Abstract
Background
Many pediatricians recommend, and many parents administer, alternating or combined doses of ibuprofen and acetaminophen for fever. Limited data support this practice with standard US doses.
Objective
This study compared the antipyretic effect of 3 different treatment regimens in children, using either ibuprofen alone, ibuprofen combined with acetaminophen, or ibuprofen followed by acetaminophen over a single 6-hour observation period.
Methods
Febrile episodes from children aged 6 to 84 months were randomized into the 3 treatment groups: a single dose of ibuprofen at the beginning of the observation period; a single dose of ibuprofen plus a single dose of acetaminophen at the beginning of the observation period; or ibuprofen followed by acetaminophen 3 hours later. Ibuprofen was administered at 10 mg/kg; acetaminophen at 15 mg/kg. Temperatures were measured hourly for 6 hours using a temporal artery thermometer. The primary outcome was temperature difference between treatment groups. Adverse-event data were not collected in this single treatment period study.
Results
Sixty febrile episodes in 46 children were assessed. The mean (SD) age of the children was 3.4 (2.2) years, and 31 (51.7%) were girls. Differences among temperature curves were significant (P < 0.001; the combined and alternating arms had significantly better antipyresis compared with the ibuprofen-alone group at hours 4 to 6 (hour 4, P < 0.005; hours 5 and 6, P < 0.001). All but one of the children in the combined and alternating groups were afebrile at hours 4, 5, and 6. In contrast, for those receiving ibuprofen alone, 30%, 40%, and 50% had temperatures >38.0°C at hours 4, 5, and 6, respectively (hour 4, P < 0.002; hours 5 and 6, P < 0.001).
Conclusion
During a single 6-hour observation period for these participating children, combined and alternating doses of ibuprofen and acetaminophen provided greater antipyresis than ibuprofen alone at 4 to 6 hours.
Keywords: fever, antipyretic, acetaminophen, ibuprofen
INTRODUCTION
Despite a lack of evidence to support their fears, a majority of parents1–7 and pediatric health care providers8–11 believe that fever can be dangerous to a child. This “fever phobia” has caused caregivers to treat fever aggressively with antipyretics such as ibuprofen and acetaminophen, often in combination.12 Although there are limited data to support the use of these medications together for the treatment of fever,13–18 it has been reported that 50% of pediatric practitioners advise parents to alternate acetaminophen and ibuprofen in an attempt to achieve greater antipyresis.12
Pharmacologic evidence suggests that acetaminophen and ibuprofen may be well tolerated when used together because the 2 medications have different pathways of metabolism that are not affected by each other.19–21 Both drugs are generally well tolerated,22–24 with wide therapeutic margins if proper dosing is used, and a combined preparation of the 2 compounds has been available outside the United States for more than a decade.25 Furthermore, despite theoretical concerns regarding misdosing, reports of adverse events from alternating or combined use of acetaminophen and ibuprofen are rare given the frequency of coadministration of the 2 drugs.12 Because acetaminophen and ibuprofen are commonly coadministered to febrile children, further evidence on this practice is required. Thus, we conducted a 3-arm, randomized, controlled trial comparing the effectiveness of a single dose of ibuprofen with the combined administration of ibuprofen and acetaminophen, as well as ibuprofen followed 3 hours later by acetaminophen. Ibuprofen was chosen as the comparator instead of acetaminophen because clinicians prefer it for high fevers12 and because the single study that compared the 2 drugs when administered at their maximum recommended dose found no significant difference between them.26 We hypothesized that the 2 treatment groups using both ibuprofen and acetaminophen would experience greater antipyresis than would the group using ibuprofen alone during the 6-hour observation period.
SUBJECTS AND METHODS
Participants
From March 2006 through July 2009, children aged 6 months through 8 years were recruited from outpatient clinics and child day-care facilities to participate in this 6-hour-long study at a single academic medical center in Hershey, Pennsylvania. Inclusion criteria for the study required an initial temperature of ≥38.0°C when measured by a temporal artery thermometer (TemporalScanner, Exergen Corporation, Watertown, Massachusetts). A temperature of 38.0°C was chosen because it is the most widely accepted temperature definition of fever.27 Participating children were also required to demonstrate an ability to cooperate with serial temporal artery temperature measurements and to take medications by mouth. Children were excluded from participating if they had received acetaminophen within 6 hours of presentation or ibuprofen, aspirin, or other NSAIDs within 8 hours of presentation. Other major exclusions included weight >60 kg (to avoid surpassing 600 mg of ibuprofen or 1000 mg of acetaminophen in a single dose), a history of adverse reaction to any study medication ingredient, diabetes mellitus, renal dysfunction, hepatic dysfunction, thrombocytopenia, or presence of moderate or severe dehydration. Children were also excluded if medical judgment determined that the severity of the underlying illness prohibited inclusion or if the child had already participated in the trial on 3 previous occasions. Although individual children could participate in the trial more than once, it was required that 2 weeks pass between each date of participation. This decision was made to aid recruitment, but the time span between participation ensured that an illness distinct from that of prior enrollment was involved and that there was a more than sufficient washout period from study drug consumption.19
The study was approved by the Penn State College of Medicine’s Human Subjects Protection Office, and the trial was registered at www.clinicaltrials.gov in December 2005. Informed consent was obtained from the parents of all participants; obtaining verbal assent from participating children was not required.
