Abstract
Behavioral inhibition (BI) is a temperament characterized in young children by a heightened sensitivity to novelty, social withdrawal, and anxious behaviors. For many children, these social difficulties dissipate over time. For others, patterns of social withdrawal continue into adolescence. Over time, attention biases to threat may influence the stability of behavioral inhibition and its association with social withdrawal, ultimately modulating the risk for anxiety disorders in BI children. However, we know relatively little about the cognitive processes that accompany BI and shape later socio-emotional functioning. We examined the relations among BI in childhood, attention biases to threat in adolescence, and adolescent social withdrawal in a longitudinal study (N=126, Mean age=15 years). As has been reported in anxious adults, adolescents who were behaviorally inhibited as toddlers and young children showed heightened attention bias to threat. In addition, attention bias to threat moderated the relation between childhood BI and adolescent social withdrawal.
Keywords: Temperament, Behavioral Inhibition, Social Withdrawal, Attention Biases
Recent studies suggest that threat-related attention biases play an important role in supporting the expression of anxious behaviors in both children and adults (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). Individuals who display attention biases to threat report increased levels of anxiety, compared to counterparts with no attention bias to threat. Experimentally manipulating threat-related attention bias in non-anxious adults (MacLeod, Rutherford, Campbell, Ebsworthy, & Holker, 2002; Mathews & MacLeod, 2002) and children (Eldar, Ricon, & Bar-Haim, 2008) influences subsequent sensitivity to stress. Taken together, these data have led some researchers to argue that threat-related attention bias plays a causal role in the expression of anxious behaviors.
Finally, emerging imaging data suggest that the relation between attention bias and anxiety is influenced by enhanced sensitivity to threat in the functional connections within a neural “fear circuit” that encompasses both the amygdala and ventro-lateral prefrontal cortex (Monk et al., 2006; Monk et al., 2008). A rapid, but crude, signaling process transfers information directly to the thalamus and amygdala (Phelps & LeDoux, 2005), while a subsequent complex processing system engages both the amygdala and cortical regions, such as the ventro-lateral prefrontal cortex. As such, the initial response to a perceived threat may shift as new, more refined information is received and perceptions are reconsidered. These data build on basic work linking the neural circuits underlying attention and affect, illustrated in work showing that the orienting response often follows direct activation of the amygdala (Davis & Whalen, 2001).
The temperament literature has long shown that attention underlies individual differences in both the reactive and regulative tendencies of young children. Infants vary in their attention to stimuli in the environment, and young children's developing abilities to control attention correlate with adaptive social development (Rothbart & Posner, 2006; Ruff & Rothbart, 1996). This relation is evident in the temperament of behavioral inhibition.
Behaviorally inhibited children have been described as hyper-vigilant to their environments and prone to exhibit a heightened sensitivity to novelty (Kagan, Reznick, & Snidman, 1988), particularly if social in nature (Kagan, 2001). These children are often slow-to-warm-up (Kagan et al., 1988), shy and reticent in novel or unfamiliar social situations (Coplan, Girardi, Findlay, & Frohlick, 2007; Coplan, Rubin, Fox, Calkins, & Stewart, 1994; Fox, Schmidt, Calkins, Rubin, & Coplan, 1996), and are autonomically over-aroused (Henderson, Fox, & Rubin, 2001; McDermott et al., 2009; Pérez-Edgar, Schmidt, Henderson, Schulkin, & Fox, 2008; Reeb-Sutherland et al., 2009; L. A. Schmidt et al., 1997; L. A. Schmidt, Fox, Schulkin, & Gold, 1999).
The precursors to this behavioral profile are evident in elevated levels of negative reactivity to novel sensory stimuli in the first months of life (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). Indeed, recent evidence (Marshall, Reeb, & Fox, 2009) has found that as early as nine months of age, infants selected for temperamental behavioral inhibition display heightened sensitivity to novel auditory stimuli. These early appearing behavioral and physiological responses to novelty are thought to be driven by increased reactivity in the amygdala (Pérez-Edgar et al., 2007; Schwartz, Wright, Shin, Kagan, & Rauch, 2003), a brain region also implicated in threat-related attention bias (Monk et al., 2008).
Recent data suggest that behavioral inhibition is marked by perturbations in attention control (Fox, Hane, & Pine, 2007; Fox, Henderson, Pérez-Edgar, & White, 2008). For example, behaviorally inhibited children show greater interference effects in Stroop-like emotion processing tasks (Pérez-Edgar & Fox, 2007; Schwartz, Snidman, & Kagan, 1996) and greater difficulty controlling selective attention when under stress (Pérez-Edgar & Fox, 2005a). However, these initial studies assessed only broad indices of attention and cannot speak to the specific mechanisms of attention (e.g., orienting vs. attention control) that might shape behavior.
The available data in behavioral inhibition suggest that attention difficulties might sustain early patterns of behavioral inhibition over the course of development (Fox et al., 2007). A stable pattern of behavioral inhibition from childhood into adolescence may manifest significant social withdrawal (Caspi et al., 2003; Caspi & Silva, 1995). The issue of sustained behavioral inhibition over time takes on added significance when considering that social withdrawal in adolescence, with all of the social pressures associated with this age period, places an individual at risk for high levels of trait anxiety and clinical diagnoses at later points in life (Chronis-Tuscano et al., in press; Hirshfeld et al., 1992; Pine, Helfinstein, Bar-Haim, Nelson, & Fox, 2009). Both extreme temperamental withdrawal and clinical anxiety can have long-lasting developmental consequences (Caspi, Bem, & Elder Jr, 1988; Caspi et al., 2003; Pine, Cohen, Gurley, Brook, & Ma, 1998). To date, no studies have directly examined the role of attention in moderating early childhood temperament over time. In particular, studies are needed that probe the orienting component of attention given evidence linking threat-related orienting biases to anxiety, social withdrawal, and perturbed limbic circuitry overly-sensitive to perceived threat (Bar-Haim et al., 2007; Monk et al., 2008).
