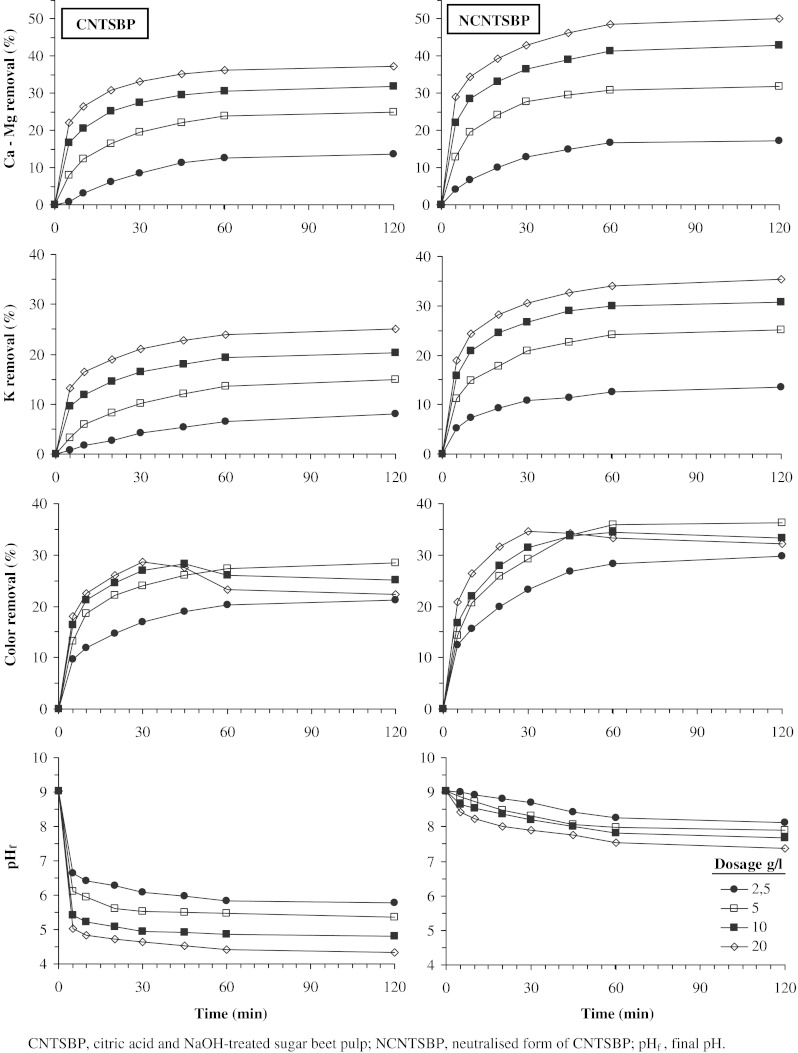
Time-dosage- removal-pH change relationships for CNTSBP and NCNTSBP (Experimental conditions: temperature, 20°C; shaking rate, 200 rpm)
The results represented in Fig. 1 show that Ca-Mg (in the Ca equivalent) and K removals increased with material dosage and contact time. As seen in the figure that removals were rapid for the first 20 min and thereafter those proceeded at a lower rate. Also, as expected, removals are dependent on sorbent dosage and the higher the dosage, the better the removal. This is due to the increase in the number of active sites as the amount of sorbent increases. It is very clear that NCNTSBP exhibits higher cations removal efficiency than CNTSBP at every corresponding dosage and this may be owed to its improved active cation binding sites (Fig. 1).
Additonally, Fig. 1 shows that the pH of thin juices contacted with CNTSBP at all dosages studied dropped to acidic region. In this ion exchange process a portion of the cations is replaced by hydrogen, which results a drop in the pH of thin juice. This cation removal process aims to remove molasses forming substances but causes sucrose inversion. In acidic media, under even slightly acidic conditions, sucrose undergoes inversion through acid hydrolysis (Edye and Clarke 1998), which results sugar loss. Bodamer and Kunin (1951) has noted that strong acid resins (sulfonic type) cause rapid sucrose inversion but a carboxylic type resin is a relatively poor catalyst for inversion reaction. In practice, after such a cation removal treatment of thin juice, pH is kept in slightly alkaline region (pH 7-9) by using a basic anion exchanger.