Procedures
Febrile children began participation through either presentation to the General Clinical Research Center of the Penn State College of Medicine or by a research coordinator traveling to the on-campus child day-care center where the febrile child was located. After confirmation that the participant met enrollment criteria, each child was randomly assigned to 1 of 3 treatment groups according to a computer-generated log and then was followed for 6 hours. Baseline measurements included weight and temporal artery temperature. In addition to the baseline measurement, temporal artery temperatures were taken hourly over the 6-hour study period. At each time point, temperatures were measured by unblinded research nurses at least twice until 2 consecutive measurements were within 0.2°C. The decision to not mask or conceal study nurses reflects the fact that the outcome of temperature is objective.
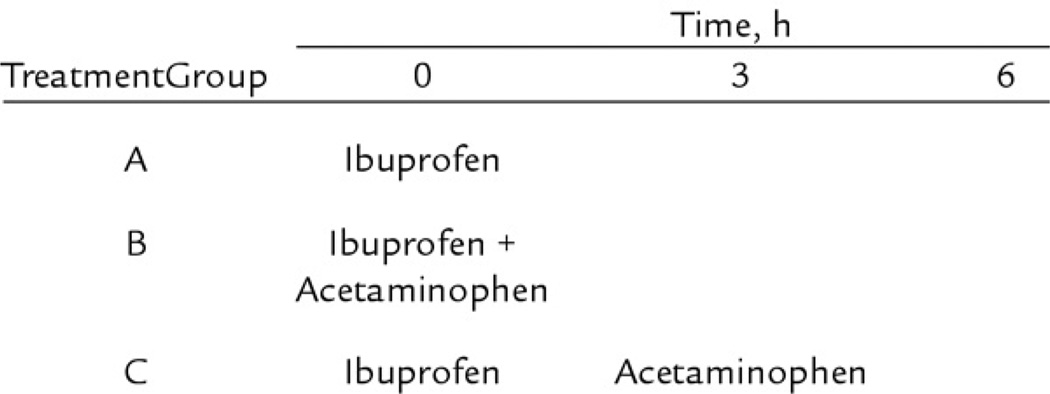
As shown in Figure 1, participants in treatment group A received a single dose of ibuprofen* (oral suspension 100 mg/5 mL), at a dose of 10 mg/kg at the beginning of the 6-hour observation period. Those in treatment group B received a single dose of 10 mg/kg of ibuprofen plus a single dose of 15 mg/kg of acetaminophen† (oral solution USP 160 mg/5 mL) at the beginning of the observation period, while those in treatment group C received 10 mg/kg of ibuprofen at the beginning of the study followed by 15 mg/kg of acetaminophen 3 hours later. The “alternating” schedule for group C was chosen as opposed to other regimens that have been described12,14,16,17 because it allows for the most frequent pattern of alternating drugs without exceeding the maximum recommended dose for either compound (40 mg/kg/d of ibuprofen, 75 mg/kg/d of acetaminophen). Medication volumes were rounded to the nearest 0.1 mL by the research nurse who administered the drugs.
Figure 1.
Study treatment arms with dosing schedule.
Sample Size and Statistical Analysis
Before initiating the study, it was determined that a total of 120 subjects, randomly assigned to 3 treatment groups, would have 80% power to detect a 0.5°C difference in temperature reduction between treatment arms with α = 0.05. Recruitment challenges arose because of the necessity of a morning fever in participants. This requirement was necessary to ensure research staff coverage for the 6-hour study period. Furthermore, administrative changes at an on-campus child-care facility led to unexpectedly poor recruitment from that center. These barriers resulted in a reduced sample size of 46 participants that took 3 years to recruit. Among the 46 participating children, 8 participated twice, 3 participated 3 times, and 35 participated only once. In total, these 46 participants contributed 60 febrile episodes that were randomly assigned into the 3 treatment groups with 20 episodes per group.
