Abstract
Objective
The aim of the study was to characterize the expression of IL-7 and IL-7R in rheumatoid arthritis (RA) synovial tissue and to examine their regulation and pathogenic role in macrophages, endothelial cells and RA synovial tissue fibroblasts.
Methods
Expression of IL-7 and IL-7R was demonstrated in RA and normal synovial tissues employing immunohistochemistry. Expression and regulation of IL-7 and IL-7R was determined in RA peripheral blood in vitro differentiated macrophages, RA synovial tissue fibroblasts and human microvascular endothelial cells (HMVECs) by real-time RT-PCR and/or flow cytometry. Next, IL-7 activated macrophages, RA fibroblasts and endothelial cells were examined for production of proangiogenic factors employing ELISA.
Results
IL-7 and IL-7R were coexpressed on RA synovial tissue lining and sublining macrophages and endothelial cells. Consistently, expression of IL-7 and its receptor were significantly elevated in RA synovial fluid and peripheral blood macrophages as well as RA fibroblasts compared to normal cells. TLR4 ligation and stimulation with TNF-α modulated expression of IL-7 and IL-7R on RA macrophages and HMVECs. However, in RA fibroblasts only expression of IL-7R was increased by LPS and TNF-α activation. IL-7 also mediated RA pathogenesis by inducing production of potent proangiogenic factors from macrophages and endothelial cells.
Conclusion
We identify, for the first time, regulators of IL-7 and IL-7R expression in RA fibroblasts, RA peripheral blood in vitro differentiated macrophages and endothelial cells and we document a novel role of IL-7 in RA angiogenesis.
Keywords: IL-7, IL-7R, RA synovial tissue fibroblast, macrophages and proangiogenic factors
IL-7 is a member of the IL-2/IL-15 family of cytokines which can be secreted or presented on stromal cells, epithelial cells, endothelial cells, fibroblasts and smooth muscle cells (1-4). It has been shown that rheumatoid arthritis (RA) patients have elevated levels of circulating IL-7 compared to normal individuals (5, 6). Additionally, an increased concentration of IL-7 has been detected in RA compared to osteoarthritis (OA) synovial fluid (7, 8). However, the cells responsible for producing IL-7 in circulation as well as in RA synovial fluid are unknown.
IL-7, in concert with other growth factors, can contribute to the expansion of T cell precursors (9). Mature T cells are also modulated by IL-7, first by costimulation of T cells through cytokine production, second by promoting TH1 differentiation, and last by inhibiting programmed cell death through the proteins of the Bcl-2 family, thereby maintaining T cell homeostasis (10-13). Further, dendritic cell development, maturation and antigen presentation are partially controlled by IL-7 (14).
It has been shown that RA synovial fluid macrophages activated with IL-7 differentiate into osteoclasts (15). IL-7 was also the most potent factor to induce differentiation of bone-resorbing cells compared to a panel of 16 cytokines (TNF-α, IL-1, 6 and 8 and others) and growth factors (GM-CSF and M-CSF and others) (15, 16). Consistently, IL-7-/- mice have a significant increase in bone mass due to reduced RANKL concentration (17). These results suggest that IL-7 plays an important role in bone resorption by inducing osteoclast differentiation as well as RANKL production.
Co-culture of T cells and monocytes stimulated by IL-7 is associated with TNF-α production by monocytes (5, 6). However, IL-7 activated T cells or monocytes cultured separately fail to produce TNF-α. Interestingly, the same group of investigators have shown that RA patients who are responsive to anti-TNF-α therapy have significantly lower circulating IL-7 levels compared to anti-TNF-α nonresponders (6).
In RA synovial tissue, IL-7R expression correlated with number of T cells and IL-7 expression level (18). Further, blockade of IL-7R in RA peripheral blood and synovial fluid significantly reduced endogenous IL-7 induced IFN-γ production (18) suggesting that IL-7 and IL-7R play an important role in RA pathology by activating T cells. Consistently, blockade of IL-7R ameliorates collagen induced arthritis (CIA) joint inflammation by reducing T cell trafficking and proinflammatory factors produced by macrophages such as TNF-α, IL-1β, IL-6 and MMPs (19).
In this paper, we demonstrate that IL-7 and IL-7R are coexpressed on RA synovial tissue lining and sublining macrophages and endothelial cells thereby identifying novel target cells responsive to IL-7 activation. Further, IL-7 and IL-7R expression is greatly increased in RA synovial fluid and peripheral blood macrophages as well as in RA peripheral blood monocytes compared to normal cells. We also show that activation with TLR4 ligand, IL-1β, and TNF-α can modulate expression of IL-7 and IL-7R in RA peripheral blood in vitro differentiated macrophages. Both IL-7 and IL-7R expression are markedly higher in RA compared to normal fibroblasts, however, only expression of IL-7R is affected by lipopolysaccharide (LPS) and TNF-α stimulation in RA fibroblasts. In contrast, TLR4 ligation and TNF-α stimulation of human microvascular endothelial cells (HMVECs) can significantly induce expression of IL-7 and its receptor. Last, we demonstrate that IL-7 can exert its pathological role by activating macrophages and endothelial cells to produce pro-angiogenic factors such as IL-8 and Ang-1. Hence, therapy directed against IL-7R ligation may reduce leukocyte migration by inhibiting angiogenesis in RA.