The behavioral inhibition—attention bias link could take on a number of forms. The effect of behavioral inhibition on social withdrawal could be carried forward via its impact on patterns of attention bias. In this case any functional relation between early behavioral inhibition and later social withdrawal would fall away when attention bias is taken into account. Alternately, variations in the pattern of attention bias could modify the relation between behavioral inhibition and social withdrawal. Attention bias to threat would then ‘intensify’ underlying temperamental biases, increasing the likelihood of social withdrawal in adolescence.
The current study was designed to examine this issue, posing three questions: (1) Do adolescents with a childhood history of behavioral inhibition show perturbations in attention orienting bias to threat? (2) Are these attention biases linked to individual differences in social withdrawal among adolescents with a history of behavioral inhibition? (3) If linked, does the relation take the form of mediation (behavioral inhibition affects social withdrawal via it affect on attention biases) or moderation (behavioral inhibition and attention bias interact to shape levels of social withdrawal)?
We used the dot-probe task (MacLeod, Mathews, & Tata, 1986; Mogg, Philippot, & Bradley, 2004), a standard measure of attention orienting, to assess attention biases in adolescents with a history of behavioral inhibition. The dot-probe task presented participants with two faces side-by-side (one emotionally evocative and one neutral). The faces were then followed by a target (an arrow pointing up or down) appearing at the location of one of the faces. Participants were asked to press one of two buttons to indicate the direction of the target arrow. On half the trials the emotionally evocative face was congruent with the target. On the other half of trials the face and target were in incongruent locations.
By examining reaction time (RT) patterns based on the spatial relations between the emotion stimuli and the target, we inferred the pattern and magnitude of attention biases to the emotion cues. If an individual was faster to respond when the target probe appeared in the spatial location of the threat cue (congruent) versus when the target probe appeared at the location of the neutral cue (incongruent), we inferred a bias toward threat. The opposite pattern indicated an avoidance of threat. Both the large adult literature and the more modest pediatric literature suggest a stable link between anxiety and attention biases to threat (Bar-Haim et al., 2007). However, no studies to date have reported on attentional threat biases in adolescents marked by early behavioral inhibition.
The current study examined adolescents (mean age 15 years) who were identified in infancy for temperamental reactivity. We noted levels of behavioral inhibition as we followed the cohort through childhood and assessed their social responsivity to unfamiliar adults and same age peers. In adolescence, we assessed their attention biases to both threatening (angry faces) and positive (happy faces) stimuli. Attention patterns to positive stimuli was assessed because recent work suggests that both behavioral inhibition and anxiety may be linked to perturbations in processing positive and negative stimuli (Bar-Haim et al., in press; Frenkel, Lamy, Algom, & Bar-Haim, 2008; Guyer et al., 2006; Pérez-Edgar et al., 2007).
We also employed two face presentation times (500 ms and 1500 ms) to examine the chronometry of the attention bias as studies suggest that attention mechanisms underlying observed bias patterns are engaged very early in processing, but that the pattern of bias (towards or away from threat) can shift with prolonged exposure (Heim-Dreger, Kohlmann, & Eschenbeck, 2006; Mogg, Bradley, Miles, & Dixon, 2004; Monk et al., 2006; Monk et al., 2008).
Lastly, to examine the role attention biases may play in social functioning, we asked parents to assess their adolescents for signs of social withdrawal. This outcome measure and reporting source was chosen for two reasons. First, social withdrawal is tightly linked to temperamental antecedents and thus provides the strongest theoretical context in which to extend the developmental trajectory between early behavioral inhibition and later socioemotional functioning. In contrast, characterizing anxiety in this sample would have added a second, potentially conceptually distinct, construct to the analyses. Second, when faced with low levels of agreement across parent- and self-report measures of internalizing difficulties, parent-report has often proven to be the more reliable source of information (Edelbrock, Costello, Dulcan, & Conover, 1986; Kolko & Kazdin, 1993; Salbach-Andrae, Lenza, & Lehmkuhl, in press).
In summary, this study examined the relations between early behavioral inhibition and patterns of attention bias to threat in adolescence, as well as the potential impact of these two factors on adolescent social withdrawal. We hypothesized that (1) adolescents who were behaviorally inhibited in childhood would show heightened attention bias to threatening stimuli, (2) this bias would be most evident after short exposure to the threat cues, and (3) bias levels to threat would affect the relation between childhood behavioral inhibition and adolescent social withdrawal. To address this third point we employed a moderated mediation model (Preacher, Rucker, & Hayes, 2007) allowing us to compare two statistical models (mediation vs. moderation) directly within the same analysis.
Methods
Participants
Participants (N=153, 73 male) were assessed during mid-adolescence (Mean=15.06 years, SD=1.05) as part of a larger longitudinal study of temperament and socioemotional development (Fox et al., 2001; Fox et al., 1995). A large community sample was initially screened for levels of reactivity at 4 months of age. Three groups were chosen for the study due to extreme profiles: Negative reactivity (N=56), positive reactivity (N=45), low reactivity (N=52). Subsequent assessments (see below) focused on emerging patterns of BI in the full cohort. The cohort was Caucasian from generally middle to upper-middle class families in the greater Washington DC metropolitan area.
138 of the 153 adolescents completed the dot-probe task. Fifteen adolescents did not provide data due to either mechanical (N=4) or scheduling difficulties (N=11). There were no significant differences between the participants who completed the task and those who did not on measures of behavioral inhibition, age, or gender (p's > 0.30).
An additional 12 subjects were excluded from the final analyses due to poor performance on the task (less than 63% accuracy, N=3) or missing data that prevented creating a temperament composite score (N=9). The excluded participants did not differ from the larger sample on age or gender (p's > 0.49). The final sample included 126 participants (61 male, Mean=15.04 years, SD=0.97).