Demographic and baseline features were analyzed through χ2 test, Fisher exact test, and ANOVA. Using an assumption that there was no dependence among the multiple fever events from a single child because each fever event was part of a distinct illness (separated by at least 2 weeks), the repeated measures of temperature were analyzed by mixed-model methodology through PROC MIXED software, version 9.2 (SAS Institute Inc., Cary, North Carolina), including modeling the polynomial trends over time and comparisons of temperatures among the 3 groups at different time points.28,29 Time of medication dosing was not included as a covariate.
The binary outcome of temperature ≥38.0°C and <38.0°C at each of the hourly time points was also evaluated because this standard temperature threshold may be used by schools and day-care facilities in determining whether a child may remain at the facility or must be taken home. Although repeated-measures analysis techniques were also preferable for this binary outcome, χ2 or Fisher exact tests were used at each of the time points because of the limitations of the data, where the majority were either 0%(0) or 100%.20 A Bonferroni adjustment was used to control the inflation of type I error, where the α level was set to 0.05/6 = 0.008 for these binary outcome comparisons.
RESULTS
Children with 60 febrile episodes meeting eligibility criteria were enrolled in this trial, with all 60 completing the 6-hour observation study. The mean (SD) age of participants was 3.4 (2.2) years and 31 (51.7%) were girls, with no significant difference between treatment groups (Table I). Forty-nine (81.7%) of the febrile episodes occurred in children who were white and non-Hispanic. The mean temperature at baseline was 38.7°C, again with no significant difference among treatment groups (Table II). The most common presenting diagnoses were upper respiratory infection (n = 27), fever without a source (n = 12), and acute otitis media (n = 8).
Table I.
Demographic variables by treatment group (N=60). Data are number (%), unless otherwise indicated.
| Ibuprofen Alone (n = 20) |
Ibuprofen + Acetaminophen (n = 20) |
Ibuprofen Followed by Acetaminophen (n =20) |
P | |
|---|---|---|---|---|
| Age, mean (SD), y | 3.2 (1.9) | 3.0 (1.9) | 4.0 (2.8) | 0.35 |
| Age distribution | 0.94 | |||
| <3 y | 11 (55) | 11 (55) | 10 (50) | |
| ≥3 y | 9 (45) | 9 (45) | 10 (50) | |
| Sex | 0.28 | |||
| Female | 8 (40) | 10 (50) | 13 (65) | |
| Male | 12 (60) | 10 (50) | 7 (35) | |
| Race/ethnicity | 0.59 | |||
| White, non-Hispanic | 18 (90) | 15 (75) | 16 (80) | |
| Other | 2 (10) | 5 (25) | 4 (20) | |
| Weight, mean (SD), kg | 15.3 (6.4) | 13.6 (4.3) | 16.8 (6.8) | 0.26 |
Table II.
Study outcomes related to temperature (N = 60).
| Temperature | Ibuprofen Alone (n = 20) |
Ibuprofen + Acetaminophen (n = 20) |
Ibuprofen Followed by Acetaminophen (n = 20) |
P*† |
|---|---|---|---|---|
| Hour 0 (baseline) | ||||
| °C, mean (SD) | 38.8 (0.9) | 38.6 (0.4) | 38.7 (0.9) | 0.60 |
| <38.0°C, no. (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Hour 1 | ||||
| °C, mean (SD) | 37.6 (0.5) | 37.4 (0.5) | 37.6 (0.4) | 0.57 |
| <38.0°C, no. (%) | 16 (80) | 18 (90) | 16 (80) | 0.75 |
| Hour 2 | ||||
| °C, mean (SD) | 37.1 (0.4) | 37.0 (0.5) | 37.2 (0.3) | 0.52 |
| <38.0°C, no. (%) | 19 (95) | 20 (100) | 20 (100) | 1.00 |
| Hour 3 | ||||
| °C, mean (SD) | 37.2 (0.6) | 36.9 (0.4) | 36.9 (0.4) | 0.20 |
| <38.0°C, no. (%) | 18 (90) | 20 (100) | 20 (100) | 0.32 |
| Hour 4 | ||||
| °C, mean (SD) | 37.5 (1.1) | 36.9 (0.3) | 36.9 (0.3) | 0.002 |
| <38.0°C, no. (%) | 14 (70) | 20 (100) | 20 (100) | 0.002 |
| Hour 5 | ||||
| °C, mean (SD) | 38.0 (1.1) | 36.9 (0.5) | 36.8 (0.3) | <0.001 |
| <38.0°C, no. (%) | 12 (60) | 20 (100) | 20 (100) | <0.001 |
| Hour 6 | ||||
| °C, mean (SD) | 38.5 (1.5) | 37.2 (0.6) | 36.9 (0.3) | <0.001 |
| <38.0°C, no. (%) | 10 (50) | 19 (95) | 20 (100) | <0.001 |
P values for the comparisons of the mean temperatures were from the repeated measures analysis using mixed models.