MATERIALS AND METHODS
Antibodies and immunohistochemistry
The studies were approved by the Institutional Review Board, and all donors gave informed written consent. Since the RA synovial tissues are recruited from the practices of orthopedic surgeons these samples are de-identified therefore the disease severity and the treatment information is unavailable. RA and normal (NL) synovial tissues were formalin fixed, paraffin embedded, and sectioned in the pathology core facility. Synovial tissues were immunoperoxidase-stained using Vector Elite ABC Kits (Vector Laboratories, Burlingame, CA), with diaminobenzidine (Vector Laboratories) as a chromogen. Briefly, slides were deparaffinized in xylene for 15 min at room temperature, followed by rehydration by transfer through graded alcohols. Antigens were unmasked by incubating slides in Proteinase K digestion buffer (Dako, Carpinteria, CA) for 10 min at room temperature. Endogenous peroxidase activity was blocked by incubation with 3% H2O2 for 5 min. Nonspecific binding of avidin and biotin was blocked using an avidin/biotin blocking kit (Dako). Tissues were incubated with antibodies to human IL-7 (1:80, R & D Systems, Minneapolis, MN) or IL-7R (1:100, Santa Cruz Biotechnology, Santa Cruz, CA) or an IgG control antibody (Beckman Coulter, Brea, CA). Slides were counterstained with Harris hematoxylin and treated with lithium carbonate for bluing. Each slide was evaluated by two blinded observer (20-23) (A.M.M. and M.V.V.). Tissue sections were scored for lining, sublining macrophages and endothelial cell staining on a 0-5 scale, where 0=no staining, 1=few cells stained, 2=some (less than half) cells stained, 3= around half of the cells were stained positively 4= majority or more than half of the cells were positively stained and 5= all cells were positively stained. Scored data were pooled, and the mean ± SEM was calculated in each data group.
To localize IL-7 or IL-7R to macrophages or endothelial cells in RA synovial tissues, slides were deparaffinized as mentioned above and the antigen was unmasked by incubating slides in Proteinase K digestion buffer (Dako) for 10 min at rooms temperature. Using an Invision G2 kit (Dako) RA synovial tissues were stained with IL-7 (1:25 dilution, Santa Cruz Biotechnology) or IL-7R (1:100 dilution, Santa Cruz Biotechnology) employing DAB (brown staining) as a chromogen. Thereafter tissues were blocked (double staining blocker included in the Invision G2 kit) and stained with Von wille brand factor (1:1000 dilution, Dako) or CD68 (1:100 dilution, Dako) employing Fast red (red staining) as a chromogen following manufacture’s instruction (Dako).
Flow cytometry
In order to determine IL-7R+ cells, normal and RA monocytes and macrophages were washed with FACS buffer (5% FBS in PBS). Thereafter cells were blocked with 50% human serum and 0.5% bovine serum albumin in phosphate buffered saline for 30 minutes at room temperature. Cells were then stained for PE labelled anti-CD127 (IL-7R, BD Pharmingen) and FITC conjugated anti-CD14 (Becton Dickinson Immunocytometry lab) or isotype control antibodies (BD Pharmingen). Percent IL-7R+ cells were identified as those that were CD14+CD127+.
Cell isolation, culture and procedures
NL and RA peripheral blood and RA synovial fluid mononuclear cells were isolated by Histopaque gradient centrifugation (Sigma-aldrich, St. Louis, MO, USA) as previously described (24, 25). Monocytes/macrophages were isolated from normal and RA peripheral blood or RA synovial fluid employing a negative selection kit (StemCell Technologies, Vancouver, Canada) according to the manufacturers’ instructions(26). Monocytes were subsequently differentiated to macrophages by culturing in RPMI containing 20% FBS for 7 days.
Quantification of chemokines and cytokines
Human IL-8, Ang-1, Ang-2, VEGF, CXCL16, IL-1β, TNF-α and IL-6 (R&D Systems, Minneapolis, MN, USA) ELISA kits were used according to the manufacturers’ instructions.
Isolation of RA synovial tissue fibroblasts
Synovial tissue fibroblasts were isolated from fresh RA synovial tissues by mincing and digestion in a solution of dispase, collagenase, and DNase (25). Cells were used between passages 3 and 9 and cultured in DMEM containing 10% FBS.
Cell treatment
RA peripheral blood in vitro differentiated macrophages, RA synovial tissue fibroblasts and HMVECs were treated with LPS (Sigma, 10 ng/ml), IL-1β (R&D Systems, 10 ng/ml), TNF-α (R&D Systems, 10 ng/ml), IL-17 (R&D Systems, 50 ng/ml), or RA synovial fluid (1:4 dilution). Cells were harvested after 6 h and the IL-7R and IL-7m RNA levels were quantified by real-time RT-PCR. RA synovial tissue fibroblasts, normal peripheral blood in vitro differentiated macrophages or human microvascular endothelial cells (HMVECs) (Lonza, Walkersville, Maryland) were treated with IL-7 (R&D Systems, 10 ng/ml) and the cell-conditioned media was harvested following 24 or 48 h treatment. Alternatively, normal peripheral blood differentiated macrophages were treated with IL-1β (10 ng/ml) for 24h thereafter cells were washed and were either untreated or treated with IL-7 (10 ng/ml) and the cell-conditioned media was harvested following 24h or 48h treatment and concentration of IL-8 and Ang-1 was quantified.
Real-time RT-PCR
Total cellular RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) from the different cell types. Subsequently, reverse transcription and real-time RT-PCR were performed to determine IL-7 and IL-7R expression level as described previously (24, 25, 27). Relative gene expression was determined by the Ct method, and results were expressed as fold increase above conditions indicated in the figure legends.