As part of the larger longitudinal study, 121 of the adolescents in this sample were clinically assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSADS; Kaufman et al., 1997; Orvaschel, Lewinsohn, & Seeley, 1995), a semi-structured diagnostic interview assessing DSM-IV disorders. Of these, 20% had either a current (N=16) or lifetime (N=8) presence of social anxiety disorder. Although the relatively small number of cases precluded us from including diagnostic history as a full factor in our analyses, we did look to see if the pattern of findings noted below changed when excluding the affected adolescents. The findings remained significant (p's < 0.04); as such, the findings are presented for the full sample.
The Institutional Review Board at the University of Maryland, College Park, approved all procedures and adolescents were compensated for their participation.
Behavioral Inhibition Composite Score
Individual differences in early childhood temperament were assessed via a composite measure based on laboratory observations and maternal reports of behavior at four time points (14 months, 24 months, 4 years, and 7 years). Laboratory observations employed Kagan's (Calkins, Fox, & Marshall, 1996; Kagan et al., 1988) protocol at 14 and 24 months, presenting the children with novel and unfamiliar objects and people. At ages 4 and 7, children's reticent behavior with unfamiliar peers was noted using the Play Observation Scale (Rubin, 1989). Maternal ratings of social fear were collected at 14 and 24 months with the Toddler Behavior Assessment Questionnaire (TBAQ; Goldsmith, 1996). Mothers rated shyness at ages 4 and 7 with the Colorado Child Temperament Inventory (CCTI; Rowe & Plomin, 1977).
To capture a broad measure of behavioral inhibition, we created a composite score using both behavioral observation and maternal report data at each of the four assessment points. Individual scores were standardized and then averaged to create a single measure (Cronbach's alpha =0.83) with higher scores reflecting higher levels of inhibition (Full sample: Mean=0.019, SD=0.60; Current sample: Mean=-0.002, SD=0.58; p=0.20).
Dot-Probe Task
The dot-probe task consisted of 16 practice and 160 experimental trials, which were presented in two blocks. There were three pseudo-random orders for the individual trials and participants were randomly assigned to one of the three orders.
Each trial began with a central fixation cross for 500 ms. A face pair was then shown for either 500 or 1500 ms. Studies suggest that as the presentation times lengthen from moderate (500 ms) to prolonged (e.g., 1500 ms) exposures, response patterns shift from bias toward threat to avoidance of threat (Mogg, Bradley et al., 2004). However, this prolonged exposure variant of the dot-probe task is less studied and has produced less consistent findings than with stimulus exposures of 500 ms or less. We used the unique opportunity presented by the present sample to directly compare attention patterns to both moderate and prolonged exposure to threat.
The emotional and neutral face pictures were each 11.1 cm × 8.9 cm and were presented side-by-side, with a distance of 20.1 cm between their centers. Immediately after a face pair disappeared, a small white arrow appeared for 500 ms in the location just occupied by one of the face pictures. Participants pressed one of two response keys to indicate whether the arrow pointed up or down. The inter-trial interval varied randomly at either 1900 or 2900 ms.
Participants were seated 210 cm from the screen so that the stimuli were at 5.2 degrees of visual angle. Stimuli were presented on a NANAO FlexScan 550i monitor and presentation was controlled by the STIM stimulus presentation system from the James Long Company (Caroga Lake, NY). There were three types of face pairs presented: Angry/Neutral (64 trials), Happy/Neutral (64 trials), and Neutral/Neutral (32 trials). There were 16 male and 16 female faces used from the NimStim face stimulus set (Tottenham et al., 2009) and each face was seen five times. Trials were designated as congruent if the arrow appeared in the same location as the affective face (i.e., Angry or Happy) and incongruent if appearing in the location of the neutral face. The task was designed so that face sex, trial congruency, probe presentation time (500/1500 ms), probe direction (up/down), and probe location (right/left) were counterbalanced throughout the 160 test trials.
Reaction times (RTs) and response errors were collected for each trial.
Social Withdrawal in Adolescence
At the time of the adolescent assessment parents completed the Child Behavior Checklist (CBCL) for 6 to 18-year-olds (Achenbach, 2001). The CBCL is a 118-item measure that asks parents to rate how descriptive a series of behavior problems are of their own child. The CBCL yields eight narrow-band factors and the current study focused on the ‘Withdrawn’ factor. This scale is a reliable index of social behavior in children (Achenbach, Edelbrock, & Howell, 1987) and reflects many of the concerns associated with temperamentally withdrawn behavior (Degnan & Fox, 2007; Pérez-Edgar & Fox, 2005b). It is also more narrowly focused in scope than alternate scales, such as anxious/depressed. Indeed, parallel analyses with this scale were in line with the data below, but did not reach statistical significance, reflecting the larger variance in scores and the broader range of concerns addressed.
Data were available for 115 of the 126 adolescents in the sample. Here we present the results from analyses using the raw data from the CBCL in order to have a full range of scores. We also carried out the full models using T-scores and found the same pattern of results (p's<0.04 for the significant findings below). At the assessment, 25 (21.7%, 13 male) adolescents met the borderline cut-off with T-scores greater than or equal to 60. Of these, 4 (all males) had scores greater than or equal to 70, the clinical cutoff.
Statistical Analyses
Trials with incorrect responses or RTs less than 200 ms were removed before analyses began. As in previous dot-probe studies (Bradley, Mogg, White, Groom, & de Bono, 1999; Mogg, Bradley et al., 2004), analyses focused on the relative bias patterns evident across the emotional (Angry or Happy) faces. Bias scores were calculated by subtracting the mean RT when the arrow replaced the emotion face (congruent trials) from the mean RT when the arrow replaced the neutral face (incongruent trials). Positive values indicate vigilance for the emotion stimuli and negative scores indicate avoidance of the emotion stimuli. The Neutral-Neutral trials served as ‘catch-trials’ during testing to help insure that adolescents did not come to expect or seek out an emotion face in every trial. This helped keep the focus on the location and direction of the arrow cue. Reaction times to the Neutral-Neutral trials were not used in the calculation of the attention bias scores.