P values for the comparisons of the binary outcome (<38.0°C vs ≥38.0°C) were from the χ2 or Fisher exact test at each of the time points except at hour 0. The significance level was set toα = 0.05/6 = 0.008.
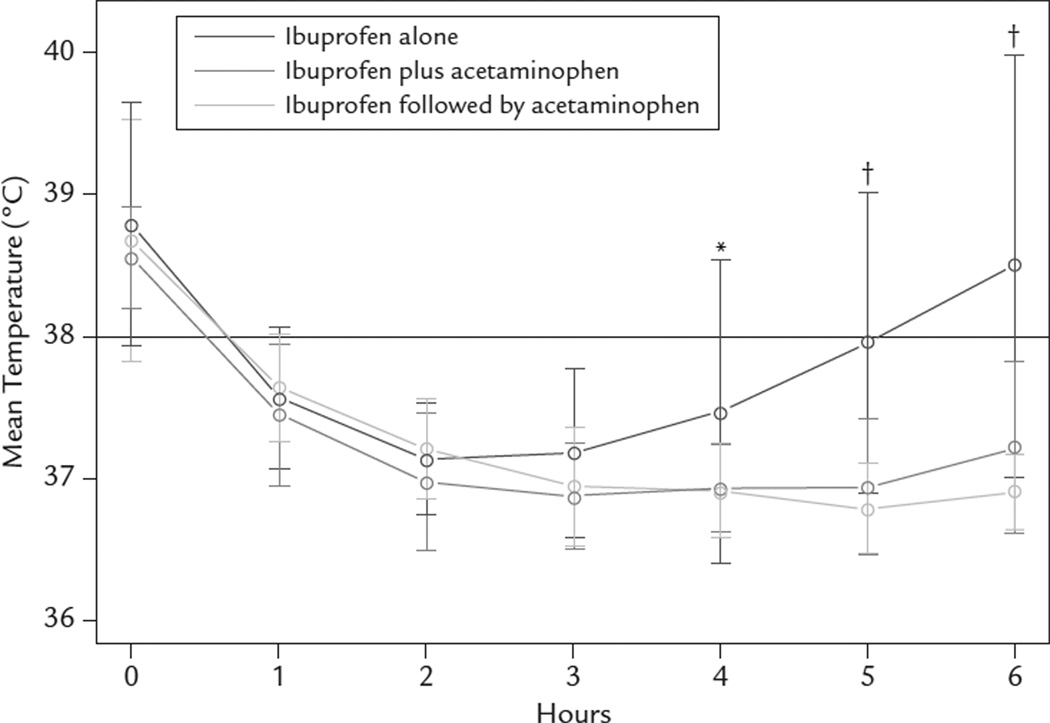
Assessing the effect of treatment on temperature over 6 hours of observation (Figure 2), there were significant temperature curve differences among the 3 treatment groups (P < 0.001); the combined and alternating treatment arms had greater antipyresis compared with the ibuprofen-alone group (Table III). Pairwise comparisons of the combined treatment with ibuprofen alone revealed significant differences at hours 4 (P = 0.002), 5 (P < 0.001), and 6 (P < 0.001), showing that the combined treatment had a greater antipyretic effect at these time points. Similarly, the alternating regimen also had a greater antipyretic effect than ibuprofen alone at hours 4 (P = 0.003), 5 (P < 0.001), and 6 (P < 0.001). Moreover, all subjects in the ibuprofen followed by acetaminophen group were afebrile (<38.0°C) at hours 4, 5, and 6, as were all subjects in the combined treatment group except 1, who had a fever at hour 6. In contrast, in those children treated with ibuprofen alone, 30%, 40%, and 50% had temperatures ≥38.0°C at hours 4, 5, and 6, respectively (hour 4, P = 0.002; hours 5 and 6, P < 0.001). Comparisons of temperatures between the combined and alternating regimens revealed no significant difference at any time point.
Figure 2.
Mean hourly temperatures by treatment group. *P < 0.005; †P < 0.001.
Table III.
