Abstract
The innate immune system plays an important role in rheumatoid arthritis (RA) pathogenesis. Previous studies support the role of TLR2 and 4 in RA and experimental arthritis models however the regulation and pathogenic effect of TLR5 is undefined in RA. In this study we show that TLR5 is elevated in RA and osteoarthritis (OA) synovial tissue lining and sublining macrophages and endothelial cells compared to normal individuals. Further, expression of TLR5 is elevated in RA synovial fluid macrophages and RA peripheral blood (PB) monocytes compared to RA and normal PB in vitro differentiated macrophages. We also found that TLR5 on RA monocytes is an important modulator of TNF-α in RA synovial fluid and that TLR5 expression on these cells strongly correlates with RA disease activity and TNF-α levels. Interestingly, TNF-α has a feed back regulation with TLR5 expression in RA monocytes, while expression of this receptor is regulated by IL-17 and IL-8 in RA macrophages and fibroblasts. We show that RA monocytes and macrophages are more responsive to TLR5 ligation compared to fibroblasts despite the proinflammatory response being mediated through the same signaling pathways in macrophages and fibroblasts. In conclusion we document the potential role of TLR5 ligation in modulating transcription of TNF-α from RA synovial fluid and the strong correlation of TLR5 and TNF-α with each other and with disease activity score in RA monocytes. Our results suggest that expression of TLR5 may be a predictor for RA disease progression and that targeting TLR5 may suppress RA.
Keywords: RA monocytes, TLR5, TNF-α, DAS28, RA fibroblasts and RA differentiated macrophages
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disorder in which the innate immune system plays an important role (1, 2). TLRs are pattern recognition receptors that are present in a number of cells and tissues, that recognize pattern associated molecular patterns (PAMPs) or endogenous ligands (3).
Previous studies demonstrate that expression of TLR2 and TLR4 is elevated in RA PB monocytes as well as in RA synovial fluid and synovial tissue macrophages (4–7). Increased TLR2 and TLR4 expression was detected in RA compared to OA synovial tissue fibroblasts (8). Further, the data obtained from experimental arthritis models strongly support the role of TLR2 and TLR4 in streptococcal cell wall arthritis (9, 10) while TLR4 has been implicated in collagen induced arthritis (CIA) (11) as well as in the IL-1RA−/− model (11, 12). However the role of TLR5 in RA and murine models of RA is undefined.
TLR5 is expressed on a variety of cell types such as epithelial cells, neutrophils, monocytes, macrophages and mast cells and is the receptor for the bacterial structural protein flagellin (13). Flagellin signaling via TLR5 is dependent on MyD88 and IRAK1 (14, 15) and subsequent activation of NF-κB, MAPK and PI3K pathways (16–18). Like other TLR agonists, flagellin has been shown to induce dendritic cell maturation and activation (19) thereby promoting lymphocyte migration to secondary lymphoid sites (20). Others have shown that spontaneous neutrophil apoptosis is delayed by flagellin through induction of Mcl-1 and inhibition of caspase 3 (21). What remains unclear is whether TLR5 is present in RA synovium and whether ligation of this receptor plays a role in RA pathogenesis.
In this study, we demonstrate for the first time that TLR5 is elevated in RA and OA synovial tissue lining and sublining macrophages and endothelial cells compared to normal controls. Consistently, our data demonstrates that TLR5 expression is greatly elevated in RA synovial fluid macrophages and PB monocytes compared to their normal counterparts. In RA monocytes, patients with higher expression of TNF-α expressed elevated levels of TLR5 and the concentration of both of these factors strongly correlated with increased disease activity score (DAS28). The role of TLR5 expression in RA pathogenesis was documented when blockade of TLR5 on monocytes significantly reduced synovial fluid mediated TNF-α transcription by 80%. Interestingly, we demonstrate a feedback modulation between TNF-α production and TLR5 ligation and expression in RA monocytes. While in RA macrophages, TLR5 expression is induced by IL-17 and IL-8, it is significantly reduced by TLR4 ligation in both RA monocytes and macrophages. Higher expression of TLR5 was detected in RA compared to normal fibroblasts, which was upregulated by a variety of inflammatory factors excluding LPS. Hence, our data demonstrates the expression of TLR5 in RA and further documents its importance in RA disease activity and TNF-α modulation.
MATERIALS AND METHODS
Antibodies and immunohistochemistry
The studies were approved by the Institutional Review Board, and all donors gave informed written consent. Since the RA synovial tissues are recruited from the practices of orthopedic surgeons these samples are de-identified therefore the disease severity and the treatment information is unavailable. RA, OA and normal (NL) synovial tissues were formalin fixed, paraffin embedded, and sectioned in the pathology core facility. Synovial tissues were immunoperoxidase-stained using Vector Elite ABC Kits (Vector Laboratories, Burlingame, CA), with diaminobenzidine (Vector Laboratories) as a chromogen. Briefly, slides were deparaffinized in xylene for 15 min at room temperature, followed by rehydration by transfer through graded alcohols. Antigens were unmasked by incubating slides in Proteinase K digestion buffer (Dako, Carpinteria, CA) for 10 min at room temperature. Tissues were incubated with antibodies to human TLR5 (1:50, Santa Cruz Biotechnology, Santa Cruz, CA) or an IgG control antibody (Beckman Coulter, Brea, CA). Slides were evaluated by blinded observers (22–25) (A.M.M. and M.V.V.). Tissue sections were scored for lining, sublining macrophages and endothelial cell staining on a 0–5 scale (26, 27). Scored data were pooled, and the mean ± SEM was calculated in each data group. To demonstrate location of TLR5 in RA synovial tissue, serial tissue sections were stained with anti-TLR5 (1:50), anti-CD68 (Vector Laboratories; 1:100) and anti-VWF (Vector Laboratories; 1:1000) antibodies. To localize TLR5 to macrophages in RA synovial tissues, slides were deparaffinized and unmasked as mentioned above. Using an Invision G2 kit (Dako) RA synovial tissues were stained with anti-TLR5 antibody (1:50 dilution, Santa Cruz Biotechnology) employing DAB (brown staining) as a chromogen. Thereafter tissues were blocked (double staining blocker included in the Invision G2 kit) and stained with anti-CD68 antibody (1:100 dilution, Dako) employing Fast red (red staining) as a chromogen following manufacturers' instructions (Dako).
RA patient population
RA specimens were obtained from patients with RA, diagnosed according to the 1987 revised criteria of the American College of Rheumatology (28). PB was obtained from 44 women and 4 men (mean age 53.7 ± 2.7 years). At the time of treatment, patients were receiving no treatment (n=7), taking non-biological disease-modifying anti-rheumatic drug (DMARD)s (methotrexate, leflunomide, sulfasalizine azathioprine) alone (n=5), taking DMARDS plus hydroxychloroquine (n=9), taking DMARDs plus prednisone (n=5), taking DMARDs plus rituximab (n=3), taking DMARDs plus hydroxychloroquine plus minocycline (n=2), or taking a TNF-α inhibitor either alone (n=6), with a (DMARD) (n = 8), a DMARD plus prednisone (n=1), or with a DMARD plus hydroxychloroquine and prednisone (n=2). These studies were approved by the University of Illinois at Chicago Institutional Ethics Review Board and all donors gave informed written consent. Maximum number of patients was 48 however please refer to figure legends for exact number of patients in each experiment.
Cell isolation, culture and procedures
NL and RA PB and RA synovial fluid mononuclear cells were isolated by Histopaque gradient centrifugation (Sigma-aldrich, St. Louis, MO, USA) as previously described (29, 30). Monocytes/macrophages were isolated from normal and RA PB or RA synovial fluid employing a negative selection kit (StemCell Technologies, Vancouver, Canada) according to the manufacturers' instructions (26, 27). Monocytes were subsequently differentiated to macrophages by culturing in RPMI containing 20% FBS for 7 days.