A 2 × 2 × 2 × 2 repeated measures ANOVA was used to examine bias patterns as a function of BI group (Median split: Low vs. High), face emotion (Angry vs. Happy), presentation time (500 ms vs. 1500 ms), and sex (Male vs. Female). Sex was included as a between-subjects factor based on previous data suggesting that the interaction between BI and behavior may differ in boys and girls (Fox et al., 2005; Henderson et al., 2001; Pérez-Edgar et al., 2008). Post-hoc independent-sample and one-sample t-tests were used to assess the pattern of attention biases.
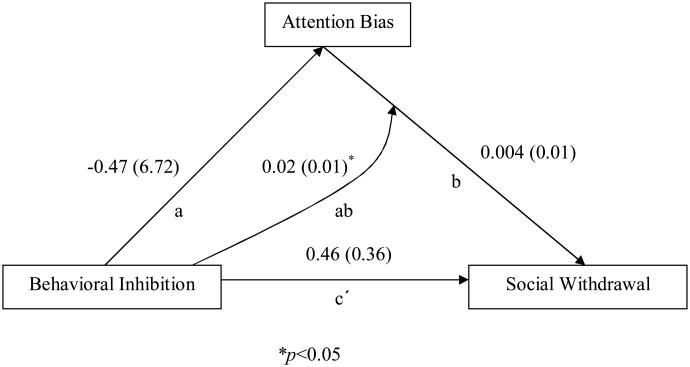
The potential role of attention biases in the relation between early temperament to social withdrawal in adolescence was then evaluated using a moderated mediation model based on the work of Preacher, Rucker and Hayes (2007). The standard approach (Baron & Kenny, 1986) for testing mediation requires three linear models to estimate (1) the relation between behavioral inhibition and social withdrawal (parameter c), (2) the relation between attention bias and social withdrawal (parameter b), (3) the relation between behavioral inhibition and attention bias (parameter a), and finally, (4) the residualized effect between behavioral inhibition and social withdrawal (parameter c′). All paths must be significantly different from zero for mediation to be possible. In a moderated mediation model, one can see if attention biases moderate the relation between behavioral inhibition and social withdrawal (parameter ab). In this instance, moderation is present when the relation between behavioral inhibition and social withdrawal varies as a function of attention bias. Given the constraints of the model, we ran the analyses separately for each of the four face (Angry vs. Happy) by presentation time (500 ms vs. 1500ms) combinations. See Figure 2 for a graphical presentation of the analyses for attention bias to angry faces at 500 ms presentation.
Figure 2.
Path results for the moderated mediation model involving attention biases to threat at 500 ms presentation. Noted are the effect coefficients with standard errors in parentheses.
Results
Relation between Behavioral Inhibition and Attention Biases to Emotion Faces
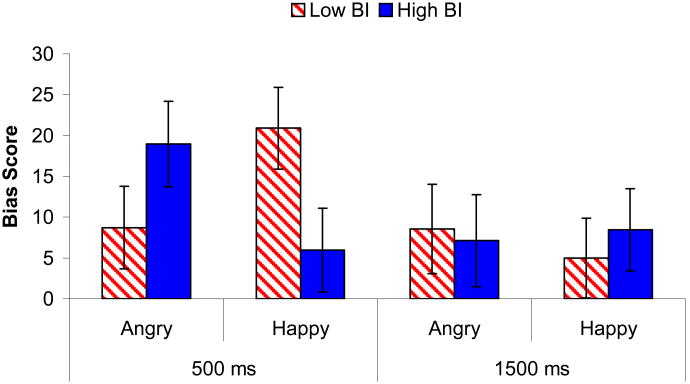
An initial repeated-measures ANOVA found a significant emotion by BI by presentation time interaction, F(1, 122)=3.78, p=0.05, f=0.18 (see Figure 1; Table 1). To examine the three-way interaction, two separate ANOVAs were completed for the 500 ms and 1500 ms presentation times, using a 2 (Emotion) × 2 (BI) × 2 (Sex) design.
Figure 1.
Bias scores for the BI groups for the Angry and Happy face stimuli at the 500 ms and 1500 ms presentation times. Mean scores reflect the magnitude of vigilance for the emotion presented.
Table 1.
Relations between BI and the central measures of the study. BI was assessed over the course of early childhood, while attention biases and social withdrawal were measured in adolescence. Mean scores are given for the full sample as well as for the BI groups created by median split of the composite score (standard deviations in parentheses).
| Overall | Low BI | High BI | |
|---|---|---|---|
| Angry Bias (500 ms) | 13.59 | 8.68 | 18.83 |
| (41.05) | (38.19) | (43.59) | |
|
| |||
| Happy Bias (500 ms) | 13.53 | 20.73* | 5.86* |
| (40.61) | (42.48) | (37.35) | |
|
| |||
| Angry Bias (1500 ms) | 7.72 | 8.23 | 7.17 |
| (44.05) | (47.76) | (40.10) | |
|
| |||
| Happy Bias (1500 ms) | 6.74 | 5.11 | 8.47 |
| (38.90) | (35.39) | (42.54) | |
|
| |||
| Social Withdrawal | 54.87 | 54.08 | 55.79 |
| (6.07) | (5.88) | (6.22) | |
p<0.05
For the 500 ms presentation, the Emotion by BI interaction was significant, F(1, 122)=5.92, p=0.02, f=0.22. Post-hoc independent-sample t-tests found that the two groups did not differ significantly in the level of bias to angry faces, t(124)=-1.39, p=0.17, d=0.25. However, the adolescents high in BI had significantly smaller bias scores to happy faces, t(124)=2.08, p=0.04, d=0.37.