Estimated pairwise comparisons of mean (SD) temperatures (in °C) between treatment groups.
| Ibuprofen Alone Versus Ibuprofen+ Acetaminophen |
Ibuprofen Alone Versus Ibuprofen Followed by Acetaminophen |
Ibuprofen+ Acetaminophen Versus Ibuprofen Followed by Acetaminophen |
||||
|---|---|---|---|---|---|---|
| Hour | Temperature Difference |
P | Temperature Difference |
P | Temperature Difference |
P |
| 0 | 0.22 (0.22) | 0.32 | 0.08 (0.22) | 0.71 | −0.13 (0.22) | 0.54 |
| 1 | 0.16 (0.18) | 0.38 | −0.01 (0.18) | 0.94 | −0.17 (0.18) | 0.34 |
| 2 | 0.19 (0.18) | 0.29 | 0.03 (0.18) | 0.86 | −0.16 (0.18) | 0.38 |
| 3 | 0.33 (0.19) | 0.08 | 0.22 (0.19) | 0.24 | −0.11 (0.19) | 0.56 |
| 4 | 0.56 (0.18) | 0.002 | 0.55 (0.18) | 0.003 | −0.01 (0.18) | 0.95 |
| 5 | 0.89 (0.18) | <0.001 | 1.02 (0.18) | <0.001 | 0.13 (0.18) | 0.47 |
| 6 | 1.31 (0.22) | <0.001 | 1.63 (0.22) | <0.001 | 0.31 (0.22) | 0.15 |
DISCUSSION
This study found that over a single 6-hour observation period, combined and alternating doses of ibuprofen and acetaminophen provided greater antipyresis than did ibuprofen alone. The findings from the last 3 hours of observation confirmed the hypothesis at the study’s outset and are consistent with other studies that tested combinations of acetaminophen and aspirin decades ago.30,31 Additionally, the data describing greater antipyresis in the treatment group in which ibuprofen and acetaminophen were coadministered are highly relevant to parents who give medication to their children before day care, school, or sleep.
The present findings can be compared with the few other studies attempting to evaluate the antipyretic effect of alternating or combined doses of acetaminophen and ibuprofen. However, 3 of the 6 previous studies used doses or dosing regimens not consistent with the current standard of care in the United States, such as the use of a 25-mg/kg loading dose of acetaminophen, 14 TID dosing along with cold sponge baths as needed,13 or using medication amounts below the widely recognized single dose amounts of 10 mg/kg of ibuprofen or 15 mg/kg of acetaminophen.13–15
Three previous studies have similarities to ours. Over an 8-hour observation period, Nabulsi et al16 followed a single dose of ibuprofen (10 mg/kg) with either acetaminophen (15 mg/kg) or placebo 4 hours after the ibuprofen dose in a randomized trial involving 70 children. The investigators found that the group receiving acetaminophen experienced a greater antipyretic effect at hours 6 through 8. In a randomized trial that involved 38 children and had a 6-hour observation period similar to the present study, Kramer et al18 compared acetaminophen administered every 4 hours with acetaminophen followed by ibuprofen 3 hours later and found greater antipyresis for the latter treatment when assessed at hours 4 and 5 of the study. Hay et al17 compared a regimen using both ibuprofen and acetaminophen with each drug taken alone in a randomized trial with 156 participating children. The initial dose of each drug in the combination arm was given together, followed by acetaminophen every 6 hours and ibuprofen every 8 hours. That study found that combined treatment resulted in substantially less time with fever for children over 24 hours, but there was insufficient power to detect differences in discomfort.
Similar to the trial by Hay et al,17 the present study was underpowered to evaluate the effect of treatment group on discomfort and pain, which may be preferable primary outcome measures for future studies. Nonetheless, there is evidence that combinations of acetaminophen and NSAIDs are more effective for the treatment of pain32–36 and can reduce opioid use37,38 compared with a single agent. Improved activity and alertness in children have been reported after antipyretic administration.39
We acknowledge several limitations in addition to the relatively small sample size of this trial. First, the 6-hour observation period allowed for evaluation of only a single cycle of drug administration and also did not evaluate the full potential duration of ibuprofen’s effects. This choice was made for logistic reasons along with the fact that ibuprofen can be safely readministered 6 hours after a previous dose. Our study also did not evaluate the effect of multiple doses over a longer observation period or adverse events that could occur from this practice. Nephrotoxicity, for example, is a theoretical possibility that could result from coadministration of ibuprofen and acetaminophen, particularly in the setting of a dehydrated child. It is noted, however, that despite the high frequency of coadministration in real-world settings,22 there are very few case reports of nephrotoxicity linked to this practice.40–42 Nonetheless, if pediatricians recommend, or caregivers administer, combined or alternating doses, careful attention must be paid to avoid overdose or use in dehydrated children.
Another limitation of the study is that no attempt was made to blind the participants or research nurses because we believed that temperatures could not be influenced in any way by knowledge of study treatment.