It is worthwhile to note that, after using NCNTSBP treatment, final pHs of thin juices are in slightly alkaline region, as shown in Fig. 1. In such a case, Ca, Mg and K ions present in thin juice are partially exchanged with Na ions in NCNTSBP and thus molassigenic substance equivalence of thin juice remains almost the same. However, the marked decrease in Ca-Mg content could be advantageous in point of the less scale forming on the evaporator, heat exchanger and crystallizer surface.
Though color removal tendency is similar to that of cation removal up to 5 g/l dosage, thereafter, CNTSBP and NCNTSBP both exhibit lower color removal values for contact time more than 30 min (Fig. 1). This may be due to color caused by sorbents themselves at these high dosages. In fact, it was found that water contacted with these materials at the dosage of 10 and 20 g/l had 30–40 absorbance units, in blank experiments. Hence, in all the subsequent experiments 5 g/l of sorbent was fixed as a favourable dosage which could give reasonably good removal efficiency.
The percent removal of metal cations increases progressively with the gradual increase in temperature from 20°C to 60°C, as shown in Table 4. Results indicate that the cations removal process on modified sugar beet pulps is endothermic in nature. However, the range of increase of percent removal of Ca-Mg is greater than those of K and Na for CNTSBP. The experimental results under different temperatures indicated that the color removal ratio decreased slightly along with increasing experimental temperature. This may be stemmed from a competitive sorption between cations and colorant. As temperature increases, adsorption capabilities of metal cations may become higher than those of colorants. Also, ionic charge, structural configuration, steric hinderance, size and surface area of the colorant molecule may play role in sorption process.
Table 4.
Results of temperature study (Experimental conditions: 1 g material, 200 ml thin juice, shaking rate, 200 rpm; initial pH, 9.0 ± 0.05)
| Material | Temp. (°C) | Time (min) | pHaf | Cation removal (%) | Initial color of thin juice (ICUMSA Unit) | Color removal (%) | ||
|---|---|---|---|---|---|---|---|---|
| Na | K | Ca-Mgb | ||||||
| CNTSBP | 20 | 30 | 5.5 | 14.4 | 9.2 | 18.9 | 2998 | 23.8 |
| 20 | 60 | 5.5 | 16.9 | 12.8 | 23.3 | 2998 | 27.2 | |
| 40 | 30 | 5.3 | 15.6 | 12.3 | 22.9 | 3013 | 17.3 | |
| 40 | 60 | 5.1 | 18.2 | 15.7 | 29.1 | 3013 | 21.8 | |
| 60 | 30 | 5.0 | 18.6 | 15.7 | 28.0 | 3034 | 13.4 | |
| 60 | 60 | 4.9 | 20.7 | 18.9 | 33.5 | 3034 | 17.9 | |
| NCNTSBP | 20 | 30 | 8.3 | nd | 21.5 | 28.6 | 3034 | 28.4 |
| 20 | 60 | 8.0 | nd | 24.8 | 33.2 | 3034 | 33.1 | |
| 40 | 30 | 8.1 | nd | 24.8 | 36.2 | 3048 | 22.1 | |
| 40 | 60 | 7.7 | nd | 28.7 | 41.3 | 3048 | 27.4 | |
| 60 | 30 | 7.8 | nd | 28.4 | 44.5 | 3070 | 16.7 | |
| 60 | 60 | 7.4 | nd | 31.6 | 49.1 | 3070 | 22.4 | |
Each value is the mean of duplicate experiments
aFinal pH, bin the base of Ca
ICUMSA International Commission for Uniform Methods of Sugar Analysis; CNTSBP citric acid and NaOH- treated sugar beet pulp; NCNTSBP neutralised form of CNTSBP
It should be noted that pH drops are much higher at higher temperature for acidic type modified product (CNTSBP) implying the removal process is not favourable at raised temperature because of sucrose inversion. NCNTSBP exhibits slightly alkaline or near-neutral pH values at elevated temperatures.