Statistical analysis
The data were analyzed using two-tailed Student t tests for paired and unpaired samples. Values of p < 0.05 were considered significant.
RESULTS
IL-7 and IL-7R are elevated in RA synovial tissues
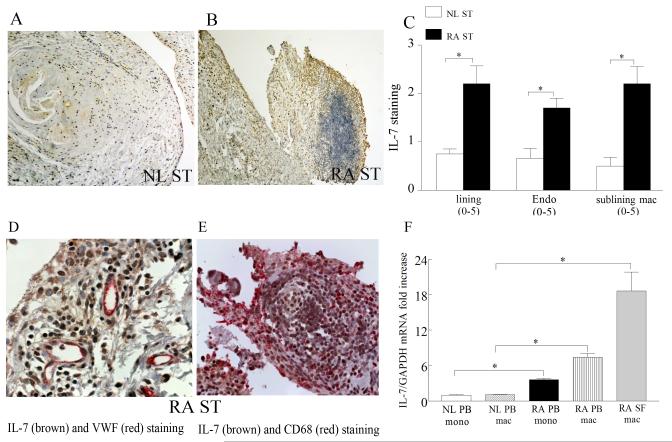
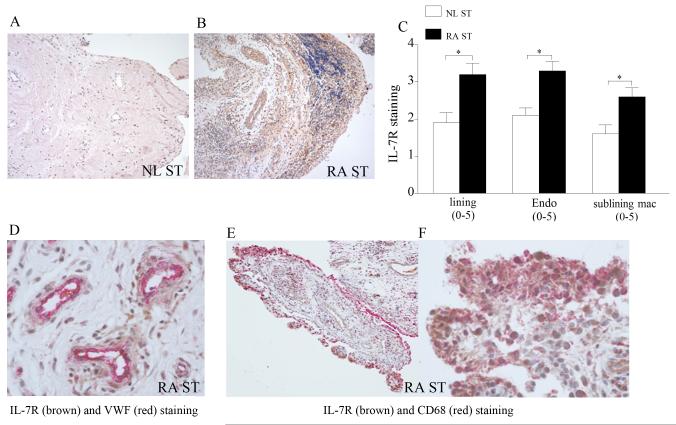
To characterize the expression pattern of IL-7 and IL-7R in RA patients compared to normal individuals, RA and NL synovial tissue were stained with antibodies against IL-7 and IL-7R. We found that IL-7 immunostaining was markedly higher on RA synovial tissue lining and sublining macrophages, and endothelial cells compared to normal synovial tissue (Fig. 1). Further, RA synovial tissue lining and sublining macrophages, and endothelial cells had significantly greater IL-7R expression levels compared to NL synovial tissue (Fig. 2). Our results also show that both IL-7 and IL-7R co-localize on VWF+ and CD68+ cells suggesting that IL-7 and its receptor is expressed in RA synovial tissue macrophages (in lining and sublining) as well as endothelial cells in the sublining (Figs. 1D-E and 2D-F). Since IL-7R and its ligand are coexpressed on the same cell types, this suggests that cells producing IL-7 may also be responsive to its activation. Further, upregulation of IL-7 on the surface of endothelial cells may play an important role in mediating transendothelial migration.
Figure 1. IL-7 is increased in RA synovial tissue (ST) compared to normal (NL) ST and macrophages from RA peripheral blood and synovial fluid are an important source for IL-7 expression.
NL (A) and RA ST (B) were stained with anti-human IL-7 (original magnification × 200) and positive immunostaining was scored on a 0-5 scale (C). ST lining and sublining macrophages and endothelial immunostaining are shown as mean ±SEM, (n=8-15). RA synovial tissues were stained for IL-7 (brown staining) and Von wille brand factor (fast red staining) (original magnification × 400) (D) or for IL-7 (brown staining) and CD68 (fast red staining) (original magnification × 400) (E) in order to distinguish endothelial cells or macrophages that express IL-7. IL-7 (F) mRNA levels were determined in NL and RA peripheral blood (PB) monocytes and macrophages as well as in RA synovial fluid (SF) macrophages by employing real-time RT-PCR (n=8-22). The data are shown as fold increase above NL PB monocytes and are normalized to GAPDH. * represents p <0.05.
Figure 2. IL-7R is elevated in RA ST compared to NL ST.
NL (A) and RA ST (B) were stained with anti-human IL-7R (original magnification × 200) and positive immunostaining was scored on a 0-5 scale (C). ST lining and sublining macrophages and endothelial immunostaining are shown as mean ±SEM, (n=8-15). RA ST were stained for IL-7R (brown staining) and Von wille brand factor (fast red staining) (original magnification × 400) (D) or for IL-7R (brown staining) and CD68 (fast red staining) (original magnification × 200) (E) and (original magnification × 400) (F) in order to distinguish endothelial cells or macrophages that express IL-7R.