Temporal artery thermometry was chosen because it is noninvasive, easily and quickly measured, and has become popular among families and professionals, with some data to support its use.43,44 Although there have been questions regarding its reliability in comparison with rectal temperature measurements,45,46 a debate regarding the most appropriate temperature measurement is beyond the scope of this article. However, the one larger study describing this topic used axillary temperatures for study outcomes related to fever.17
CONCLUSIONS
The findings of this study suggest that combined and alternating doses of ibuprofen and acetaminophen provided greater antipyresis than ibuprofen alone at 4 to 6 hours in these children. Future studies may be needed to evaluate the safety of the antipyretic regimens described in this study and of repeated doses of these 2 drugs when used in combination.
ACKNOWLEDGMENTS
This study was supported by a research grant from the George L. Laverty Foundation and in part by a General Clinical Research Center (GCRC) grant from the National Institutes of Health (M01RR10732) and a CGRC Construction Grant (C06RR016499) awarded to the Pennsylvania State University College of Medicine.
Dr. Paul had full access to all the data in the study and is responsible for the integrity of the data and the accuracy of the data analysis. Dr. Paul supervised all aspects of the project, designed the study, and was responsible for drafting the manuscript. Ms. Sturgis led participant recruitment efforts and clinical care of the participants, and assisted with the design and manuscript. Dr. Yang led the statistical analysis and assisted with portions of the manuscript. Ms. Engle performed many of the statistical analyses and helped edit the manuscript. Ms. Watts performed much of the clinical care of the study and led much of the study coordination. Dr. Berlin provided mentorship and content expertise and assisted in the development of both the study protocol and manuscript.
Dr. Paul has been a paid consultant for the Consumer Healthcare Products Association; McNeil Consumer Healthcare; Novartis Consumer Health, Inc.; Procter & Gamble; and Reckitt Benckiser Healthcare International Ltd., but no industry employees were involved in any aspect of the study.
Footnotes
Trademark: Children’s Motrin®, McNeil-PPC, Inc., Fort Washington, Pennsylvania.
Manufactured by Goldline Laboratories, Inc., Miami, Florida.
The authors have indicated that they have no other conflicts of interest regarding the content of this article.
REFERENCES
- 1.Schmitt BD. Fever phobia: Misconceptions of parents about fevers. Am J Dis Child. 1980;134:176–181. [PubMed] [Google Scholar]
- 2.Casey R, McMahon F, McCormick MC, et al. Fever therapy: An educational intervention for parents. Pediatrics. 1984;73:600–605. [PubMed] [Google Scholar]
- 3.Kramer MS, Naimark L, Leduc DG. Parental fever phobia and its correlates. Pediatrics. 1985;75:1110–1113. [PubMed] [Google Scholar]
- 4.Kai J. What worries parents when their preschool children are acutely ill, why: A qualitative study. BMJ. 1996;313:983–986. doi: 10.1136/bmj.313.7063.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly L, Morin K, Young D. Improving caretakers’ knowledge of fever management in preschool children: Is it possible? J Pediatr Health Care. 1996;10:167–173. doi: 10.1016/S0891-5245(96)90040-1. [DOI] [PubMed] [Google Scholar]
- 6.Impicciatore P, Nannini S, Pandolfini C, Bonati M. Mother’s knowledge of, attitudes toward, and management of fever in preschool children in Italy. Prev Med. 1998;27:268–273. doi: 10.1006/pmed.1998.0262. [DOI] [PubMed] [Google Scholar]
- 7.Crocetti M, Moghbeli N, Serwint J. Fever phobia revisited: Have parental misconceptions about fever changed in 20 years? Pediatrics. 2001;107:1241–1246. doi: 10.1542/peds.107.6.1241. [DOI] [PubMed] [Google Scholar]