In an attempt to see the results of continuous column tests, a fixed bed column study was conducted. In these runs a regular flow regime could not be achieved since the beads swelled in the column. Upon this problem, successive batch runs resembling a column treatment to some extent were done. In this group of experiments, a thin juice sample was successively contacted with solid materials at a 5 g/l sorbent dosage at 20°C for a period of 20 min in each run. For this purpose, firstly, 5 g material was contacted with 1000 ml thin juice by shaking. After 20 min, the whole mixture was filtered. A 50 ml sample was taken from treated thin juice and put aside for analyses. The volume of remained thin juice was measured. A fresh solid material corresponding to the 5 g/l dosage was added to this treated thin juice. The mixture was shaken for a period of 20 min. At the end of second period, another sample of 50 ml was taken. According this procedure, six successive runs were conducted. The results of successive batch treatment were shown in Fig. 2.
Fig. 2.
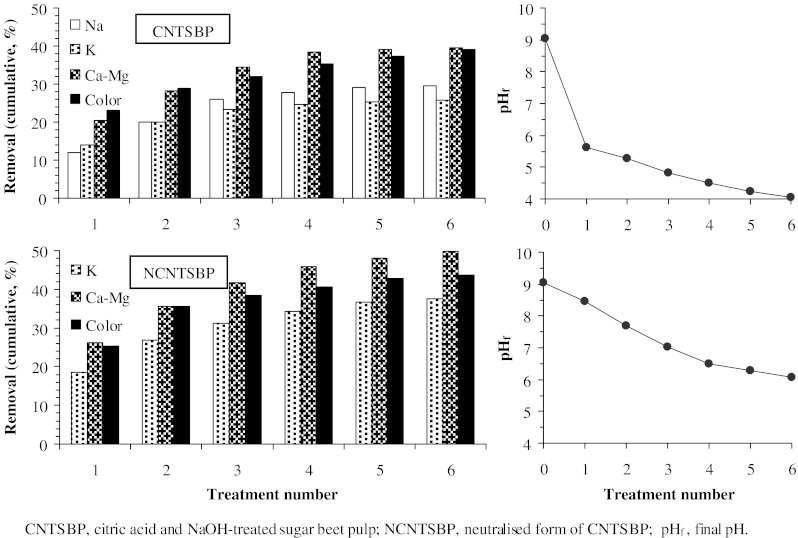
Removal efficiencies for multistage contact (Experimental conditions: material dosage, 5 g/l (in each stage); contact time, 20 min (for each stage); temperature, 20°C; shaking rate, 200 rpm)
The removal rates of cations and color were very rapid during the initial stages of the stage-wise process. As can be seen from Fig. 2, metal cations and color removal percentages increased up to 4th stage, thereafter, remained almost constant for CNTSBP. In these runs, pH of thin juice decreased from 9.0 to 5.6 in the second stage and to 4.1 in the sixth stage. Starting from the 4th stage, lower removal efficiency obtained may be stemmed from acidic medium. Low pH value causes to an increase in H+ ion concentration in the system and the surface of the sorbent acquires positive charge by adsorbing H+ ions. As the sorbent surface is positively charged at low pH value, an electrostatic repulsion appears between the positively charged sorbent surface and cationic species leading to insignificant uptake of metal cations and cationic colorants. The sorption on NCNTSBP, on the other hand, increased slightly and monotonously with increasing stage number, probably due to decreasing in the pH is not so much.
The strong pH dependent sorption of metal ions on modified sugar beet pulps suggests that an anion exchange process should be conducted as a following step. This application could provide a removal of anionic species (such as Cl−, SO2−4 etc.) and colorants in anionic structure. Thus, pH of thin juice can be kept within the safety limits as well.
Conclusions
Carboxylate functional group-rich materials were prepared by treating sugar beet pulp with NaOH and citric acid. Modified sugar beet pulps obtained were characterized and tested to remove cations and colorants from sugar thin juice. The tests confirmed that modified sugar beet pulps exhibited rather stable properties and the enhanced cation exchange capacity. Neutralised type modified sugar beet pulp exhibited significantly enhanced metal ions removal without causing a noticable pH drop in thin juice. The modified sugar beet pulps also exhibited a remarkable color removal capability. They are easily prepared by utilizing the waste heat and the condensed water in a sugar factory, and therefore they may be considered as potential agents for purifying sugar juices. Furthermore, modification agent citric acid is a harmless and cheap material, which is produced from molasses that is another by-product of sugar industry.