Quantification of chemokines and cytokines
Human TNF-α, IL-6 and CCL2 (R&D Systems, Minneapolis, MN) ELISA kits were used according to the manufacturers' instructions.
Isolation of RA synovial tissue fibroblasts
Synovial tissue fibroblasts were isolated from fresh RA synovial tissues by mincing and digestion in a solution of dispase, collagenase, and DNase (30). Cells were used between passages 3 and 9 and cultured in DMEM containing 10% FBS and cell purity was validated by CD90 staining.
Cell treatment
RA PB monocytes and in vitro differentiated macrophages or RA synovial tissue fibroblasts were treated with Poly I:C (only in RA monocytes, Invivogen, 10 ng/ml), LPS (Sigma, 10 ng/ml), IL-1β (R&D Systems, 10 ng/ml), TNF-α (R&D Systems, 10 ng/ml), IL-17 (R&D Systems, 50 ng/ml), IL-6 (R&D Systems, 10 ng/ml), IL-8 (R&D Systems, 10 ng/ml), or RA synovial fluid (10%). Cells were harvested after 6 h and the TLR5 mRNA levels were quantified by real-time RT-PCR. RA synovial tissue fibroblasts, RA PB monocytes and differentiated macrophages were treated with flagellin Ultra pure (10 and 100 ng/ml)(endotoxin levels<50EU/mg) (InvivoGen, San Diego, CA) and cells (6h; for real-time RT-PCR) or conditioned media (24h; for ELISA) were harvested following treatment and TNF-α, IL-6 and CCL2 mRNA production was quantified. In a different experiment, RA monocytes from 6 different patients were treated with anti-TLR5 antibody or IgG (10 µg/ml; InvivoGen) for 1h prior to being treated with RA synovial fluid (10%; n=6) for 6h. To demonstrate that reduction of RA synovial fluid mediated TNF-α levels are due to blockade of TLR5 and not to the necrotic effect of this antibody in RA monocytes, cells were pretreated with anti-TLR5 antibody or IgG control 1h prior to treating the cells with PBS or flagellin for 6h. Subsequently, the TNF-α mRNA levels were quantified by real-time RT-PCR for experiments performed for Figs. 3A and B.
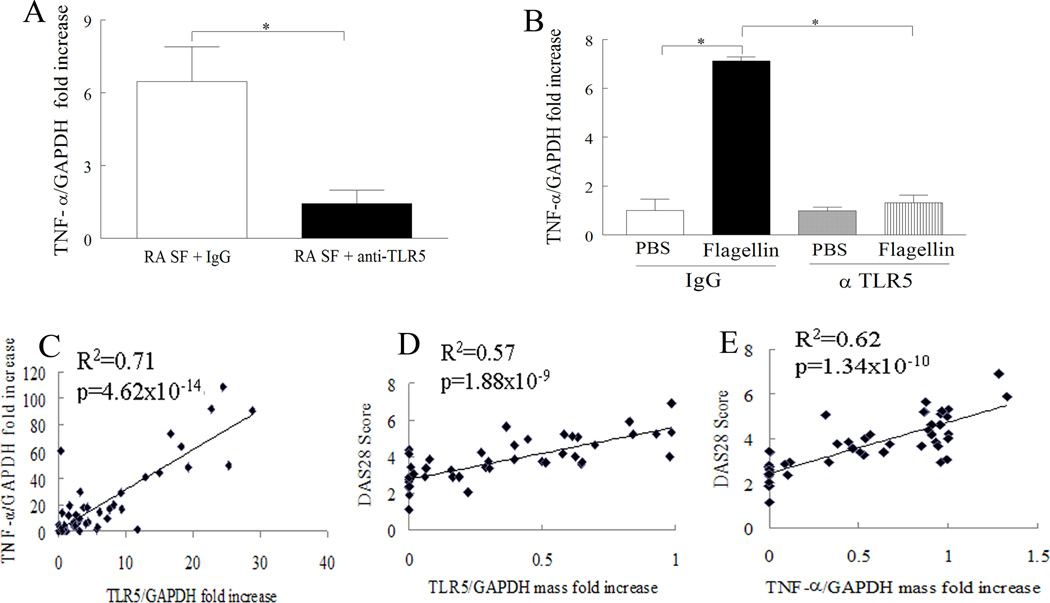
Figure 3. TLR5 ligation can regulate synovial fluid induced TNF-α transcription in RA monocytes and expression of TLR5 on these cells strongly correlates with DAS28 and TNF-α levels.
A. RA monocyte from 6 different patients were treated with anti-TLR5 antibody or IgG (10 µg/ml; InvivoGen) for 1h prior to being treated with RA synovial fluid (10%; n=6) for 6h. B. RA monocytes were pretreated with anti-TLR5 antibody or IgG control 1h prior to treating the cells with PBS or flagellin for 6h. Subsequently, the TNF-α mRNA levels were quantified in A and B by real-time RT-PCR and normalized to GAPDH value. In A the data is shown as fold increase above RA monocytes treated with RA synovial fluid plus anti-TLR5 antibody. Whereas in B the data is shown as fold increase above IgG pretreated PBS group. Linear regression analysis was used to compare TNF-α levels with TLR5 (C) (n=48 RA patients) as well as DAS28 score with expression of TLR5 (RNA mass normalized to GAPDH mass) (D) (n=45 RA patients) or TNF-α (RNA mass normalized to GAPDH mass) (E) (n=45 RA patients) in RA monocytes. The mRNA expression in RA monocytes is shown as fold increase above NL PB monocytes and is normalized to GAPDH. Values of p < 0.05 were considered significant.
Real-time RT-PCR
Total cellular RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) from the different cell types. Subsequently, reverse transcription and real-time RT-PCR were performed to determine TLR5, TNF-α, IL-6 and CCL2 expression levels as described previously (29–31). Relative gene expression was determined by the ΔΔCt method, and results were expressed as fold increase above conditions indicated in the figure legends.
Flow cytometry
In order to determine TLR5+ cells, normal and RA monocytes and differentiated macrophages were washed with FACS buffer (5% FBS in PBS). Thereafter cells were blocked with 50% human serum and 0.5% BSA. Cells were then stained for PE conjugated anti-TLR5 (Imgenex, San Diego, CA) and FITC labeled anti-CD14 (Becton Dickinson Immunocytometry lab, Franklin Lakes, NJ) or isotype control antibodies (BD Pharmingen). Percent TLR5+ cells were identified as those that were CD14+TLR5+. Because of limited access to RA synovial fluid macrophages these cells were not included in the FACS analysis.
Flagellin signaling pathways in RA macrophages or RA fibroblasts
RA synovial tissue fibroblasts and macrophages (2×106/ml) were untreated or treated with flagellin (100 ng/ml) for 0 to 65 min. Cell lysates were examined by Western blot analysis (30). Blots were probed with phospho (p)-ERK, p-p38 MAPK, p-AKT1, p-JNK (Cell Signaling; 1:1000 dilution) or degradation of IκB (Santa Cruz; 1:3000 dilution) overnight or probed with ERK, p38, AKT and JNK or actin (Cell Signaling or Sigma; 1:3000 dilution).