- 8.Weiss J, Herskowitz L. House officer management of the febrile child.Asurvey. Clin Pediatr (Phila) 1983;22:766–769. doi: 10.1177/000992288302201106. [DOI] [PubMed] [Google Scholar]
- 9.May A, Bauchner H. Fever phobia: The pediatrician’s contribution. Pediatric. 1992;90:851–854. [PubMed] [Google Scholar]
- 10.Ipp M, Jaffe D. Physicians’ attitudes toward the diagnosis and management of fever in children 3 months to 2 years of age. Clin Pediatr (Phila) 1993;32:66–70. doi: 10.1177/000992289303200201. [DOI] [PubMed] [Google Scholar]
- 11.Poirier MP, Davis PH, Gonzalez-del Rey JA, Monroe KW. Pediatric emergency department nurses’ perspectives on fever in children. Pediatr Emerg Care. 2000;16:9–12. doi: 10.1097/00006565-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Mayoral CE, Marino RV, Rosenfeld W, Greensher J. Alternating antipyretics: Is this an alternative? Pediatrics. 2000;105:1009–1012. doi: 10.1542/peds.105.5.1009. [DOI] [PubMed] [Google Scholar]
- 13.Lal A, Gomber S, Talukdar B. Antipyretic effects of nimesulide, paracetamol and ibuprofen-paracetamol. Indian J Pediatr. 2000;67:865–870. doi: 10.1007/BF02723945. [DOI] [PubMed] [Google Scholar]
- 14.Sarrell EM, Wielunsky E, Cohen HA. Antipyretic treatment in young children with fever: Acetaminophen, ibuprofen, or both alternating in a randomized, double-blind study. Arch Pediatr Adolesc Med. 2006;160:197–202. doi: 10.1001/archpedi.160.2.197. [DOI] [PubMed] [Google Scholar]
- 15.Erlewyn-Lajeunesse MD, Coppens K, Hunt LP, et al. Randomised controlled trial of combined paracetamol and ibuprofen for fever. Arch Dis Child. 2006;91:414–416. doi: 10.1136/adc.2005.087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nabulsi MM, Tamim H, Mahfoud Z, et al. Alternating ibuprofen and acetaminophen in the treatment of febrile children: A pilot study [ISRCTN30487061] BMC Med. 2006;4:4. doi: 10.1186/1741-7015-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay AD, Costelloe C, Redmond NM, et al. Paracetamol plus ibuprofen for the treatment of fever in children (PITCH): Randomised controlled trial. BMJ. 2008;337:a1302. doi: 10.1136/bmj.a1302. [Published corrections appear in BMJ. 2009;339:b3295] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer LC, Richards PA, Thompson AM, et al. Alternating antipyretics: Antipyretic efficacy of acetaminophen versus acetaminophen alternated with ibuprofen in children. Clin Pediatr (Phila) 2008;47:907–911. doi: 10.1177/0009922808319967. [DOI] [PubMed] [Google Scholar]
- 19.Wright CE 3rd, Antal EJ, Gillespie WR, Albert KS. Ibuprofen and acetaminophen kinetics when taken concurrently. Clin Pharmacol Ther. 1983;34:707–710. doi: 10.1038/clpt.1983.237. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics. Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108:1020–1024. doi: 10.1542/peds.108.4.1020. [DOI] [PubMed] [Google Scholar]
- 21.Aronoff DM, Neilson EG. Antipyretics: Mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304–315. doi: 10.1016/s0002-9343(01)00834-8. [DOI] [PubMed] [Google Scholar]
- 22.Lesko SM, Mitchell AA. An assessment of the safety of pediatric ibuprofen. A practitioner-based randomized clinical trial. JAMA. 1995;273:929–933. [PubMed] [Google Scholar]
- 23.Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics. 1999;104:e39. doi: 10.1542/peds.104.4.e39. [DOI] [PubMed] [Google Scholar]
- 24.Perrott DA, Piira T, Goodenough B, Champion GD. Efficacy and safety of acetaminophen vs ibuprofen for treating children’s pain or fever: A meta-analysis. Arch Pediatr Adolesc Med. 2004;158:521–526. doi: 10.1001/archpedi.158.6.521. [DOI] [PubMed] [Google Scholar]
- 25. [Accessed December 10, 2010];Sanofi-aventis. Emerging markets IR seminar. 2009 http://en.sanofiaventis.com/binaries/IR_Seminar-Emerging_markets_tcm28-25561.