Specificity of the bias scores was examined with one-sample t-tests. Adolescents who showed high levels of BI in childhood produced bias scores that were significantly greater than zero for the angry faces, t(60)=3.37, p=0.001, d=0.87, but not the happy faces, t(60)=1.23, p=0.23, d=0.32. In contrast, the adolescents who were low in BI showed only a trend for a bias to angry faces, t(64)=1.83, p=0.07, d=0.46, but significant vigilance for happy faces, t(64)=3.94, p=0.001, d=0.98.
For the 1500 ms presentation, there were no significant main or interaction effects.
The relation between Behavioral Inhibition, Attention Biases, and Social Withdrawal
Figure 2 presents the results of the moderated mediator analysis for the angry faces at 500 ms. The results for all four analyses are presented in Table 2.
Table 2.
Predicting Social Withdrawal in adolescence using measures of early temperament (BI composite) and attention bias to emotional stimuli (Angry and Happy faces) in adolescence. The table presents the path coefficients (standard errors) and t-values for the separate moderated mediation models. BI-AB: Relation between BI and attention bias score; AB-SW: Relation between attention bias score and social withdrawal; BI-SW: Residualized effect of BI on social withdrawal; BI × AB-SW: The interaction of BI and attention bias on social withdrawal.
| BI-AB | AB-SW | BI-SW | BI × AB-SW | |||||
|---|---|---|---|---|---|---|---|---|
| a | b | c′ | ab | |||||
|
| ||||||||
| β (SE) | t | β (SE) | t | β (SE) | t | β (SE) | t | |
| Angry Faces | -0.465 | -0.07 | 0.004 | 0.74 | 0.459 | 1.27 | 0.023 | 2.33* |
| 500ms | (6.721) | (0.005) | (0.361) | (0.010) | ||||
|
| ||||||||
| Happy Faces | -6.76 | -1.05 | 0.006 | 1.01 | 0.345 | 0.83 | 0.016 | 1.47 |
| 500 ms | (6.45) | (0.006) | (0.414) | (0.011) | ||||
|
| ||||||||
| Angry Faces | 6.115 | 0.855 | 0.001 | 0.27 | 0.496 | 1.28 | 0.007 | 0.88 |
| 1500ms | (7.14) | (0.005) | (0.388) | (0.008) | ||||
|
| ||||||||
| Happy Faces | 1.82 | 0.29 | -0.001 | -0.25 | 0.519 | 1.35 | 0.006 | 0.82 |
| 1500 ms | (6.26) | (0.006) | (0.384) | (0.008) | ||||
p<0.05
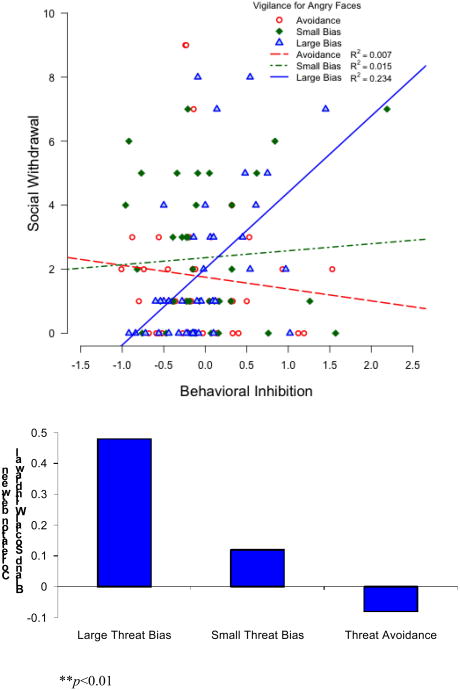
For bias to angry faces at 500 ms, none of the direct or mediated path analyses reached statistical cut-offs. However, the moderation path found a significant behavioral inhibition by attention bias interaction. Here, the effect was estimated at 0.0228 with a standard error estimate of 0.0098 (t=2.33, p=0.02). To interpret this interaction, the adolescents were divided into terciles based on the magnitude of their attention bias to threat: no bias or avoidance (Mean= −30.94 ms, SD=24.81), small bias (Mean= 13.98 ms, SD=10.03) and large bias (Mean= 57.74, SD=19.81). The correlations between early behavioral inhibition and adolescent social withdrawal were then calculated separately for each group (See Figure 3). For the children with a large bias to threat, there was a significant positive correlation between behavioral inhibition and social withdrawal, r(39) = 0.48, p = 0.002. The relation was not evident for children with a small bias, r(38) = 0.12, p = 0.47, or avoidance, r(38) = -0.08, p = 0.62. Fisher's Z-scores indicated that the correlations were significantly different between the large bias and avoidance groups, Z = -2.58, p = 0.01, and trended in the same direction for the small bias group, Z = -1.71, p = 0.09.
Figure 3.
Relation between BI in early childhood and social withdrawal in adolescence as a function of attention patterns to threat. Adolescents are grouped based on terciles of bias scores to angry faces at the 500 ms presentation time.
For the remaining models, none of the direct or mediated path analyses reached statistical cut offs. The interaction effects between behavioral inhibition and attention biases were also non-significant.