Inhibition of the signaling pathways in RA synovial tissue fibroblasts and macrophages
To define which signaling pathways mediate flagellin-induced CCL2 secretion, RA macrophages and fibroblasts were incubated with DMSO or 10 µM of inhibitors to p38 (SB203580), ERK (PD98059), JNK (SP600125), PI3K (LY294002), or NF-κB (MG-132) for 1h in RA differentiated macrophages or fibroblasts. Cells were subsequently activated with flagellin (100 ng/ml) for 24h and the media was collected in order to quantify the levels of CCL2 employing ELISA.
Statistical analysis
The data was analyzed employing 1-way ANOVA followed by a post hoc two-tailed Student’s t-test for paired and unpaired samples. In RA monocytes TLR5 and TNF-α mRNA expression was correlated with each other using the ΔΔCt method. Further TLR5 or TNF-α mRNA mass was normalized to its GAPDH mass and values were correlated with DAS28 score employing linear regression analysis in RA monocytes. Values of p < 0.05 were considered significant.
RESULTS
TLR5 elevated in RA and OA synovial tissues
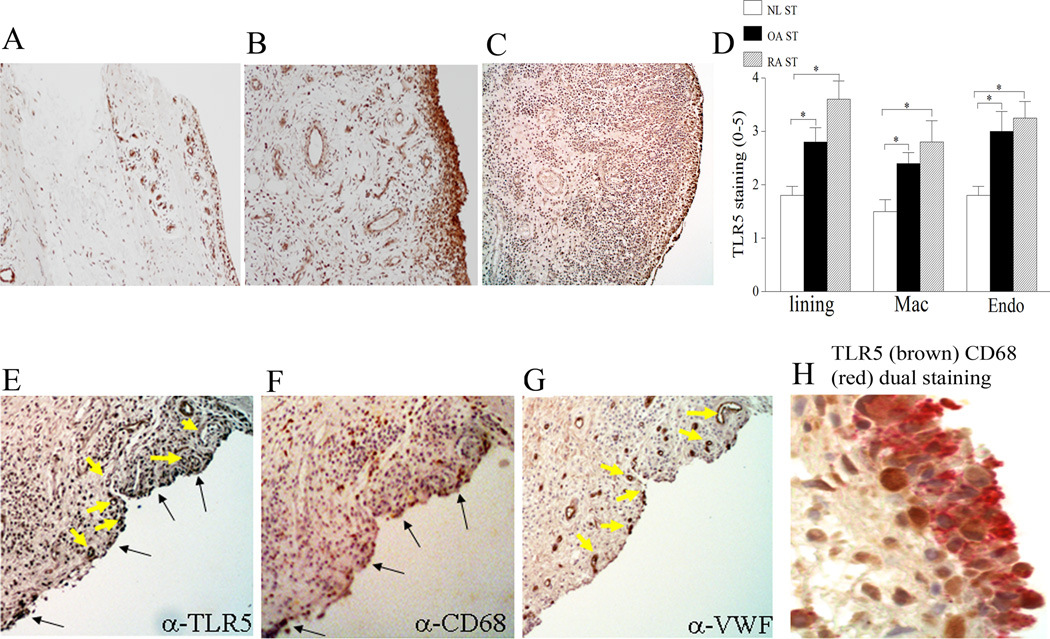
To characterize the expression pattern of TLR5 in RA and OA compared to controls, synovial tissues were stained with antibodies against TLR5. We found that both in RA and OA, TLR5 immunostaining was significantly higher on synovial tissue lining and sublining macrophages, and endothelial cells compared to normal synovial tissue (Figs. 1A–D). Consistently, TLR5 staining was co-localized to RA synovial tissue CD68+ (Figs. 1E–F, 1H) and VWF+ cells (Figs. 1E, 1G). Although previous studies demonstrate that fibroblasts in the lining and macrophages in the lining and sublining express TLR2 and 4 (32), expression of these receptors has not been reported in endothelial cells. Therefore TLR5 may be a member of the TLR family that is uniquely elevated on RA and OA endothelial cells.
Figure 1. TLR5 expression is increased in RA and OA synovial tissue (ST) lining and sublining macrophages and endothelial cells compared to normal (NL) ST.
NL (A), OA (B) and RA (C) ST were stained with anti-human TLR5 (A–C) (original magnification × 200) and positive immunostaining was scored on a 0–5 scale (D). ST lining and sublining macrophage (Mac) and endothelial (Endo) immunostaining are shown as mean ±SEM, (n=5–7). * represents p <0.05. RA serial sections were stained with anti-TLR5 (E), anti-CD68 (F) and anti-VWF (G) antibodies in order to distinguish TLR5 immunostaining on RA synovial tissue macrophages and endothelial cells (original magnification × 400). Black (E, F) and yellow arrows (E, G) demonstrate colocalization of TLR5 on CD68+ and VWF+ cells. H. RA synovial tissues were stained for anti-TLR5 (brown staining) and anti-CD68 antibodies (fast red staining) (original magnification × 800) in order to demonstrate TLR5 co-staining on lining macrophages.
RA synovial fluid macrophages and RA PB monocytes express uupregulated levels of TLR5
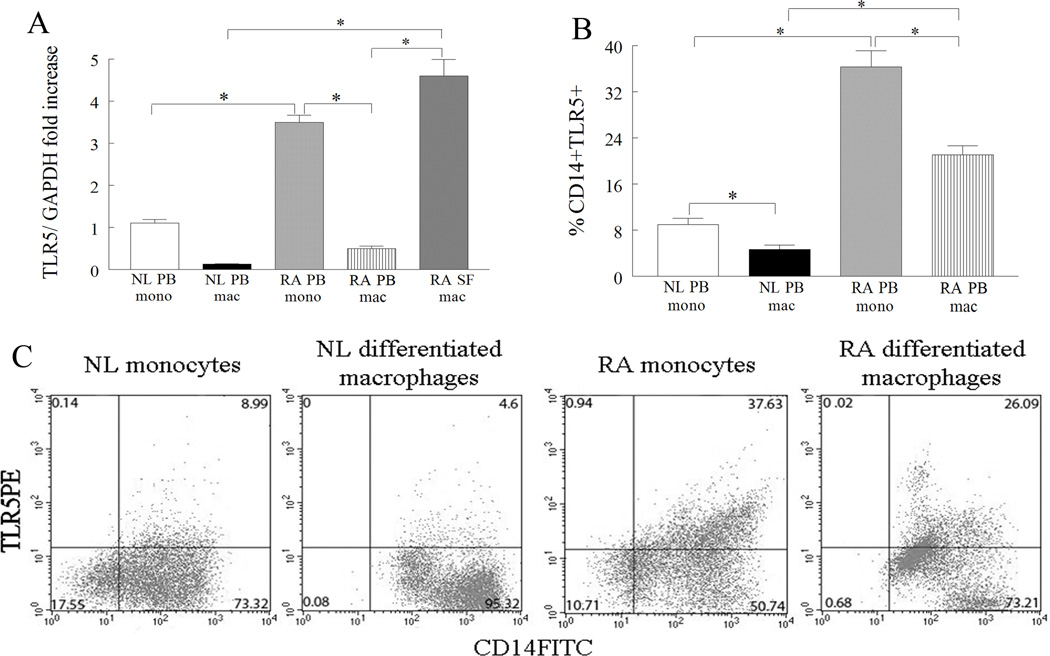
Since TLR5 immunostaining was elevated in RA synovial tissue lining and sublining macrophages, we asked whether mRNA and/or cell surface expression of this receptor was increased in RA synovial fluid macrophages compared to RA and NL PB monocytes or differentiated macrophages. Employing microarray analysis, TLR5 was identified as one of the genes (3.2 fold higher, p=1.58 × 10−10) that was greatly increased in RA synovial fluid macrophages compared to normal macrophages. These results were confirmed when real time RT-PCR demonstrated that the expression of TLR5 was elevated 9 and 35 fold in RA synovial fluid macrophages compared to RA and normal PB differentiated macrophages respectively (Fig. 2A). Further, levels of TLR5 were 7 and 3 fold greater in RA PB monocytes compared to RA PB differentiated macrophages and normal monocytes (Fig. 2A). Consistent with our mRNA results, FACS analysis demonstrated that percent TLR5 was significantly reduced both in RA and normals when monocytes were differentiated into macrophages, however, percent TLR5 was 4 fold higher in RA monocytes and macrophages compared to normal counterpart cells (Figs. 2B and 2C). Despite reduction of TLR5 expression during monocyte to macrophage differentiation, TLR5 expression is significantly increased in macrophages isolated from RA joints, compared to control or RA PB macrophages. Altogether, our results suggest that RA synovial tissue and fluid macrophages as well as RA PB monocytes may be an important source for TLR5 response.