Acknowledgement
Authors thank to Elazig Sugar Factory (in Turkey) for providing sugar beet pulp and thin juice and doing some analyses in their laboratories. H. Arslanoglu is indebted to Assoc.Prof.Dr. H.Soner Altundogan for providing a scholarship.
References
- Agudo JAG, Cubero MTG, Benito GG, Miranda MP. Removal of coloured compounds from sugar solutions by adsorption onto anionic resins: equilibrium and kinetic study. Sep Purif Technol. 2002;29:199–205. doi: 10.1016/S1383-5866(02)00083-7. [DOI] [Google Scholar]
- Ahmedna M, Johns MM, Clarke SJ, Marshall WE, Rao RM. Potential of agricultural by-product-based activated carbons for use in raw sugar decolourisation. J Sci Food Agr. 1997;75:117–124. doi: 10.1002/(SICI)1097-0010(199709)75:1<117::AID-JSFA850>3.0.CO;2-M. [DOI] [Google Scholar]
- Ahmedna M, Marshall WE, Rao RM (2000a) Granular activated carbons from agricultural by products: Preparation, properties and application in cane sugar refining. Lousiana State University Agricultural Center Bulletin number 869:1–56
- Ahmedna M, Marshall WE, Rao RM. Surface properties of granular activated carbons from agricultural by-products and their effect on raw sugar decolorization. Bioresour Technol. 2000;71:103–112. doi: 10.1016/S0960-8524(99)90069-X. [DOI] [Google Scholar]
- Aksu Z, Isoglu A. Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem. 2005;40:3031–3044. doi: 10.1016/j.procbio.2005.02.004. [DOI] [Google Scholar]
- Aksu Z, Isoglu A. Use of agricultural waste sugar beet pulp for the removal of Gemazol turquoise blue-G reactive dye from aqueous solution. J Hazard Mater. 2006;137:418–430. doi: 10.1016/j.jhazmat.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Aksu Z, Isoglu A. Use of dried sugar beet pulp for binary biosorption of Gemazol Turquoise Blue-G reactive dye and copper(II) ions: equilibrium modeling. Chem Eng J. 2007;127:177–188. doi: 10.1016/j.cej.2006.09.014. [DOI] [Google Scholar]
- Altundogan HS, Arslan NE, Tumen F. Copper removal from aqueous solutions by sugar beet pulp treated by NaOH and citric acid. J Hazard Mater. 2007;149:432–439. doi: 10.1016/j.jhazmat.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Asadi M. Beet-sugar handbook. Hoboken: Wiley; 2006. [Google Scholar]
- ASTM D 2187-74 Standard methods of test for physical and chemical properties of particulate ion-exchange resins. Philadelphia: American Society of Testing and Materials; 1974. [Google Scholar]
- Bento LSM. Ion exchange resins for sugar decolorization. Int Sugar J. 1998;100:111–117. [Google Scholar]
- Bento LSM (2008) Softening, Sucropedia (Sugar Industry Encyclopedia) E 0057 http://www.sucropedia.com/entries.php?accao=ler_entrada&id_entrada=68, accesed in Dec 17, 2008
- Bodamer G, Kunin R. Heterogeneous catalytic inversion of sucrose with cation exchange resins. J Ind Eng Chem. 1951;43:1082–1085. doi: 10.1021/ie50497a025. [DOI] [Google Scholar]
- Clarke SJ (2001) Application of a different type of ion-exchange material to sugar operations. Proc Sugar Industry Technologists (SIT) Conf, May 2001, Taipei, Taiwan, Presentation number 790
- Coca M, Garcia MT, Mato S, Carton A, Gonzales G. Evolution of colorants in sugarbeet juices during decolorization using styrenic resins. J Food Eng. 2008;89:429–434. doi: 10.1016/j.jfoodeng.2008.05.025. [DOI] [Google Scholar]