RA synovial fluid macrophages express high levels of IL-7 and IL-7R
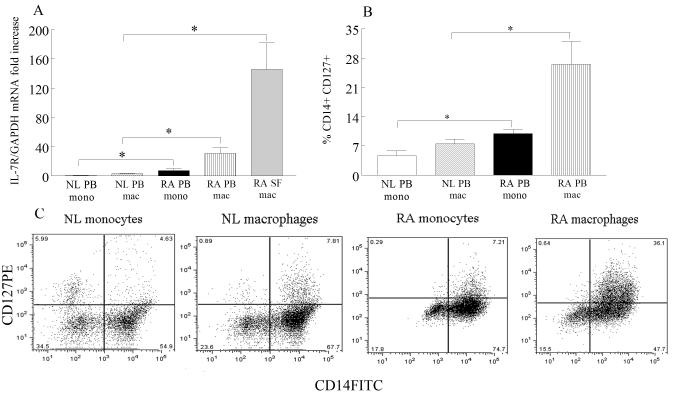
Since expression levels of IL-7 and IL-7R were elevated in RA synovial tissue lining and sublining macrophages, we asked whether expression of these factors was increased in RA peripheral blood and synovial fluid macrophages compared to normal peripheral blood monocytes and macrophages. Expression of IL-7 was elevated 17-fold in RA synovial fluid macrophages and 7 fold in RA peripheral blood macrophages compared to their counterpart normal cells by real-time RT-PCR (Fig. 1F). Levels of IL-7 were 4 fold greater in RA peripheral blood monocytes compared to normal monocytes (Fig. 3A). Employing microarray analysis, IL-7R was identified as one of the most greatly altered genes (5 fold higher, p=1.10×10−8) in RA synovial fluid macrophages compared to normal macrophages (data not shown). Further concentrations of IL-7R were significantly higher in RA synovial fluid (45 fold) and RA peripheral blood (10 fold) macrophages compared to normal macrophages employing real-time RT-PCR (Fig. 3B). Also, RA peripheral blood monocytes expressed 8 fold greater levels of IL-7R compared to normal monocytes (Fig. 3A). Consistent with our mRNA results, flow cytometry experiments demonstrate that IL-7R is significantly elevated in RA monocytes and macrophages compared to control counterparts (Fig. 3B-C). Our results suggest that RA synovial fluid and peripheral blood macrophages may be an important source for IL-7 production and response.
Figure 3. IL-7R is upregulated in RA SF and PB macrophages compared to NL PB macrophages.
A. IL-7R mRNA levels were determined in NL and RA PB monocytes and macrophages as well as in RA SF macrophages by employing real-time RT-PCR (n=8-22). The data are shown as fold increase above NL PB monocytes and are normalized to GAPDH. Values demonstrate mean ± SEM. B. Normal and RA PB monocyte and macrophages were immunostained with CD14 and CD127 in order to determine % IL-7R positive cells. The values are presented as mean ± SEM of % CD14+IL-7R+ in each cell population (n=4-5). C. Representative flow cytometry histogram showing CD14+CD127+ NL and RA monocytes and macrophages. * represents p<0.05.
Proinflammatory factors regulate expression of IL-7 and IL-7R in RA macrophages
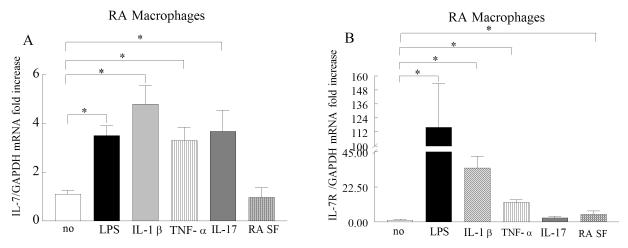
To determine which factors modulate expression of IL-7 and IL-7R in RA peripheral blood in vitro differentiated macrophages, cells were either untreated or treated with LPS, IL-1β, TNF-α, IL-17 or RA synovial fluid. Our results demonstrate that both IL-7 and IL-7R expression were greatly induced by LPS, TNF-α and IL-1β activation of RA peripheral blood macrophages compared to untreated cells (Fig. 4A-B). However only expression levels of IL-7 were upregulated (3.5-fold) by IL-17 stimulation, while RA synovial fluid activation in macrophages resulted in increased IL-7R expression compared to untreated cells (Fig. 4A-B). Our results suggest that expression of IL-7 and IL-7R are to some extent similarly modulated in RA peripheral blood in vitro differentiated macrophages.
Figure 4. Proinflammatory factors induce the expression of IL-7 and IL-7R in RA PB in vitro differentiated macrophages.
RA PB in vitro differentiated macrophages were untreated (PBS) or treated with LPS (10 ng/ml), IL-1β (10 ng/ml), TNF-α (10 ng/ml), IL-17 (50 ng/ml), or RA SF (1:4 dilution) and expression of IL-7 (A) and IL-7R (B) was measured by real-time RT-PCR (n=4-9). The data are shown as fold increase above untreated RA PB macrophages and are normalized to GAPDH. Values demonstrate mean ± SEM, * represents p<0.05.
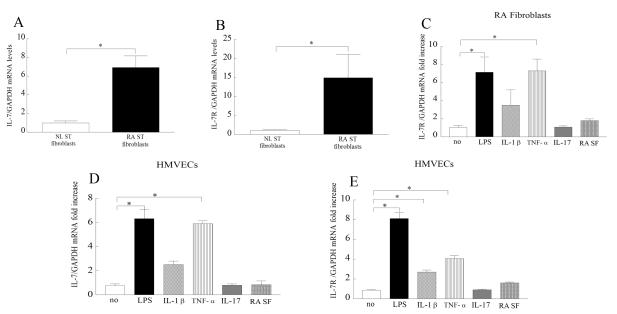
IL-7 and IL-7R expression levels are elevated in RA synovial tissue fibroblasts and expression of IL-7R is induced by proinflammatory factors
Based on our histological data, we asked whether expression of IL-7 and IL-7R was elevated in RA compared to NL synovial tissue fibroblasts. Results obtained from real-time RT-PCR demonstrate that IL-7 (Fig. 5A) and IL-7R (Fig. 5B) expression was 7 and 15 fold greater in RA respectively compared to normal fibroblasts. We next show that while LPS and TNF-α upregulate the expression of IL-7R in RA fibroblasts (Fig. 5C), expression of IL-7 was unaffected by LPS, IL-1β, TNF-α, IL-17 and RA synovial fluid activation of RA fibroblasts (data not shown). Since expression of IL-7R in RA fibroblasts is greater and more responsive to activation compared to IL-7, these cells may be activated by IL-7R ligation but are not the main production source for IL-7.