Figure 2. TLR5 is upregulated in RA synovial fluid (SF) compared to RA and NL PB macrophages.
A. TLR5 mRNA levels were determined in NL (n for monocytes or macrophages=11 or 18) and RA PB monocytes (n=11) and differentiated macrophages (n=15) as well as in RA SF macrophages (n=10) by employing real-time RT-PCR. The data are shown as fold increase above NL PB monocytes and are normalized to GAPDH. B. Normal and RA PB monocytes and differentiated macrophages were immunostained with CD14 labeled with FITC and TLR5 conjugated with PE in order to determine % TLR5 positive cells (n=6–10). The values are presented as mean ± SEM of % CD14+TLR5+ in each cell population. C. Representative flow cytometry histograms showing CD14+TLR5+ in NL and RA PB monocytes and differentiated macrophages. * represents p<0.05.
TLR5 ligation can modulate synovial fluid induced TNF-α transcription in RA monocytes and expression of TLR5 on these cells strongly correlates with DAS28 and TNF-α
Since expression of TLR5 was higher in RA PB monocytes compared to differentiated macrophages we asked whether ligation of TLR5 in RA monocytes may affect disease pathogenesis. Previous studies have identified a number of endogenous TLR2 and/or TLR4 ligands in RA synovial fluid (33, 34). Hence synovial fluid mediated TNF-α transcription in RA monocytes was examined to determine if endogenous TLR5 ligand(s) were present in RA synovial fluid. Our results show that blockade of TLR5 on RA monocytes greatly downregulates (5 fold decrease; 80% reduction) TNF-α transcription activated by RA synovial fluid (Fig. 3A) suggesting that ligation of TLR5 by potential endogenous ligands expressed in RA synovial fluid may be partially responsible for joint TNF-α modulation. We further validated that the inhibitory effect of anti-TLR5 antibody on RA synovial fluid mediated TNF-α was specifically due to blockade of TLR5 ligation and had no effect on cell necrosis (Fig. 3B). Given that ligation of TLR5 plays a role in joint TNF-α regulation we asked whether expression of this these two factors correlate with each other and/or DAS28. We found that the levels of TLR5 and TNF-α in RA monocytes were closely related (R2=0.71, p=4.62×10−14) (Fig. 3C). Further, data analyzed by regression analysis demonstrated that patients with greater levels of DAS28 had increased expression of TLR5 (R2=0.57, p=1.88×10−9) (Fig. 3D) and TNF-α (R2=0.62, p=1.34×10−10) (Fig. 3E) in RA monocytes. These results suggest that RA disease expression is related to ligation of TLR5 and production of TNF-α from RA monocytes.
Proinflammatory factors regulate expression of TLR5 in RA monocytes and macrophages
To determine which factors modulate expression of TLR5 in RA PB monocytes or in vitro differentiated macrophages, cells were either untreated or treated with Poly I:C (only in RA monocytes), LPS, IL-1β, TNF-α, IL-17, IL-6, IL-8 or RA synovial fluid. Results from these experiments demonstrate TLR5 expression was modulated by TNF-α in RA monocytes and by IL-17 and IL-8 in RA macrophages, however, expression levels of TLR5 were suppressed by TLR3 and TLR4 ligation in RA monocytes and/or differentiated macrophages (Figs. 4A and 4B). Hence the data suggest that with the exception of LPS, expression of TLR5 in RA monocytes and macrophages is differentially regulated in RA monocytes and differentiated macrophages.
Figure 4. Proinflammatory factors induce the expression of TLR5 in RA PB monocytes and in vitro differentiated macrophages.

RA PB monocytes (A) or in vitro differentiated macrophages (B) were untreated (PBS) or treated with Poly I:C (10 ng/ml; only in RA monocytes) LPS (10 ng/ml), IL-1β (10 ng/ml), TNF-α (10 ng/ml), IL-17 (50 ng/ml), IL-6 (10 ng/ml), IL-8 (10 ng/ml), or RA SF (10%) for 6h and expression of TLR5 was measured by real-time RT-PCR (n=5–12). The data are shown as fold increase above untreated RA PB monocytes or macrophages and are normalized to GAPDH. Values demonstrate mean ± SEM, * represents p<0.05.
Ligation of TLR5 induces production of proinflammatory factors in RA PB monocytes and macrophages
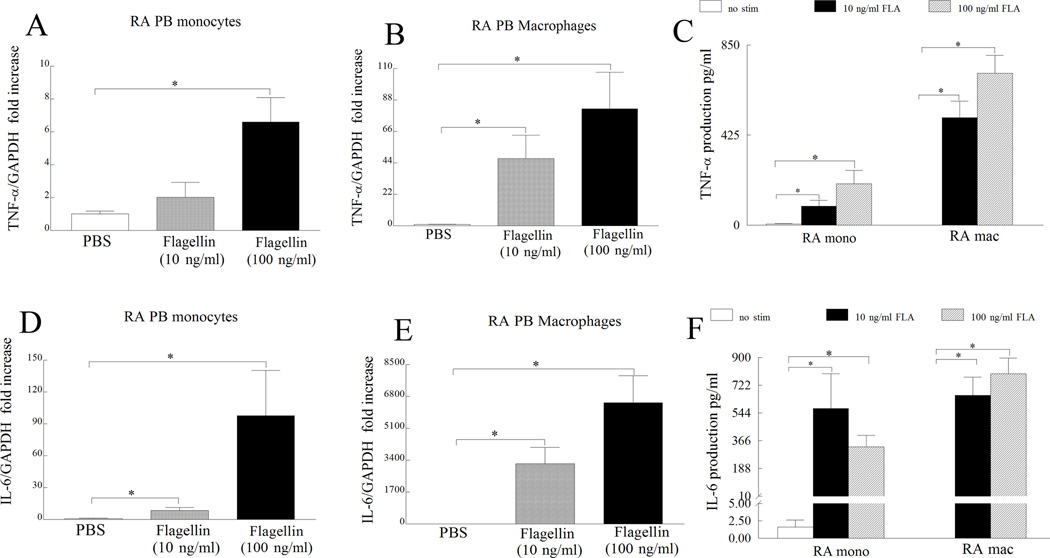