- 26.Walson PD, Galletta G, Chomilo F, et al. Comparison of multidose ibuprofen and acetaminophen therapy in febrile children. Am J Dis Child. 1992;146:626–632. doi: 10.1001/archpedi.1992.02160170106025. [DOI] [PubMed] [Google Scholar]
- 27.Adam HM. Physiology and management of fever. In: McInerny TK, Adam HM, Campbell DE, et al., editors. Textbook of Pediatric Care. Elk Grove Village, Ill: American Academy of Pediatrics; 2009. pp. 418–422. [Google Scholar]
- 28.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. StatMed. 2000;19:1793–1819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Littell RC, Milliken GA, Stroup WW, et al. SAS for Mixed Models. 2nd ed. Cary, North Carolina: SAS Institute Inc; 2006. [Google Scholar]
- 30.Steele RW, Young FS, Bass JW, Shirkey HC. Oral antipyretic therapy: Evaluation of aspirin-acetaminophen combination. Am J Dis Child. 1972;123:204–206. doi: 10.1001/archpedi.1972.02110090074006. [DOI] [PubMed] [Google Scholar]
- 31.Simila S, Keinanen S, Kouvalainen K. Oral antipyretic therapy: Evaluation of benorylate, an ester of acetylsalicylic acid and paracetamol. Eur J Pediatr. 1975;121:15–20. doi: 10.1007/BF00464391. [DOI] [PubMed] [Google Scholar]
- 32.Seideman P, Samuelson P, Neander G. Naproxen and paracetamol compared with naproxen only in coxarthrosis. Increased effect of the combination in 18 patients. Acta Orthop Scand. 1993;64:285–288. doi: 10.3109/17453679308993626. [DOI] [PubMed] [Google Scholar]
- 33.Breivik EK, Barkvoll P, Skovlund E. Combining diclofenac with acetaminophen or acetaminophen-codeine after oral surgery: A randomized, double-blind single-dose study. Clin Pharmacol Ther. 1999;66:625–635. doi: 10.1053/cp.1999.v66.103629001. [DOI] [PubMed] [Google Scholar]
- 34.Homer JJ, Swallow J, Semple P. Audit of pain management at home following tonsillectomy in children. J Laryngol Otol. 2001;115:205–208. doi: 10.1258/0022215011907208. [DOI] [PubMed] [Google Scholar]
- 35.Mehlisch DR, Aspley S, Daniels SE, et al. Asingle-tablet fixed-dose combination of racemic ibuprofen/paracetamol in the management of moderate to severe postoperative dental pain in adult and adolescent patients: A multicenter, two-stage, randomized, double-blind, parallel-group, placebo-controlled, factorial study. Clin Ther. 2010;32:1033–1049. doi: 10.1016/j.clinthera.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Mehlisch DR, Aspley S, Daniels SE, Bandy DP. Comparison of the analgesic efficacy of concurrent ibuprofen and paracetamol with ibuprofen or paracetamol alone in the management of moderate to severe acute postoperative dental pain in adolescents and adults: A randomized, double-blind, placebo-controlled, parallel-group, single-dose, twocenter, modified factorial study. Clin Ther. 2010;32:882–895. doi: 10.1016/j.clinthera.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Stambaugh JE, Jr, Drew J. The combination of ibuprofen and oxycodone/ acetaminophen in the management of chronic cancer pain. Clin Pharmacol Ther. 1988;44:665–669. doi: 10.1038/clpt.1988.209. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery JE, Sutherland CJ, Kestin IG, Sneyd JR. Morphine consumption in patients receiving rectal paracetamol and diclofenac alone and in combination. Br J Anaesth. 1996;77:445–447. doi: 10.1093/bja/77.4.445. [DOI] [PubMed] [Google Scholar]
- 39.Kramer MS, Naimark LE, Roberts- Brauer R, et al. Risks and benefits of paracetamol antipyresis in young children with fever of presumed viral origin. Lancet. 1991;337:591–594. doi: 10.1016/0140-6736(91)91648-e. [DOI] [PubMed] [Google Scholar]
- 40.Moghal NE, Hegde S, Eastham KM. Ibuprofen and acute renal failure in a toddler. Arch Dis Child. 2004;89:276–277. doi: 10.1136/adc.2002.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaffanello M, Brugnara M, Angeli S, Cuzzolin L. Acute non-oliguric kidney failure and cholestatic hepatitis induced by ibuprofen and acetaminophen: A case report. Acta Paediatr. 2009;98:903–905. doi: 10.1111/j.1651-2227.2008.01209.x. [DOI] [PubMed] [Google Scholar]
- 42.Del Vecchio MT, Sundel ER. Alternating antipyretics: Is this an alternative? Pediatrics. 2001;108:1236–1237. [PubMed] [Google Scholar]
- 43.Greenes DS, Fleisher GR. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr Adolesc Med. 2001;155:376–381. doi: 10.1001/archpedi.155.3.376. [DOI] [PubMed] [Google Scholar]
- 44.Lawson L, Bridges EJ, Ballou I, et al. Accuracy and precision of noninvasive temperature measurement in adult intensive care patients. Am J Crit Care. 2007;16:485–496. [PubMed] [Google Scholar]
- 45.Siberry GK, Diener-West M, Schappell E, Karron RA. Comparison of temple temperatures with rectal temperatures in children under two years of age. Clin Pediatr (Phila) 2002;41:405–414. doi: 10.1177/000992280204100605. [DOI] [PubMed] [Google Scholar]
- 46.Schuh S, Komar L, Stephens D, et al. Comparison of the temporal artery and rectal thermometry in children in the emergency department. Pediatr Emerg Care. 2004;20:736–741. doi: 10.1097/01.pec.0000144915.78124.26. [DOI] [PubMed] [Google Scholar]