Figure 5. RA ST fibroblasts have elevated levels of IL-7 and IL-7R and expression levels of IL-7 and IL-7R are modulated by TLR4 ligation and TNF-α activation in HMVECs.
IL-7 (A) and IL-7R (B) mRNA levels were determined in NL and RA ST fibroblasts employing real-time RT-PCR (n=7-15). The data are shown as fold increase above NL ST fibroblasts and are normalized to GAPDH. RA ST fibroblasts were untreated (PBS) or treated with LPS (10 ng/ml), IL-1β (10 ng/ml), TNF-α (10 ng/ml), IL-17 (50 ng/ml) or RA SF (1:4 dilution) and expression of IL-7R (C) was measured by real-time RT-PCR (n=4-9). Since IL-7 expression levels were unaffected in stimulated RA fibroblasts, the data is not shown. The data are shown as fold increase above untreated RA ST fibroblasts and are normalized to GAPDH. HMVECs were similarly treated as RA fibroblasts mentioned above in C and expression of IL-7 (D) and IL-7R (E) was measured by real-time RT-PCR (n=4-6). The data are shown as fold increase above untreated HMVECs and are normalized to GAPDH. Values demonstrate mean ± SEM, * represents p<0.05.
Proinflammatory factors regulate expression of IL-7 and IL-7R on endothelial cells
Since IL-7 and IL-7R were expressed by RA synovial tissue vascular endothelial cells, we examined factors that may modulate their expression on HMVECs. We found that both IL-7 (Fig. 5D) and IL-7R (Fig. 5E) expression levels were induced by LPS and TNF-α stimulation of HMVECs, while IL-1β activation only affected IL-7R expression. Expression of IL-7 on endothelial cells may play an important role in facilitating transendothelial migration of IL-7R+ cells.
IL-7 induces production of proangiogenic factors from macrophages and HMVECs
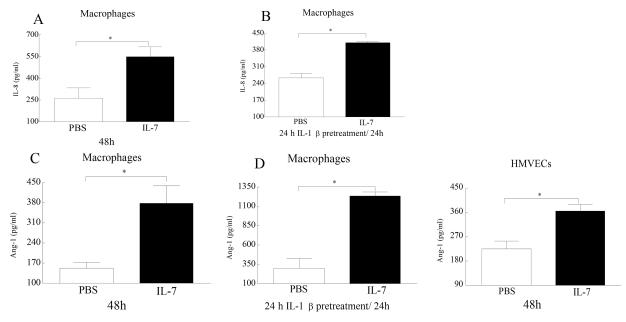
To examine the mechanism by which IL-7 mediates pathogenesis in RA, IL-7 activated macrophages, RA synovial tissue fibroblasts and HMVECs were screened for a variety of proinflammatory factors. IL-7 induced the production of IL-8 from macrophages (Fig. 6A) as well as Ang-1 from macrophages (Fig. 6B) and HMVECs (Fig. 6C) after 48h. However, IL-7 did not induce production of IL-6, CCL2/MCP-1, CCL5/ RANTES, CCL19, CCL21 from RA synovial tissue fibroblasts, macrophages or HMVECs (data not shown). Further, production of TNF-α and IL-1β by macrophages or HMVECs was not mediated by IL-7 stimulation (data not shown). Since IL-7 was capable of inducing the production of IL-8 and Ang-1 after 48h we asked whether increased levels of IL-7R in macrophages may affect the production level or the response time of these factors. To increase IL-7R levels in macrophages, cells were pretreated with IL-1β prior to IL-7 stimulation. We demonstrate that pretreatment of macrophages with IL-1β resulted in earlier detection (24h) of IL-7 induced IL-8 levels but not significantly higher levels compared to 48h when cells were untreated with IL-1β. In contrast, IL-1β pretreated macrophages produced significantly greater levels of Ang-1 following 24h of IL-7 treatment compared to IL-1β untreated cells were Ang-1 was detectable following 48h of IL-7 activation. These results indicate that the pathogenic role of IL-7 in RA is mediated through production of proangiogenic factors and that expression levels of IL-7R on macrophages plays a role on IL-7 response.
Figure 6. IL-7 activates production of proangiogenic factors from macrophages and HMVECs.
Macrophages (A-C) or HMVECs (E) were treated with PBS or IL-7 (10 ng/ml) for 24-48h and levels of IL-8 (A), and Ang-1 (C and E) were detected by ELISA utilizing the conditioned media. To determine whether elevated levels of IL-7R on macrophages would al1ow earlier detection or increase in proangiogenic factors cells were pretreated with IL-1β (10ng/ml; for 24h) prior to IL-7 (10 ng/ml) treatment. Production levels of IL-8 (B) and Ang-1 (D) was determined in condition media collected following 24h IL-7 treatment employing ELISA. Proangiogenic factors were not detected in IL-7 activated RA fibroblasts (data not shown). Values are the mean ± SEM, (n=5). * represents p <0.05.