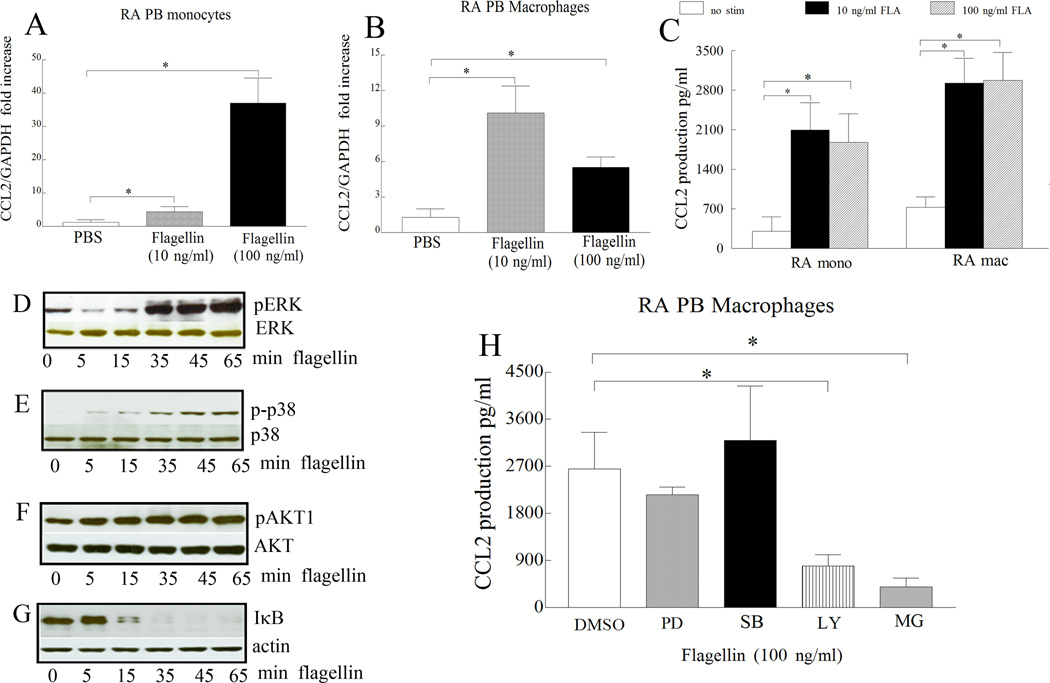
Next, we asked whether RA monocytes and differentiated macrophages respond to ligation of flagellin to TLR5. For this purpose RA monocytes and differentiated macrophages were activated with different doses of flagellin and cells were screened for transcription (6h) and production (24h) of proinflammatory factors such as TNF-α, IL-6 and CCL2. Generally, transcription but not the secretion of TNF-α, IL-6 and CCL2 was dose dependently increased with flagellin stimulation in RA monocytes and differentiated macrophages (Figs. 5 and 6A–C). Although TLR5 expression was greatly elevated in RA monocytes compared to RA differentiated macrophages, TLR5 ligation resulted in higher (TNF-α) or comparable production of proinflammatory factors (IL-6 and CCL2) in RA macrophages compared to that of monocytes. These results suggest that despite lower expression of TLR5 in RA macrophages, both monocytes and macrophages respond comparably to TLR5 ligation.
Figure 5. TLR5 ligation induces expression and production of TNF-α and IL-6 in RA monocytes and macrophages.
RA monocytes (A and D) and differentiated macrophages (B and E) were either untreated (PBS) or treated with flagellin at 10 ng/ml or 100 ng/ml for 6h and expression levels of TNF-α (A and B) and IL-6 (D and E) were quantified by real-time RT-PCR, n=6–10. The data are shown as fold increase above untreated cells and were normalized to GAPDH values. Supernatants were harvested from RA monocytes or differentiated macrophages untreated (PBS) or treated with flagellin 10 ng/ml or 100 ng/ml for 24h and TNF-α (C) and IL-6 (F) levels were determined by ELISA, n=5–8. Values demonstrate mean ± SEM, * represents p<0.05.
Figure 6. CCL2 levels are increased following TLR5 ligation in RA monocytes and macrophages. Further, in RA macrophages, flagellin-induced CCL2 production is modulated by PI3K and NF-κB pathways.
RA monocytes (A) and differentiated macrophages (B) were either untreated (PBS) or treated with flagellin at 10 ng/ml or 100 ng/ml for 6h and expression levels of CCL2 (A and B) were quantified by real-time RT-PCR, n=6–10. The data are shown as fold increase above untreated cells and were normalized to GAPDH. Supernatants were harvested from RA monocytes or differentiated macrophages untreated (PBS) or treated with flagellin at 10 ng/ml or 100 ng/ml for 24h and CCL2 (C) levels were determined by ELISA. Values are the mean ± SEM, n=5–8. In order to determine the mechanism of TLR5 activation in RA macrophages, cells were stimulated with flagellin at 100 ng/ml for 0–65 minutes, and the cell lysates were probed for, p-ERK (D), p-p38 (E), p-AKT1 (F) and degradation of IκB (G) and/or equal loading control. To examine which of the signaling pathways were associated with TLR5-induced CCL2 production, in RA macrophages, cells were untreated (DMSO) or treated with 10 µM inhibitors to ERK (PD98059), p38 (SB203580), PI3K (LY294002) or NF-κB (MG-132) for 1h. Cells were subsequently activated with flagellin (100 ng/ml) for 24h and the conditioned media was collected in order to quantify the levels of CCL2 employing ELISA (H). Values are the mean ± SEM, n=4. * represents p <0.05.
Flagellin-induced CCL2 is regulated by NF-κB and PI3K pathways in RA macrophages
We next inhibited flagellin activated pathways in RA differentiated macrophages in order to determine signaling pathways contributing to flagellin-mediated proinflammatory factor production. We found that p38 (5 min), AKT1 (5 min), ERK (35 min) and NF-κB (15 min) pathways (Figs. 6D–6G) were activated by flagellin stimulation in RA differentiated macrophages. We chose to examine the regulation of flagellin-induced CCL2 since this chemokine was detected both in RA differentiated macrophages and fibroblasts. While chemical inhibitors to NF-κB and PI3K suppressed flagellin-induced CCL2 secretion by 3–6 fold (Fig. 6H, p < 0.05), inhibition of p38 or ERK pathway did not reduce the levels of CCL2 secretion by RA differentiated macrophages. Our results suggest that activation of NF-κB and PI3K by flagellin regulates CCL2 production in RA differentiated macrophages.