- Dronnet VM, Renard CMGC, Axelos MAV, Thibault JF. Binding of divalent metal cations by sugar-beet pulp. Carbohydr Polym. 1997;34:73–82. doi: 10.1016/S0144-8617(97)00055-6. [DOI] [Google Scholar]
- Dronnet VM, Axelos MAV, Renard CMGC, Thibault JF. Improvoment of the binding capacity of metal cations by sugar-beet pulp. 1. Impact of cross-linking treatments on composition, hydration and binding properties. Carbohydr Polym. 1998;35:29–37. doi: 10.1016/S0144-8617(97)00118-5. [DOI] [Google Scholar]
- Dronnet VM, Axelos MAV, Renard CMGC, Thibault JF. Improvoment of the binding capacity of metal cations by sugar-beet pulp. 2. Binding of divalent metal cations by modified sugar-beet pulp. Carbohydr Polym. 1998;35:239–247. doi: 10.1016/S0144-8617(97)00253-1. [DOI] [Google Scholar]
- Edye L, Clarke MA. Sucrose loss and color formation in sugar manufacture. Adv Exp Med Biol. 1998;43:123–133. doi: 10.1007/978-1-4899-1925-0_12. [DOI] [PubMed] [Google Scholar]
- Erdogan B, Demirci S, Akay Y. Treatment of sugar beet juice with bentonite, sepiolite, diatomite and quartamin to remove color and turbidity. Appl Clay Sci. 1996;11:55–67. doi: 10.1016/0169-1317(96)00012-9. [DOI] [Google Scholar]
- Godshall MA (1999) Removal of colorants and polysaccharides and the quality of white sugar. Proc Sixth International Symposium Organized by Association Andrew van Hook (AvH), March 25 1999, Reims, France, 28–35
- Godshall MA, Vercellotti JR, Triche R. Comparison of cane and beet sugar macromolecules in processing. Int Sugar J. 2002;104:228–233. [Google Scholar]
- Gong R, Jin Y, Chen F, Chen J, Liu Z. Enhanced malachite green removal from aqueous solution by citric acid modified rice straw. J Hazard Mater. 2006;137:865–870. doi: 10.1016/j.jhazmat.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Gong R, Jin Y, Chen J, Hu Y, Sun J. Removal of basic dyes from aqueous solution by sorption on phosphoric acid modified rice straw. Dyes Pigm. 2007;73:332–337. doi: 10.1016/j.dyepig.2006.01.037. [DOI] [Google Scholar]
- Gong R, Jin Y, Sun J, Zhong K. Preparation and utilization of rice straw bearing carboxyl groups for removal of basic dyes from aqueous solution. Dyes Pigm. 2008;76:519–524. doi: 10.1016/j.dyepig.2006.10.013. [DOI] [Google Scholar]
- ICUMSA (1978) Method 4 for the determination of color of sugar in solution. International comission for uniform sugar analysis, Verlag Bartens, Berlin, Germany, pp 343–344
- Jaruwong P, Kiattikomol-Wibulswas R (2003) Raw sugar decolorization by montmorillonite and organo-clays (The 2nd Asian Particle Technology Symposium 2003. Dec 17–19. Penang, Malaysia) Published in: Particle Design New Technologies & Industrial Applications 385–391
- Kavas MF, Leblebici MJ (2004) Kalite ve Isletme Kontrol Laboratuvarlari El Kitabi. Turkiye Seker Fabrikalari A.S. Genel Mudurlugu Ankara (In Turkish) 129–130 and 246
- Lehrfeld J. Cation exchange resins prepared from phytic acid. J Appl Polym Sci. 1997;66:491–497. doi: 10.1002/(SICI)1097-4628(19971017)66:3<491::AID-APP9>3.0.CO;2-K. [DOI] [Google Scholar]
- L’Hermine GJA, Lundquist EG (1999) Decolorization of sugar syrups using functionalized adsorbents. US Patent Office Pat No 5 972 121