DISCUSSION
In this study, we show that RA synovial tissue lining and sublining macrophages and endothelial cells express higher levels of IL-7 and IL-7R compared to tissues of normal controls. Our data demonstrate that macrophages are an important source of IL-7 production in RA and expression levels of this cytokine and its receptor are greatly upregulated in RA synovial fluid and peripheral blood macrophages compared to their normal counterparts. LPS, IL-1β and TNF-α activation modulates expression of IL-7 and IL-7R in RA peripheral blood macrophages. Consistent with the histological data, RA synovial tissue fibroblasts express elevated levels of IL-7 and IL-7R compared to normal fibroblasts. In RA fibroblasts, while IL-7R expression is regulated by LPS and TNF-α activation, IL-7 levels were unresponsive to these stimuli. In contrast, both IL-7 and IL-7R expression are greatly increased in HMVECs treated with LPS and TNF-α versus untreated cells. Last, we document a novel role for IL-7 in the induction of key proangiogenic factors from macrophages and HMVECs. Our results suggest that macrophages in RA synovial tissue and fluid are an important production source for IL-7 and elevated levels of IL-7R on these cells allow them to respond to IL-7 stimulation by producing proangiogenic factors in RA.
Previous studies have shown that IL-7R is expressed on CD4+ and NK T cells (28). Others have shown that IL-7 is expressed by lymphoid follicles (29). In contrast, our data demonstrate that IL-7 and IL-7R are coexpressed on RA sublining macrophages and endothelial cells as well as in RA synovial tissue lining, where macrophages and RA fibroblasts are in close proximity and interact with each other. Further, it was shown by others that the number of macrophages in the lining and sublining correlates with IL-7+ cells in RA synovial tissue, suggesting that macrophages may be major producers of IL-7 (5). Since IL-7 and IL-7R are expressed by RA fibroblasts, macrophages and endothelial cells, this may suggest that these cells can be directly activated through IL-7 ligation to produce proinflammatory factors in the synovium.
Elevation of IL-7 levels in peripheral blood prior to the onset of RA suggests that IL-7 may play an important role in the initiation of disease (30). Additionally, IL-7 plasma levels correlate with C reactive protein (CRP) suggesting that IL-7 may also play an essential role in established disease (31). Similar to findings in RA synovial tissue, our results demonstrate that IL-7 and IL-7R expression are elevated in RA synovial fluid and peripheral blood macrophages compared to normal cells. Earlier studies have shown that RA synovial fluid macrophages activated with IL-7 differentiate to osteoclasts, suggesting that ligation of IL-7 to IL-7R expressed on RA synovial fluid macrophages plays an important role in osteoclastogenesis (15). Studies performed in human monocytes and mice demonstrated that IL-7 induced osteoclast formation is dependent on RANKL production (32-34).
Interestingly, both IL-7 and IL-7R expression in RA peripheral blood macrophages are elevated by LPS, IL-1β and TNF-α while IL-17 or RA synovial fluid stimulation only modulates expression of IL-7 or IL-7R respectively in these cells. Consistently, others have shown that elevated levels of IL-1β and TNF-α in RA synovial fluid increases IL-7 production in stromal cells (35). It has been shown that the levels of TNF-α and IL-7 in RA synovial fluid and tissue correlate with each other, suggesting that TNF-α can contribute to IL-7 production (28). IL-7 can also stimulate monocyte dependent TNF-α production in monocyte and T cell coculture (5, 6). The feedback regulation between IL-7 and TNF-α was documented in a recent study where investigators demonstrated that TNF-α blockade in RA patients reduces circulating levels of IL-7 in anti-TNF-α responders (6). This is in agreement with our findings since TNF-α in RA macrophages can potentiate IL-7 function by upregulating IL-7R on these cells. Altogether, our results suggest that macrophages are important cells types that can express and respond to IL-7 and proinflammatory factors such as LPS, IL-1β and TNF-α are in part responsible for this process.
IL-7 protein was previously detected in RA and not in OA synovial tissue fibroblasts (36). In contrast to our findings, these investigators demonstrate that IL-7 production is greatly increased by TNF-α activation in RA fibroblasts (36) and this may be due to differences in passage number, growth condition or methods employed for quantifying IL-7 levels. Since RA fibroblasts were extracted from de-identified tissues the treatment information is unknown and there is no patient information indicated in the study performed by Harada et al. (36) however one may speculate that the differences in our results may also be due to differences in treatment strategies. While our data show that both IL-7 and its receptor are elevated in RA compared to normal fibroblasts, only IL-7R expression levels are modulated by LPS and TNF-α stimulation in RA fibroblasts suggesting that although these cells are responsive to IL-7 stimulation they may not be the main source for its production.
Expression of IL-7 and IL-7R by endothelial cells is consistent with the pathological role they play in RA angiogenesis. Similar to RA macrophages, expression of IL-7 and IL-7R is regulated by LPS and TNF-α activation in HMVECs. IL-7R but not IL-7 is also modulated by IL-1β in HMVECs. When IL-7 activated RA fibroblasts, macrophages and HMVECs were screened for a variety of inflammatory factors, our results demonstrate that IL-7 was able to induce production of IL-8 and Ang-1 from macrophages and secretion of Ang-1 from HMVECs. Production of potent proangiogenic factors from macrophages and endothelial cells activated by IL-7 suggests that IL-7 is important in RA angiogenesis and is in line with another finding showing reduction of joint vascularization and FGF levels following IL-7R blockade in a collagen induced arthritis (CIA) model (19). In contrast to our data, others have found that IL-7 is capable of inducing production of TNF-α, IL-1 and IL-6 from monocytes/macrophages (37). The inconsistency is most probably due to lower dose of IL-7 (10ng/ml) employed in our studies since the authors (37) mention that higher concentration’s of IL-7 (100 ng/ml) was required to detect the aforementioned monokines.