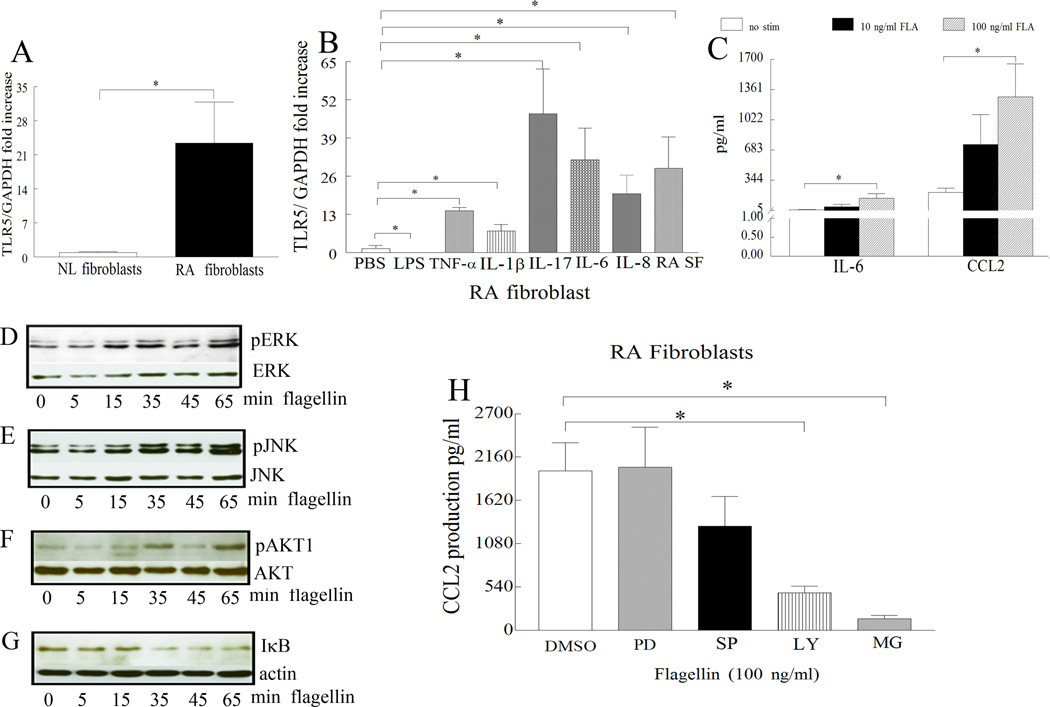
TLR5 is elevated in RA fibroblasts and its expression is responsive to stimulation
Based on our histological data, we asked whether expression of TLR5 was elevated in RA compared to NL synovial tissue fibroblasts. Results obtained from real-time RT-PCR demonstrate that TLR5 (Fig. 7A) expression was 23 fold greater in RA compared to normal synovial tissue fibroblasts. We next show that with the exception of LPS, all other proinflammatory factors such as TNF-α (14 fold), IL-1β (7 fold), IL-17 (47 fold), IL-6 (31 fold), IL-8 (20 fold), and RA synovial fluid (28 fold) greatly upregulate the expression of TLR5 in RA fibroblasts (Fig. 7B). To determine whether RA fibroblasts respond to TLR5 ligation, flagellin activated cells (at two different doses) were screened for a variety of proinflammatory factors. Unlike, RA monocytes and differentiated macrophages that are very responsive to flagellin stimulation, RA fibroblasts produce increased levels of IL-6 and CCL2 only when activated with a higher dose of flagellin (100 ng/ml). We found that flagellin activates JNK (15min), ERK (15 min), AKT1 (35min) and NF-κB (35 min) pathways in RA fibroblasts (Figs. 7D–G). Both in RA fibroblasts and macrophages ligation of TLR5-induced CCL2 production was modulated by NF-κB and PI3K activation (Figs. 6H and 7H). These data suggest that although ligation of TLR5 can induce production of proinflammatory factors through the same signaling pathways in both RA macrophages and fibroblasts, macrophages are comparatively more sensitive to TLR5 activation.
Figure 7. Expression of TLR5 in RA ST fibroblasts is very responsive to stimulation however only higher concentrations of flagellin can induce production of IL-6 and CCL2 which is modulated by PI3K and NF-κB pathways.
A. TLR5 mRNA levels were determined in NL and RA ST fibroblasts employing real-time RT-PCR (n=7). The data are shown as fold increase above NL ST fibroblasts and are normalized to GAPDH. B. RA ST fibroblasts were untreated (PBS) or treated with LPS (10 ng/ml), IL-1β (10 ng/ml), TNF-α (10 ng/ml), IL-17 (50 ng/ml), IL-6 (10 ng/ml), IL-8 (10 ng/ml), or RA SF (10%) for 6h and expression of TLR5 was measured by real-time RT-PCR (n=5–12). The data are shown as fold increase above untreated RA fibroblasts and are normalized to GAPDH. C. Supernatants were harvested from RA fibroblasts untreated (PBS) or treated with flagellin at 10 ng/ml or 100 ng/ml for 24h and IL-6 and CCL2 levels were determined by ELISA, (n=4). Values demonstrate mean ± SEM, * represents p<0.05. In order to determine the mechanism of TLR5 activation in RA fibroblasts, cells were stimulated with flagellin 100 ng/ml for 0–65 minutes, and the cell lysates were probed for, p-ERK (D), p-JNK (E), p-AKT1 (F) and degradation of IκB (G) and/or equal loading control. To examine which of the signaling pathways were associated with TLR5-induced CCL2 production, in RA fibroblasts, cells were untreated (DMSO) or treated with 10 µM inhibitors to ERK (PD98059), JNK (SP600125), PI3K (LY294002) or NF-κB (MG-132) for 1h. Cells were subsequently activated with flagellin (100 ng/ml) for 24h and the conditioned media was collected in order to quantify the levels of CCL2 employing ELISA (H). Values are the mean ± SEM, n=4. * represents p <0.05.
DISCUSSION
In the current study, we show that RA and OA synovial tissue lining and sublining macrophages and endothelial cells express higher levels of TLR5 than tissues of normal controls. We found that transcription levels of TLR5 were elevated in RA synovial fluid macrophages and RA monocytes compared to RA and normal differentiated macrophages. Confirming histological studies, TLR5 levels were also elevated in RA compared to NL fibroblasts. We show that in RA fibroblasts and macrophages, the TLR5 mRNA concentration was modulated by IL-17 and IL-8. In spite of elevated cell surface levels of TLR5 in RA PB monocytes compared to differentiated macrophages, production of proinflammatory factors were comparable in both cell types which was higher than what was secreted by RA fibroblasts following ligation. Most importantly, we document that in RA monocytes, TLR5 is a regulator of synovial fluid mediated TNF-α transcription and levels of this receptor are strongly correlated to TNF-α and DAS28 score. These results suggest that TLR5 endogenous ligand(s) in the RA joint may potentially activate TLR5+ RA monocytes and contribute to production of joint TNF-α and perpetuation of disease activity.
We show for the first time that TLR5 expression is elevated in RA and OA synovial tissue lining and sublining macrophages and endothelial cells compared to normal individuals. However, expression of TLR5 has not been associated with systemic lupus erythematosus (SLE) (35). Previous studies demonstrate that TLR5 is expressed in dendritic cells (36), neutrophils (37), synovial fibroblasts from patients with Juvenile idiopathic arthritis (JIA (38) and in a number of endothelial cell lines (39) however its expression is undefined in RA synovial tissue and blood cells.
Interestingly, we found that differentiation of RA monocytes to macrophages reduces TLR5 expression, as confirmed by both real time RT-PCR and FACS studies. The same trend was also observed in normal cells. Like TLR5, expression of TLR2 was greater in normal monocytes compared to PB differentiated macrophages whereas similar levels of TLR4 were detected in normal PB monocytes and differentiated macrophages (7). Further, elevated expression levels of TLR2 and TLR4 in RA synovial fluid macrophages compared to normal macrophages (7) is consistent with our findings with TLR5. In contrast to our results, others have shown that TLR5 is similarly expressed in normal PB monocytes and macrophages (37). The discrepancy in the data may be due to monocyte isolation technique as well as employing 100 ng/ml M-CSF for macrophage differentiation studies (37). Additionally, we demonstrate that TLR3 and TLR4 ligation reduced TLR5 expression on RA monocytes and/or macrophages or fibroblasts. TIR domain containing adaptor inducing interferon beta (TRIF), is an adaptor protein that is shown to degrade TLR5 expression through a caspase dependent manner (40). Hence, suppression of TLR5 expression in RA cells may be due to activation of TRIF by TLR3 or TLR4 ligation. In RA fibroblasts, while expression of TLR5 is reduced by TLR4 ligation, stimulation with IL-1β has a reverse effect and this may be due to its lack of association with the TRIF pathway (41). With the exception of LPS, TLR5 expression is differentially regulated in monocytes and macrophages. Others have shown that in human monocytes, expression of TLR5 is suppressed by TLR2 ligation as well as stimulation with IFN-γ and GM-CSF, however TLR5 expression is greatly increased by flagellin ligation (37, 42). In RA macrophages and fibroblasts, expression of TLR5 was modulated by IL-17. Previous studies have shown that TLR5 ligation can induce TH-17 cell differentiation in normal PB mononuclear cells (43) as well as production of IL-17 in splenocytes (44). IL-17 can also enhance TLR5-induced TNF-α and IL-1β production in epithelial cells (45). These results suggest that expression and ligation of TLR5 on cells present in RA synovial tissue lining may be in feed back regulation with TH-17 cell differentiation and production of joint IL-17.