Discussion
The current study provides two important additions to our understanding of the mechanisms underlying the stability of temperament from behavioral inhibition in childhood to social withdrawal in adolescence. First, the data show that adolescents with a history of behavioral inhibition show heightened levels of attention bias to threat, while their non-inhibited counterparts displayed a bias toward positive (happy) stimuli. To the best of our knowledge, this is the first study to demonstrate the presence of this attention bias, manifest during adolescence, within a prospectively followed, non-clinical sample selected for a temperamental trait in infancy and childhood. Second, the study finds that the relation between the early-childhood temperament of behavioral inhibition and the adolescent construct of social withdrawal is moderated by the magnitude and direction of attention bias to socially salient emotional stimuli (faces). Namely, early behavioral inhibition is linked to adolescent social withdrawal only among those adolescents who also manifest attention biases to threat. These data add to the growing evidence that perturbations in attention orienting towards emotion-laden social stimuli may play an important role in shaping the trajectory of social behavior into adolescence and may help explain the link between early temperament, social withdrawal, and anxiety.
These findings are in line with data indicating that attention biases may play a causal role in an individual's vulnerability to stress. For example, McLeod and colleagues (MacLeod, Campbell, Rutherford, & Wilson, 2004; MacLeod et al., 2002; See, MacLeod, & Bridle, 2009) trained non-anxious subjects using a dot-probe task to adopt an attention bias to threat. Subsequent to this training, subjects were assessed in a stress inducing procedure. Those previously trained to threat were more likely to report heightened distress and negative affect compared to those who received a placebo condition not intended to alter attention patterns. In a recent extension of this work, Eldar and colleagues (Eldar et al., 2008) completed a similar study with 7- to 13-year-old children, finding again that those children trained to attend to threat exhibited heightened stress reactivity in a subsequent stressor task. These data suggest that biases in orienting to threat may play a critical role in an individual's reactivity to uncertainty, novelty, and stress. When coupled with a temperamental proclivity to a heightened response to novelty and stress, a child may be more ‘resistant’ to the ameliorating influences either in the environment (e.g., sensitive parenting) or within the child (e.g., more efficient self-regulation mechanisms) that lead most behaviorally inhibited children to shed their extreme discomfort with social interactions.
Our data form a link to the findings on attention bias in clinically anxious and high trait-anxiety samples, demonstrating a similar pattern in temperamentally extreme children. Indeed, the magnitude of the bias to threat in this sample was comparable to the bias found in the most comprehensive study to date of clinically anxious adolescents (Krain Roy et al., 2008). Although childhood temperament can shape behavior across a wide range of contexts, these tendencies are by no means immutable. While a significant minority of children identified with behavioral inhibition may develop anxiety disorders (Chronis-Tuscano et al., in press), most do not (Degnan & Fox, 2007). Thus, one important goal is to identify specific mechanisms that are involved in moderating childhood temperament towards or away from adaptive social outcomes. The present findings suggest that attentional components, particularly orienting toward threat, may be important candidate mechanisms.
Individual differences in attention may go hand-in-hand with a child's initial temperamental disposition to heightened reactivity to novelty. Such a disposition and concomitant perturbations in attention are evident as early as the first years of life. As mentioned above, temperamentally reactive nine-month-olds were more likely to exhibit greater mismatch negativity between novel and standard tones compared to non-reactive infants (Marshall et al., 2009). Such differences in attention to novelty may bias a child's attention to aspects of the environment, including social interactions that are novel. This selection process may subsequently define processing and evaluation of the environment, as well as any accompanying behavioral responses. One can then imagine a self-reinforcing cycle in which a behaviorally inhibited child notes a threat in his environment, presumes a potential danger, and then withdraws. This withdrawal limits his interaction with the environment and further predisposes him to detect and attend to threat (Fox et al., 2007).
Alternately, attention bias may be the initial predisposing factor that places a child at increased risk for social withdrawal. Bishop (2008) has shown that trait anxious individuals have difficulty engaging the dorsolateral prefrontal cortex (DLPFC) even when responding to neutral stimuli under conditions with a low perceptual load. This places the individual at risk for interruption and intrusion from non-relevant stimuli, echoing recent work showing that individual differences in sustained attention in infancy are linked to increasing levels of social withdrawal in childhood despite comparable levels in initial negative reactivity (Pérez-Edgar, McDermott, Pine, & Fox, 2009). A processing style (Bishop, 2008) marked by increased levels of vigilance and an attention bias to threat may be a root cause of social withdrawal and anxiety in children (Eldar et al., 2008) and adults (Amir, Beard, Burns, & Bomyea, 2009).
Most interventions with temperamentally at-risk children have focused on social skill training (Rapee, Kennedy, Ingram, Edwards, & Sweeney, 2005). However, these data provide initial suggestions that targeting attention biases may prove effective in shaping broad patterns of behavior. Indeed, attention training paradigms can both exacerbate (Eldar et al., 2008) and ameliorate (N. Schmidt, Richey, Buckner, & Timpano, 2009) pre-existing patterns of attention biases and subsequent stress sensitivity. Given the interaction patterns presented here, attention training paradigms may also prove to be an effective and efficient intervention tool for temperamentally at risk children.
The focus need not be exclusively on threat stimuli. In this sample, the non-inhibited adolescents displayed a large attention bias toward happy faces. Recent work shows that biases to happy stimuli may aid emotion regulation under stress (Frenkel et al., 2008; Wadlinger & Isaacowitz, 2008), suggesting that this may help protect children from negative developmental outcomes. A bias toward positive affect, rather than simply the absence of a threat bias, may act as an active mechanism for buffering early dispositions towards maladaptive social outcomes.
Our findings were limited to the 500 ms presentation of the emotion faces. This is in line with previous work finding peak threat biases in this range (Mogg, Bradley et al., 2004). Although only a few studies have systematically manipulated presentation times within a single task, the consensus across studies is that exposure time plays an important role in the form and magnitude of attention biases observed in the laboratory (Bar-Haim et al., 2007). At relatively long presentation times, attention biases to threat are often non-evident or reversed such that individuals now avoid threat. In these cases, it may be that any initial attention biases are colored by subsequent processing and responses, including inhibition of return (Bar-Haim et al., 2007). Clearly, the attention biases noted in the current study are part of a larger, ongoing, evaluative process that will be evident in different forms at specific points in a task's time line.