In conclusion, fibroblasts and endothelial cells from RA synovial tissue and macrophages from RA synovial fluid, peripheral blood and tissue express higher levels of IL-7 and IL-7R compared to control cells. We show for the first time that LPS and TNF-α can regulate expression of IL-7 and IL-7R in RA macrophages and HMVECs as well as IL-7R in RA fibroblasts. Finally, potent proangiogenic factors are secreted from IL-7 activated macrophages and endothelial cells, highlighting a novel role for IL-7 in RA angiogenesis.
Acknowledgments
This work was supported in part by awards from the National Institutes of Health (AR056099, AR055240), Arthritis National Research Foundation, grants from Within Our Reach from The American College of Rheumatology and funding provided by Department of Defense PR093477.
Footnotes
AUTHOR CONTRIBUTIONS: All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Shahrara had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Shahrara, Pickens
Acquisition of data. Pickens, Chamberlain, Volin, Mandelin, Pope, Talarico
Analysis and interpretation of data. Shahrara, Pickens, Volin, Chamberlain
Providing reagents. Pope
REFERENCES
- 1.Golden-Mason L, Kelly AM, Traynor O, McEntee G, Kelly J, Hegarty JE, et al. Expression of interleukin 7 (IL-7) mRNA and protein in the normal adult human liver: implications for extrathymic T cell development. Cytokine. 2001;14(3):143–51. doi: 10.1006/cyto.2001.0852. [DOI] [PubMed] [Google Scholar]
- 2.Kroncke R, Loppnow H, Flad HD, Gerdes J. Human follicular dendritic cells and vascular cells produce interleukin-7: a potential role for interleukin-7 in the germinal center reaction. Eur J Immunol. 1996;26(10):2541–4. doi: 10.1002/eji.1830261040. [DOI] [PubMed] [Google Scholar]
- 3.Sorg RV, McLellan AD, Hock BD, Fearnley DB, Hart DN. Human dendritic cells express functional interleukin-7. Immunobiology. 1998;198(5):514–26. doi: 10.1016/S0171-2985(98)80075-2. [DOI] [PubMed] [Google Scholar]
- 4.de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160(4):1666–76. [PubMed] [Google Scholar]
- 5.van Roon JA, Verweij MC, Wijk MW, Jacobs KM, Bijlsma JW, Lafeber FP. Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact-dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum. 2005;52(6):1700–10. doi: 10.1002/art.21045. [DOI] [PubMed] [Google Scholar]
- 6.van Roon JA, Hartgring SA, Wenting-van Wijk M, Jacobs KM, Tak PP, Bijlsma JW, et al. Persistence of interleukin 7 activity and levels on tumour necrosis factor alpha blockade in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2007;66(5):664–9. doi: 10.1136/ard.2006.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hitchon CA, Alex P, Erdile LB, Frank MB, Dozmorov I, Tang Y, et al. A distinct multicytokine profile is associated with anti-cyclical citrullinated peptide antibodies in patients with early untreated inflammatory arthritis. J Rheumatol. 2004;31(12):2336–46. [PubMed] [Google Scholar]
- 8.Ponchel F, Verburg RJ, Bingham SJ, Brown AK, Moore J, Protheroe A, et al. Interleukin-7 deficiency in rheumatoid arthritis: consequences for therapy-induced lymphopenia. Arthritis Res Ther. 2005;7(1):R80–92. doi: 10.1186/ar1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gringhuis SI, de Leij LF, Verschuren EW, Borger P, Vellenga E. Interleukin-7 upregulates the interleukin-2-gene expression in activated human T lymphocytes at the transcriptional level by enhancing the DNA binding activities of both nuclear factor of activated T cells and activator protein-1. Blood. 1997;90(7):2690–700. [PubMed] [Google Scholar]
- 10.Chazen GD, Pereira GM, LeGros G, Gillis S, Shevach EM. Interleukin 7 is a T-cell growth factor. Proc Natl Acad Sci U S A. 1989;86(15):5923–7. doi: 10.1073/pnas.86.15.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costello R, Brailly H, Mallet F, Mawas C, Olive D. Interleukin-7 is a potent co-stimulus of the adhesion pathway involving CD2 and CD28 molecules. Immunology. 1993;80(3):451–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrotra PT, Grant AJ, Siegel JP. Synergistic effects of IL-7 and IL-12 on human T cell activation. J Immunol. 1995;154(10):5093–102. [PubMed] [Google Scholar]
- 13.Borger P, Kauffman HF, Postma DS, Vellenga E. IL-7 differentially modulates the expression of IFN-gamma and IL-4 in activated human T lymphocytes by transcriptional and post-transcriptional mechanisms. J Immunol. 1996;156(4):1333–8. [PubMed] [Google Scholar]
- 14.Varas A, Vicente A, Sacedon R, Zapata AG. Interleukin-7 influences the development of thymic dendritic cells. Blood. 1998;92(1):93–100. [PubMed] [Google Scholar]
- 15.Toyosaki-Maeda T, Takano H, Tomita T, Tsuruta Y, Maeda-Tanimura M, Shimaoka Y, et al. Differentiation of monocytes into multinucleated giant bone-resorbing cells: two-step differentiation induced by nurse-like cells and cytokines. Arthritis Res. 2001;3(5):306–10. doi: 10.1186/ar320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takano H, Tomita T, Toyosaki-Maeda T, Maeda-Tanimura M, Tsuboi H, Takeuchi E, et al. Comparison of the activities of multinucleated bone-resorbing giant cells derived from CD14-positive cells in the synovial fluids of rheumatoid arthritis and osteoarthritis patients. Rheumatology (Oxford) 2004;43(4):435–41. doi: 10.1093/rheumatology/keh077. [DOI] [PubMed] [Google Scholar]