Our results suggest that TLR5 endogenous ligand(s) may be present in synovial fluid as blockade of this receptor on monocytes significantly reduces TNF-α transcription induced by synovial fluid. Interestingly, a number of endogenous TLR ligands have been identified in RA synovial tissue and fluid including fibrinogen, HSP60 and 70, 96 and EDA fibronectin that bind to TLR2 and/or TLR4 (33, 34). Previous studies demonstrate that TLR5 transfected reporter HEK 293T cells stimulated with full length HSP70 had enhanced flagellin induced NF-κB mediated luciferase activity however this effect was not detected with HSP70 treatment alone (46). These findings suggest that HSP70 expressed in RA synovial fluid (47), synovial tissue macrophages and fibroblasts (33) may be a chaperone protein for TLR5 endogenous ligand(s) (48). Lectins have also been identified as novel agonists for cell surface bound TLRs (49). Based on earlier investigations there is a possibility that HSPs (33, 34) and/or lectins (49) may be potential TLR5 endogenous ligands in RA joint. Therefore, studies are currently being conducted to identify RA synovial fluid TLR5 endogenous ligands (not within the scope of this study).
Our results suggest that ligation of synovial fluid TLR5 endogenous ligands to TLR5+ monocytes can contribute to production of joint TNF-α which in turn can further upregulate expression of TLR5 on these cells. Once RA monocytes reach their destination in the joint and differentiate to macrophages, TLR5 expression is no longer modulated by TNF-α and their levels are reduced, however, they remain at least as responsive to ligation as RA monocytes. Perhaps in RA monocytes, TLR5 levels correlate with DAS28 and TNF-α and are in a feed back regulation with TNF-α by producing and responding to this factor in order to perpetuate disease.
When RA monocytes and differentiated macrophages were stimulated with flagellin, similar levels of IL-6 and CCL2 were produced despite RA macrophages having lower TLR5 expression compared to RA monocytes. This may be due to monocytes being in circulation while macrophages are immobilized in the inflammatory milieu of RA synovial tissue in cell to cell contact with other macrophages or RA fibroblasts therefore amplifying the activation response. It is also possible that macrophages from RA synovial tissue like RA synovial fluid have higher TLR5 expression compared to RA monocytes and may be the presence of proinflammatory factors is required to enhance TLR5 expression during the differentiation process which is available in the RA joint and unavailable in the culture system. In contrast to our results, other studies were unable to detect TNF-α production when normal monocytes were activated with flagellin (50). This may be due to lower levels of TLR5 expression in normal cells compared to RA monocytes as well as isolation and culturing methods. However, consistent with our data they were able to demonstrate high levels of CCL2 following TLR5 ligation in normal monocytes (50).
Unlike RA fibroblasts where only higher concentrations of flagellin (100 ng/ml) are capable of inducing expression of IL-6 and CCL2, in RA monocytes and macrophages ligation of TLR5 with lower concentrations (10 ng/ml) can produce these factors. Conversely, in skeletal muscle cells ligation of TLR5 was unable to produce significant levels of CCL2 without IFN-γ priming (51). Despite activation of nonoverlapping pathways in RA fibroblasts and macrophages by TLR5 ligation, CCL2 production was modulated by inhibition of NF-κB and PI3K in both cell types. In contrast to our results, blockade of PI3K or use of PI3K/AKT deficient mice resulted in marked increase in flagellin-induced IL-6 or IL-8/KC levels (52) indicating that the proinflammatory factors produced as a result of TLR5 ligation are differentially regulated in mice and humans.
In conclusion, we demonstrate for the first time that TLR5 is expressed in RA synovial tissue macrophages and fibroblasts as well as RA PB monocytes. We further document modulating factors and pathways contributing to TLR5 inflammatory response. Moreover, our study highlights that there is a strong correlation between TNF-α and TLR5 expression with disease activity in RA monocytes suggesting that TLR5 may be a TNF-α responsive gene that is linked to RA progression.
Acknowledgments
This work was supported in part by awards from the National Institutes of Health (AR056099, AR055240), Arthritis National Research Foundation, grants from Within Our Reach from The American College of Rheumatology and funding provided by Department of Defense PR093477.
Abbreviation
- RA
Rheumatoid Arthritis
- OA
osteoarthritis
- NL
normal
- PB
peripheral blood
- DAS28
disease activity score based on 28 defined joints
- PAMPs
pattern associated molecular patterns
- VWF
Von willebrand Factor
- HSP
heat shock protein
REFERENCES
- 1.Brentano F, Kyburz D, Schorr O, Gay R, Gay S. The role of Toll-like receptor signalling in the pathogenesis of arthritis. Cell Immunol. 2005;233:90–96. doi: 10.1016/j.cellimm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Drexler SK, Sacre SM, Foxwell BM. Toll-like receptors: a new target in rheumatoid arthritis? Expert Rev Clin Immunol. 2006;2:585–599. doi: 10.1586/1744666X.2.4.585. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, Akashi S, Miyake K, Godowski PJ, Makino H. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50:1457–1467. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 5.Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, Joosten LA, van den Berg WB. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50:3856–3865. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen LK, Havemose-Poulsen A, Sonder SU, Bendtzen K, Holmstrup P. Blood cell gene expression profiling in subjects with aggressive periodontitis and chronic arthritis. J Periodontol. 2008;79:477–485. doi: 10.1902/jop.2008.070309. [DOI] [PubMed] [Google Scholar]
- 7.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 8.Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, Gay RE, Gay S, Kyburz D. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58:3684–3692. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- 9.Joosten LA, Koenders MI, Smeets RL, Heuvelmans-Jacobs M, Helsen MM, Takeda K, Akira S, Lubberts E, van de Loo FA, van den Berg WB. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J Immunol. 2003;171:6145–6153. doi: 10.4049/jimmunol.171.11.6145. [DOI] [PubMed] [Google Scholar]
- 10.Abdollahi-Roodsaz S, Joosten LA, Helsen MM, Walgreen B, van Lent PL, van den Bersselaar LA, Koenders MI, van den Berg WB. Shift from toll-like receptor 2 (TLR-2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 production. Arthritis Rheum. 2008;58:3753–3764. doi: 10.1002/art.24127. [DOI] [PubMed] [Google Scholar]
- 11.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, Popa C, van der Meer JW, Netea MG, van den Berg WB. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56:2957–2967. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 12.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, Heuvelmans-Jacobs M, Akira S, Nicklin MJ, Ribeiro-Dias F, van den Berg WB. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honko AN, Mizel SB. Effects of flagellin on innate and adaptive immunity. Immunol. Res. 2005;33:83–101. doi: 10.1385/IR:33:1:083. [DOI] [PubMed] [Google Scholar]
- 14.Moors MA, Li L, Mizel SB. Activation of interleukin-1 receptor-associated kinase by gram-negative flagellin. Infect Immun. 2001;69:4424–4429. doi: 10.1128/IAI.69.7.4424-4429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 16.Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol. 2004;173:2913–2917. doi: 10.4049/jimmunol.173.5.2913. [DOI] [PubMed] [Google Scholar]