The current paper's limitations include the fact that attention biases to threat were assessed at only one time point. As such, we could not employ statistical tools, such as structural equation modeling, that would have allowed us to note how changes in socioemotional adjustment over time relate to observed patterns of attention bias. A larger sample size would also have aided in this effort. Second, since attention biases were noted in adolescence, and not in early childhood, we cannot address the developmental timing of these mechanisms.
In conclusion, the current data illustrate a potential mechanism for the relation between early temperament and later social maladjustment. Future studies will need to take a prospective, longitudinal, approach that can track these mechanisms over time. The coupling between attention and emotion mechanisms may take place over development as the motivational style marked by behavioral inhibition ‘pushes’ the attention system to favor signs of threat in the environment, biasing subsequent processing. Alternately, attention systems characterized by vigilance may back propagate and sensitize the amygdala. This sensitization would then be manifest in social withdrawal. While we cannot directly assess these alternative pathways, the current study is among the first to examine this relation from a nonclinical, but at-risk, perspective. These data show that children with a history of behavioral inhibition show bias patterns that mirror biases seen in anxious children and adults. In addition, early behavioral inhibition, when coupled with attention biases to threat, is linked to sustained levels of social withdrawal in adolescence. These data point to a potentially important mechanism that sustains early underlying temperamental traits and may increase the risk for the later emergence of clinical anxiety.
Acknowledgments
The authors would like to thank Kenneth H. Rubin and Amy Kennedy for the coding and analysis of the peer interaction data at ages 4 and 7, Patrick McKnight for his suggestions for the statistical analyses, and Chris A. Monk for his invaluable help with data presentation. We would also like to thank Stacey Barton, Melissa Ghera, Dalit H. Marshall, Kirsten VanMeenen, Ariana Shahinfar, Genevieve Erb, Patricia Peters, Shari K. Young, and Lisa Perry for their assistance in the longitudinal data collection. We would especially like to thank the parents of the children who participated and continue to participate in our studies. Funding for the study was provided by grants from the NIMH (MH073569) to Koraly Pérez-Edgar and the NIH (MH074454; HD17899) and NARSAD Foundation (Distinguished Investigator Award) to Nathan A. Fox.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/emo.
References
- Achenbach TM. Child behavior checklist for ages 6 to 18. Burlington: University of Vermont, Research Center for Children, Youth, and Familieso; 2001. Document Number. [Google Scholar]
- Achenbach TM, Edelbrock C, Howell CT. Empirically based assessment of the behavioral/emotional problems of 2- and 3-year-old children. Journal of Abnormal and Child Psychology. 1987;15:629–650. doi: 10.1007/BF00917246. [DOI] [PubMed] [Google Scholar]
- Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with Generalized Anxiety Disorder. Journal of Abnormal Psychology. 2009;109:28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Benson B, Guyer AE, Pérez-Edgar K, Williams A, Nelson EE, et al. Neural correlates of reward processing in adolescents with a history of shyness and inhibited temperament. Psychological Science. doi: 10.1111/j.1467-9280.2009.02401.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg M, van IJzendoorn M. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2008;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. British Journal of Clinical Psychology. 1999;38:267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Caspi A, Bem DJ, Elder G., Jr Moving away from the world: Life-course patterns of shy children. Developmental Psychology. 1988;24(6):824–831. [Google Scholar]
- Caspi A, Harrington H, Milne B, Amell JW, Theodore RF, Moffitt TE. Children's behavioral styles at age 3 are linked to their adult personality traits at age 26. Journal of Personality and Social Psychology. 2003;71:495–513. doi: 10.1111/1467-6494.7104001. [DOI] [PubMed] [Google Scholar]
- Caspi A, Silva P. Temperamental qualities at age 3 predict personality traits in young adulthood: Longitudinal evidence from a birth cohort. Child Development. 1995;66:486–498. doi: 10.1111/j.1467-8624.1995.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan K, Pine D, Perez-Edgar K, Henderson H, Diaz Y, et al. Stable behavioral inhibition during infancy and early childhood predicts the development of anxiety disorders in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. doi: 10.1097/CHI.0b013e3181ae09df. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan RJ, Girardi A, Findlay LC, Frohlick SL. Understanding solitude: Young children's attitudes and responses toward hypothetical socially withdrawn peers. Social Development. 2007;16:390–409. [Google Scholar]
- Coplan RJ, Rubin KH, Fox NA, Calkins SD, Stewart SL. Being alone, playing alone, and acting alone: distinguishing among reticence and passive and active solitude in young children. Child Development. 1994;65:129–137. [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Degnan K, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19:729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Edelbrock C, Costello A, Dulcan M, Conover N. Parent-child agreement on child psychiatric symptoms assessed via structured interview. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1986;27:181–190. [PubMed] [Google Scholar]
- Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: Implications for stress response in children. Behaviour Research & Therapy. 2008;46:450–461. doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Fox NA, Hane AA, Pine DS. Plasticity for affective neurocircuitry: How the environment affects gene expression. Current Directions in Psychological Science. 2007;16:1–5. [Google Scholar]
- Fox NA, Henderson HA, Pérez-Edgar K, White L. The biology of temperament: An integrative approach. In: Nelson C, Luciana M, editors. The Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2008. pp. 839–854. [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin KH, Schmidt LA, Hamer DH, et al. Evidence for a gene environment interaction in predicting behavioral inhibition in middle childhood. Psychological Science. 2005;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, et al. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1771–1784. [PubMed] [Google Scholar]
- Fox NA, Schmidt LA, Calkins SD, Rubin KH, Coplan RJ. The role of frontal activation in the regulation and dysregulation of social behavior during the preschool years. Development and Psychopathology. 1996;8:89–102. [Google Scholar]