- 17.Lee SK, Kalinowski JF, Jastrzebski SL, Puddington L, Lorenzo JA. Interleukin-7 is a direct inhibitor of in vitro osteoclastogenesis. Endocrinology. 2003;144(8):3524–31. doi: 10.1210/en.2002-221057. [DOI] [PubMed] [Google Scholar]
- 18.Hartgring SA, van Roon JA, Wenting-van Wijk M, Jacobs KM, Jahangier ZN, Willis CR, et al. Elevated expression of interleukin-7 receptor in inflamed joints mediates interleukin-7-induced immune activation in rheumatoid arthritis. Arthritis Rheum. 2009;60(9):2595–605. doi: 10.1002/art.24754. [DOI] [PubMed] [Google Scholar]
- 19.Hartgring SA, Willis CR, Alcorn D, Nelson LJ, Bijlsma JW, Lafeber FP, et al. Blockade of the IL-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T-cell activity and proinflammatory mediators. Arthritis Rheum. doi: 10.1002/art.27578. [DOI] [PubMed] [Google Scholar]
- 20.Ruth JH, Volin MV, Haines GK, III, Woodruff DC, Katschke KJ, Jr., Woods JM, et al. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis and Rheumatism. 2001;44:1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Koch AE, Nickoloff BJ, Holgersson J, Seed B, Haines GK, Burrows JC, et al. 4A11, a monoclonal antibody recognizing a novel antigen expressed on aberrant vascular endothelium. Upregulation in an in vivo model of contact dermatitis. American Journal of Pathology. 1994;144(2):244–259. [PMC free article] [PubMed] [Google Scholar]
- 22.Shahrara S, Proudfoot AE, Woods JM, Ruth JH, Amin MA, Park CC, et al. Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 2005;52(6):1907–19. doi: 10.1002/art.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahrara S, Proudfoot AE, Park CC, Volin MV, Haines GK, Woods JM, et al. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180(5):3447–56. doi: 10.4049/jimmunol.180.5.3447. [DOI] [PubMed] [Google Scholar]
- 24.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182(6):3884–91. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahrara S, Pickens SR, Mandelin AM, 2nd, Karpus WJ, Huang Q, Kolls JK, et al. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 184(8):4479–87. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Mandelin AM, 2nd, Shahrara S. Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum. doi: 10.1002/art.30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickens SR, Volin MV, Mandelin AM, 2nd, Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 184(6):3233–41. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartgring SA, Bijlsma JW, Lafeber FP, van Roon JA. Interleukin-7 induced immunopathology in arthritis. Annals of the Rheumatic Diseases. 2006;65(Suppl 3):iii69–74. doi: 10.1136/ard.2006.058479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmer TC, Baltus B, Vondenhoff M, Huizinga TW, Tak PP, Verweij CL, et al. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 2007;56(8):2492–502. doi: 10.1002/art.22748. [DOI] [PubMed] [Google Scholar]
- 30.Kokkonen H, Soderstrom I, Rocklov J, Hallmans G, Lejon K, Dahlqvist S Rantapaa. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 62(2):383–91. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 31.van Roon JA, Glaudemans KA, Bijlsma JW, Lafeber FP. Interleukin 7 stimulates tumour necrosis factor alpha and Th1 cytokine production in joints of patients with rheumatoid arthritis. Annals of the Rheumatic Diseases. 2003;62(2):113–9. doi: 10.1136/ard.62.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SK, Kalinowski JF, Jacquin C, Adams DJ, Gronowicz G, Lorenzo JA. Interleukin-7 influences osteoclast function in vivo but is not a critical factor in ovariectomy-induced bone loss. J Bone Miner Res. 2006;21(5):695–702. doi: 10.1359/jbmr.060117. [DOI] [PubMed] [Google Scholar]
- 33.Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T-cell production of soluble osteoclastogenic cytokines. Blood. 2000;96(5):1873–8. [PubMed] [Google Scholar]
- 34.Churchman SM, Ponchel F. Interleukin-7 in rheumatoid arthritis. Rheumatology (Oxford) 2008;47(6):753–9. doi: 10.1093/rheumatology/ken053. [DOI] [PubMed] [Google Scholar]
- 35.Kim HR, Hwang KA, Park SH, Kang I. IL-7 and IL-15: biology and roles in T-Cell immunity in health and disease. Crit Rev Immunol. 2008;28(4):325–39. doi: 10.1615/critrevimmunol.v28.i4.40. [DOI] [PubMed] [Google Scholar]
- 36.Harada S, Yamamura M, Okamoto H, Morita Y, Kawashima M, Aita T, et al. Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(7):1508–16. doi: 10.1002/1529-0131(199907)42:7<1508::AID-ANR26>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 37.Alderson MR, Tough TW, Ziegler SF, Grabstein KH. Interleukin 7 induces cytokine secretion and tumoricidal activity by human peripheral blood monocytes. J Exp Med. 1991;173(4):923–30. doi: 10.1084/jem.173.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]