- 17.Rolli J, Rosenblatt-Velin N, Li J, Loukili N, Levrand S, Pacher P, Waeber B, Feihl F, Ruchat P, Liaudet L. Bacterial flagellin triggers cardiac innate immune responses and acute contractile dysfunction. PLoS ONE. 5:e12687. doi: 10.1371/journal.pone.0012687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng H, Wu H, Sloane V, Jones R, Yu Y, Lin P, Gewirtz AT, Neish AS. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G96–G108. doi: 10.1152/ajpgi.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 20.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 185:5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salamone GV, Petracca Y, Fuxman Bass JI, Rumbo M, Nahmod KA, Gabelloni ML, Vermeulen ME, Matteo MJ, Geffner JR, Trevani AS. Flagellin delays spontaneous human neutrophil apoptosis. Lab Invest. 2010;90:1049–1059. doi: 10.1038/labinvest.2010.77. [DOI] [PubMed] [Google Scholar]
- 22.Ruth JH, Volin MV, Haines GK, III, Woodruff DC, Katschke KJ, Jr, Woods JM, Park CC, Morel JCM, Koch AE. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44:1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Koch AE, Nickoloff BJ, Holgersson J, Seed B, Haines GK, Burrows JC, Leibovich SJ. 4A11, a monoclonal antibody recognizing a novel antigen expressed on aberrant vascular endothelium. Upregulation in an in vivo model of contact dermatitis. Am. J. Pathol. 1994;144:244–259. [PMC free article] [PubMed] [Google Scholar]
- 24.Shahrara S, Proudfoot AE, Woods JM, Ruth JH, Amin MA, Park CC, Haas CS, Pope RM, Haines GK, Zha YY, Koch AE. Amelioration of rat adjuvant-induced arthritis by Met-RANTES. Arthritis Rheum. 2005;52:1907–1919. doi: 10.1002/art.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahrara S, Proudfoot AE, Park CC, Volin MV, Haines GK, Woods JM, Aikens CH, Handel TM, Pope RM. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180:3447–3456. doi: 10.4049/jimmunol.180.5.3447. [DOI] [PubMed] [Google Scholar]
- 26.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Mandelin AM, 2nd, Shahrara S. Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum. 2011;63:914–922. doi: 10.1002/art.30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM, 2nd, Shahrara S. Characterization of interleukin-7 and interleukin-7 receptor in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2011;63:2884–2893. doi: 10.1002/art.30493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TAJ, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 29.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahrara S, Pickens SR, Mandelin AM, 2nd, Karpus WJ, Huang Q, Kolls JK, Pope RM. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184:4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickens SR, Volin MV, Mandelin AM, 2nd, Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 2010;184:3233–3241. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez-Canas I, Juarranz Y, Santiago B, Arranz A, Martinez C, Galindo M, Paya M, Gomariz RP, Pablos JL. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology. 2006;45:527–532. doi: 10.1093/rheumatology/kei219. [DOI] [PubMed] [Google Scholar]
- 33.Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–364. doi: 10.1007/s11926-009-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, 2nd, Tak PP, Haines GK, 3rd, Nicchitta CV, Pope RM. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182:4965–4973. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komatsuda A, Wakui H, Iwamoto K, Ozawa M, Togashi M, Masai R, Maki N, Hatakeyama T, Sawada K. Up-regulated expression of Toll-like receptors mRNAs in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Exp Immunol. 2008;152:482–487. doi: 10.1111/j.1365-2249.2008.03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicente-Suarez I, Brayer J, Villagra A, Cheng F, Sotomayor EM. TLR5 ligation by flagellin converts tolerogenic dendritic cells into activating antigen-presenting cells that preferentially induce T-helper 1 responses. Immunol Lett. 2009;125:114–118. doi: 10.1016/j.imlet.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Mahony DS, Pham U, Iyer R, Hawn TR, Liles WC. Differential constitutive and cytokine-modulated expression of human Toll-like receptors in primary neutrophils, monocytes, and macrophages. Int J Med Sci. 2008;5:1–8. doi: 10.7150/ijms.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal S, Misra R, Aggarwal A. Induction of metalloproteinases expression by TLR ligands in human fibroblast like synoviocytes from juvenile idiopathic arthritis patients. Indian J Med Res. 2010;131:771–779. [PubMed] [Google Scholar]
- 39.Maaser C, Heidemann J, von Eiff C, Lugering A, Spahn TW, Binion DG, Domschke W, Lugering N, Kucharzik T. Human intestinal microvascular endothelial cells express Toll-like receptor 5: a binding partner for bacterial flagellin. J Immunol. 2004;172:5056–5062. doi: 10.4049/jimmunol.172.8.5056. [DOI] [PubMed] [Google Scholar]
- 40.Choi YJ, Im E, Pothoulakis C, Rhee SH. TRIF modulates TLR5-dependent responses by inducing proteolytic degradation of TLR5. J. Biol. Chem. 2010;285:21382–21390. doi: 10.1074/jbc.M110.115022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer-Barber KD, Barber DL, Shenderov K, White SD, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Nunez G, Schlueter D, Flavell RA, Sutterwala FS, Sher A. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cabral ES, Gelderblom H, Hornung RL, Munson PJ, Martin R, Marques AR. Borrelia burgdorferi lipoprotein-mediated TLR2 stimulation causes the down-regulation of TLR5 in human monocytes. J Infect Dis. 2006;193:849–859. doi: 10.1086/500467. [DOI] [PubMed] [Google Scholar]
- 43.Evans HG, Suddason T, Jackson I, Taams LS, Lord GM. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc. Natl. Acad. Sci. USA. 2007;104:17034–17039. doi: 10.1073/pnas.0708426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, Janot L, Erard F, Bertout J, Leger H, Sebbane F, Benecke A, Renauld JC, Hardt WD, Ryffel B, Sirard JC. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol. 2010;185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beklen A, Sorsa T, Konttinen YT. Toll-like receptors 2 and 5 in human gingival epithelial cells co-operate with T-cell cytokine interleukin-17. Oral Microbiol Immunol. 2009;24:38–42. doi: 10.1111/j.1399-302X.2008.00473.x. [DOI] [PubMed] [Google Scholar]
- 46.Ye Z, Gan YH. Flagellin contamination of recombinant heat shock protein 70 is responsible for its activity on T cells. J. Biol. Chem. 2007;282:4479–4484. doi: 10.1074/jbc.M606802200. [DOI] [PubMed] [Google Scholar]
- 47.Martin CA, Carsons SE, Kowalewski R, Bernstein D, Valentino M, Santiago-Schwarz F. Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp)70 in the rheumatoid joint: possible mechanisms of hsp/DC-mediated cross-priming. J Immunol. 2003;171:5736–5742. doi: 10.4049/jimmunol.171.11.5736. [DOI] [PubMed] [Google Scholar]
- 48.Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85:905–910. doi: 10.1189/jlb.0109005. [DOI] [PubMed] [Google Scholar]
- 49.Unitt J, Hornigold D. Plant lectins are novel Toll-like receptor agonists. Biochem Pharmacol. 2011;81:1324–1328. doi: 10.1016/j.bcp.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh TK, Mickelson DJ, Fink J, Solberg JC, Inglefield JR, Hook D, Gupta SK, Gibson S, Alkan SS. Toll-like receptor (TLR) 2–9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006;243:48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Boyd JH, Divangahi M, Yahiaoui L, Gvozdic D, Qureshi S, Petrof BJ. Toll-like receptors differentially regulate CC and CXC chemokines in skeletal muscle via NF-kappaB and calcineurin. Infect Immun. 2006;74:6829–6838. doi: 10.1128/IAI.00286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu Y, Nagai S, Wu H, Neish AS, Koyasu S, Gewirtz AT. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagellin-induced proinflammatory gene expression. J Immunol. 2006;176:6194–6201. doi: 10.4049/jimmunol.176.10.6194. [DOI] [PubMed] [Google Scholar]