- Marshall WE, Wartelle LH, Boler DE, Johns MM, Toles CA. Enhanced metal adsorption by soybean hulls modified with citric acid. Bioresour Technol. 1999;69:263–268. doi: 10.1016/S0960-8524(98)00185-0. [DOI] [Google Scholar]
- Marshall WE, Wartelle LH, Boler DE, Toles CA. Metal ion adsorption by soybean hulls modified with citric acid: a comparative study. Environ Technol. 2000;21:601–607. doi: 10.1080/09593332108618075. [DOI] [Google Scholar]
- Marshall WE, Chatters AZ, Wartelle LH, McAloon A. Optimization and estimated production cost of a citricacid-modified soybean hull ion exchanger. Ind Crop Prod. 2001;14:191–199. doi: 10.1016/S0926-6690(01)00084-X. [DOI] [Google Scholar]
- Mudoga HL, Yucel H, Kincal NS. Decolorization of sugar syrups using commercial and sugar beet pulp based activated carbons. Bioresour Technol. 2008;99:3528–3533. doi: 10.1016/j.biortech.2007.07.058. [DOI] [PubMed] [Google Scholar]
- Ozer A, Tumen F, Bildik M. Cr(VI) removal from aqueous solutions by depectinated sugar beet pulp. Chim Acta Turc. 1997;25:113–118. [Google Scholar]
- Ozer A, Tumen F, Bildik M. Cr(III) removal from aqueous solutions by depectinated sugar beet pulp. Environ Technol. 1997;18:893–901. doi: 10.1080/09593331808616608. [DOI] [Google Scholar]
- Pendyal B, Johns MM, Marshall WE, Ahmedna M, Rao RM. Removal of sugar colorant by granular activated carbons made from binders and agricultural by-products. Bioresour Technol. 1999;69:45–51. doi: 10.1016/S0960-8524(98)00172-2. [DOI] [Google Scholar]
- Rousset F, Theoleyre MA, Gula F, Saska M (2003) Raffinate regeneration of ion-exchange softeners in the beet sugar industry. J Sugar Beet Res 40:193–194 [2003 Joint Meeting of the American Society of Sugar Beet Technologists (ASSBT) and International Institute for Beet Research (IIRB), Feb 26–March 1, 2003, San Antonio, Texas, USA]
- Skoog DA, West DM, Holler FJ. Fundamentals of analytical chemistry. 7. Philadelphia: Harcourt College Publishers; 1996. [Google Scholar]
- Stevens RR (1989) Process for decolorizing aqueous sugar solution. US Patent Office Pat. No 4 871 397
- Unal HI, Erdogan B. The use of sepiolite for decolorization of sugar juice. Appl Clay Sci. 1998;12:419–429. doi: 10.1016/S0169-1317(97)00023-9. [DOI] [Google Scholar]
- Vaughan T, Seo CW, Marshall WE. Removal of selected metal ions from aqueous solution using modified corncobs. Bioresour Technol. 2001;78:133–139. doi: 10.1016/S0960-8524(01)00007-4. [DOI] [PubMed] [Google Scholar]
- Wong KK, Lee CK, Low KS, Haron MJ. Removal of Cu and Pb by tartaric acid modified rice husk from aqueous solutions. Chemosphere. 2003;50:23–28. doi: 10.1016/S0045-6535(02)00598-2. [DOI] [PubMed] [Google Scholar]
- Wafwoyo W, Seo CW, Marshall WE. Utilization of peanut shells as adsorbents for selected metals. J Chem Technol Biotechnol. 1999;74:1117–1121. doi: 10.1002/(SICI)1097-4660(199911)74:11<1117::AID-JCTB151>3.0.CO;2-R. [DOI] [Google Scholar]
- Yu S, Geng Y, Li G, Gao D (2001) The utilization of bagasse exchanger in sugar industry. Proc Sugar Industry Technologists (SIT) Conf, May 2001, Taipei, Taiwan, Presentation number 789
- Yu S, Chou CC, Geng Y, Ran Y. A new cane bagasse based anion exchanger for sugar decolorization/clarification. Int Sugar J. 2002;104:313–320. [Google Scholar]