- Frenkel TI, Lamy D, Algom D, Bar-Haim Y. Individual differences in perceptual sensitivity and response bias in anxiety: evidence from emotional faces. Cognition and Emotion. 2008;22:1–13. [Google Scholar]
- Goldsmith HH. Studying temperament via construction of the toddler behavior assessment questionnaire. Child Development. 1996;67:218–235. [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim-Dreger U, Kohlmann CW, Eschenbeck H. Attentional biases for threatening faces in children: Vigilant and avoidant processes. Emotion. 2006;6:320–325. doi: 10.1037/1528-3542.6.2.320. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Fox NA, Rubin KH. Temperamental contributions to social behavior: The moderating roles of frontal EEG asymmetry and gender. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(1):68–74. doi: 10.1097/00004583-200101000-00018. [DOI] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, et al. Stable behavioral inhibition and its association with anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Kagan J. Temperamental contributions to affective and behavioral profiles in childhood. In: Holman SG, DiBartolo PM, editors. From Social Anxiety to Social Phobia: Multiple Perspectives. Needham Heights, MA, US: Allyn & Bacon; 2001. pp. 216–234. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Annual Progress in Child Psychiatry & Child Development. 1988:102–127. [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kolko DJ, Kazdin A. Emotional/Behavioral Problems in Clinic and Nonclinic Children: Correspondence Among Child, Parent and Teacher Reports. Journal of Child Psychology and Psychiatry. 1993;34:991–1006. doi: 10.1111/j.1469-7610.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Krain Roy A, Vasa RA, Bruck M, Mogg K, Bradley BP, Sweeney M, et al. Attention bias toward threat in pediatric anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Campbell L, Rutherford EM, Wilson EJ. The causal status of anxiety-linked attentional and interpretive bias. In: Yiend J, editor. Cognition, emotion and psychopathology: Theoretical, empirical and clinical directions. New York, NY: Cambridge University Press; 2004. pp. 172–189. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107–123. [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Developmental Science. 2009;12:568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition and Emotion. 2002;16:331–354. [Google Scholar]
- McDermott JM, Pérez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox N. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18(5):689–700. [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113(1):160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, et al. Ventrolateral prefrontal cortex activation and attention bias in responsive to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvaschel H, Lewinsohn P, Seeley J. Continuity of psychopathology in a community sample of adolescents. Journal of the American Academy of Chile and Adolescent Psychiatry. 1995;34:1525–1535. doi: 10.1097/00004583-199511000-00020. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. A behavioral and electrophysiological study of children's selective attention under neutral and affective conditions. Journal of Cognition and Development. 2005a;6(1):89–118. [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005b;14:681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperamental contributions to children's performance in an emotion-word processing task: A behavioral and electrophysiological study. Brain & Cognition. 2007;65:22–35. doi: 10.1016/j.bandc.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, McDermott JM, Pine DS, Fox NA. Gene-by-Attention Interaction in the Emergence of Childhood Anxiety; Paper presented at the Anxiety Disorders Association of America Annual Meeting; Santa Ana Pueblo, New Mexico. 2009. Mar, [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Schmidt LA, Henderson HA, Schulkin J, Fox NA. Salivary cortisol levels and infant temperament shape developmental trajectories in boys at risk for behavioral maladjustment. Psychoneuroendocrinology. 2008;33:916–925. doi: 10.1016/j.psyneuen.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, Fox NA. Challenges in developing novel treatments for childhood disorders: Lessons from research on anxiety. Neuropsychopharmacology. 2009;34:213–228. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Kennedy S, Ingram M, Edwards S, Sweeney L. Prevention and early intervention of anxiety disorders in inhibited preschool children. Journal of Consulting and Clinical Psychology. 2005;73:488–497. doi: 10.1037/0022-006X.73.3.488. [DOI] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Helfinstein SM, Degnan KA, Pérez-Edgar K, Henderson HA, Lissek S, et al. Startle modulation in behaviorally inhibited adolescents with a lifetime occurrence of anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:610–617. doi: 10.1097/CHI.0b013e31819f70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI. Temperament, attention, and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Handbook of developmental psychopathology. 2nd. Vol. 2. Hoboken, NJ: John Wiley & Sons; 2006. pp. 465–501. [Google Scholar]
- Rowe DC, Plomin R. Temperament in early childhood. Journal of Personality Assessment. 1977;41:150–156. doi: 10.1207/s15327752jpa4102_5. [DOI] [PubMed] [Google Scholar]
- Rubin KH. The Play Observation Scale (POS) University of Waterloo; 1989. [Google Scholar]
- Ruff H, Rothbart MK. Attention in early development: Themes and variations. Oxford: Oxford Press; 1996. [Google Scholar]
- Salbach-Andrae H, Lenza K, Lehmkuhl U. Patterns of agreement among parent, teacher and youth ratings in a referred sample. European Psychiatry. doi: 10.1016/j.eurpsy.2008.07.008. in press. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC, et al. Behavioral and neuroendocrine responses in shy children. Developmental Psychobiology. 1997;30(2):127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology. 1999;35:119–135. [PubMed] [Google Scholar]
- Schmidt N, Richey J, Buckner J, Timpano K. Attention training for Generalized Social Anxiety Disorder. Journal of Abnormal Psychology. 2009;118:5–14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Early temperament predictors of Stroop interference to threatening information at adolescence. Journal of Anxiety Disorders. 1996;10(2):89–96. [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants grown up: Adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- See J, MacLeod C, Bridle R. The reduction of anxiety vulnerability through the modification of attentional bias: A real-world study using a home-based cognitive bias modification procedure. Journal of Abnormal Psychology. 2009;109:65–75. doi: 10.1037/a0014377. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon A, McCarry T, Nurse M, Hare T, et al. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Looking happy: The experimental manipulation of a positive visual attention bias. Emotion. 2008;8:121–126. doi: 10.1037/1528-3542.8.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]