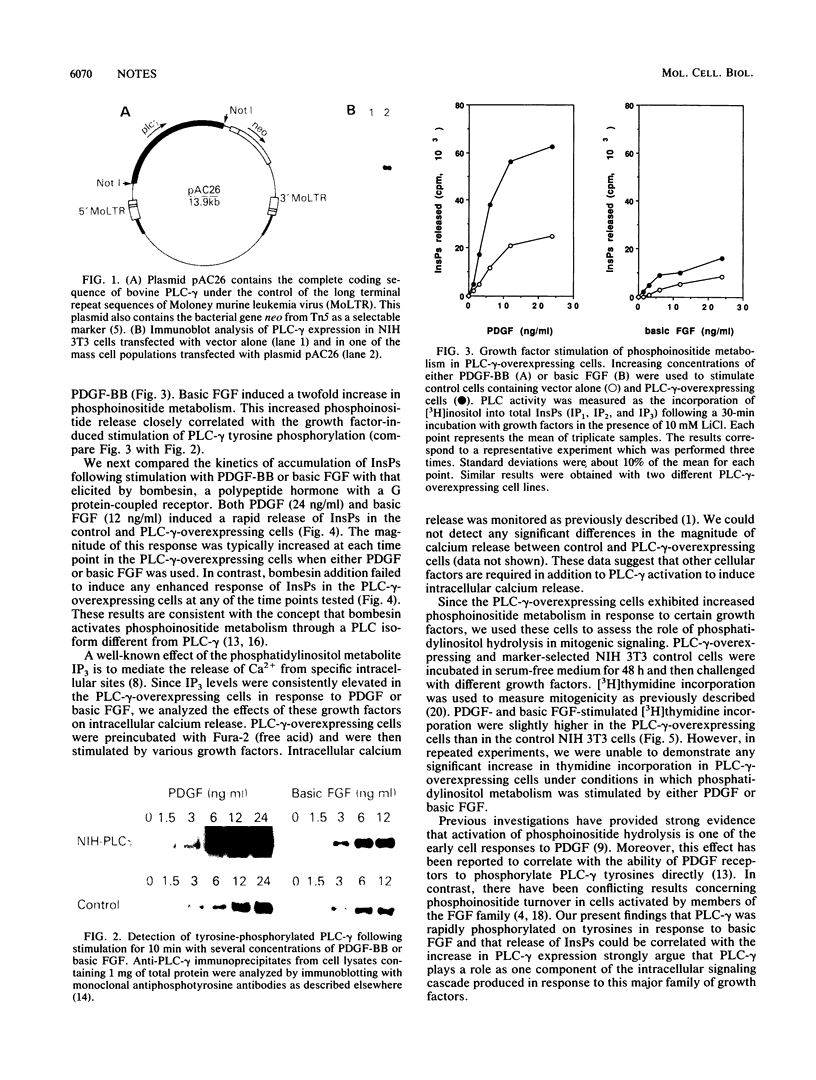
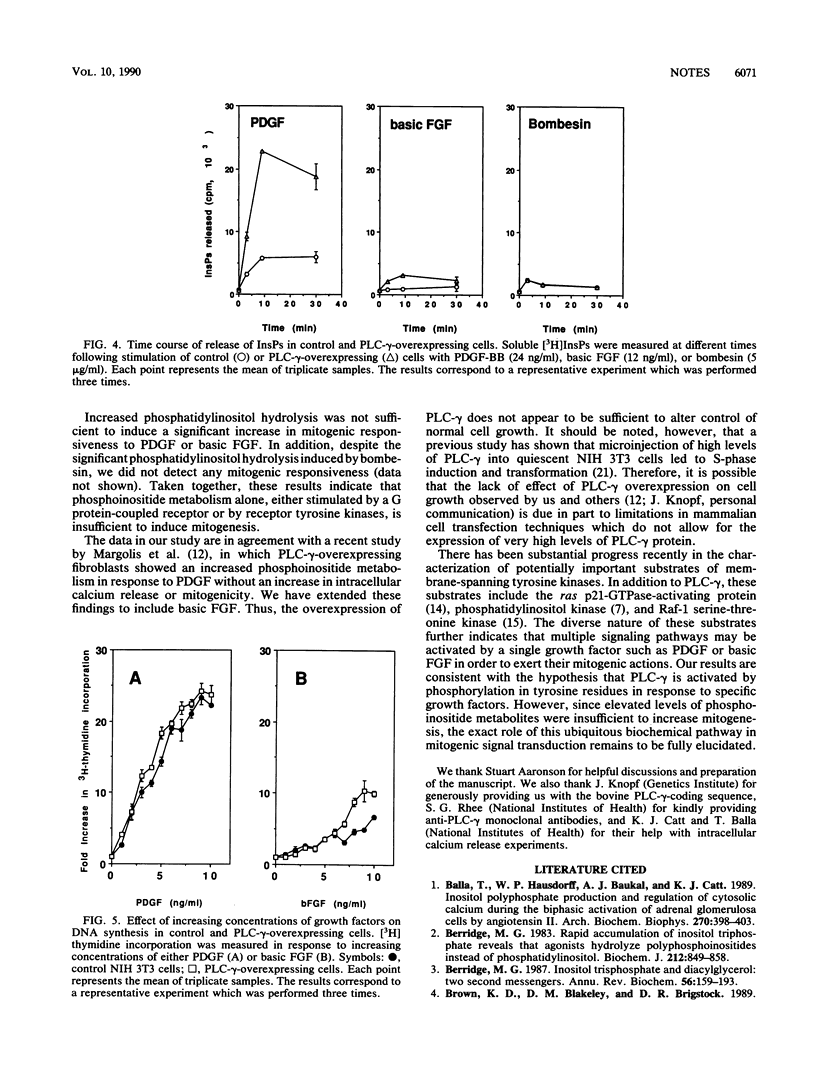
Abstract
Overexpression of phospholipase C-gamma in fibroblasts led to increased tyrosine phosphorylation of this enzyme in response to platelet-derived growth factor and basic fibroblast growth factor. This correlated with increased phosphoinositide release but not with enhanced mitogenicity. Thus, phospholipase C-gamma-mediated phosphoinositide metabolism may not be limiting in the signaling pathways initiated by these growth factors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balla T., Hausdorff W. P., Baukal A. J., Catt K. J. Inositol polyphosphate production and regulation of cytosolic calcium during the biphasic activation of adrenal glomerulosa cells by angiotensin II. Arch Biochem Biophys. 1989 Apr;270(1):398–403. doi: 10.1016/0003-9861(89)90043-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Hasegawa-Sasaki H. Early changes in inositol lipids and their metabolites induced by platelet-derived growth factor in quiescent Swiss mouse 3T3 cells. Biochem J. 1985 Nov 15;232(1):99–109. doi: 10.1042/bj2320099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Margolis B., Zilberstein A., Franks C., Felder S., Kremer S., Ullrich A., Rhee S. G., Skorecki K., Schlessinger J. Effect of phospholipase C-gamma overexpression on PDGF-induced second messengers and mitogenesis. Science. 1990 May 4;248(4955):607–610. doi: 10.1126/science.2333512. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nånberg E., Rozengurt E. Temporal relationship between inositol polyphosphate formation and increases in cytosolic Ca2+ in quiescent 3T3 cells stimulated by platelet-derived growth factor, bombesin and vasopressin. EMBO J. 1988 Sep;7(9):2741–2747. doi: 10.1002/j.1460-2075.1988.tb03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S., Chambard J. C., Pouysségur J. Tyrosine kinase-activating growth factors potentiate thrombin- and AIF4- -induced phosphoinositide breakdown in hamster fibroblasts. Evidence for positive cross-talk between the two mitogenic signaling pathways. J Biol Chem. 1988 Sep 15;263(26):12893–12900. [PubMed] [Google Scholar]
- Rhee S. G., Suh P. G., Ryu S. H., Lee S. Y. Studies of inositol phospholipid-specific phospholipase C. Science. 1989 May 5;244(4904):546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Ryu S. H., Suh P. G., Rhee S. G., Kung H. F. S-phase induction and transformation of quiescent NIH 3T3 cells by microinjection of phospholipase C. Proc Natl Acad Sci U S A. 1989 May;86(10):3659–3663. doi: 10.1073/pnas.86.10.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M. L., Ferenz C. R., Kelleher K. L., Kriz R. W., Knopf J. L. Sequence similarity of phospholipase C with the non-catalytic region of src. Nature. 1988 Mar 17;332(6161):269–272. doi: 10.1038/332269a0. [DOI] [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Choi W. C., Lee K. Y., Rhee S. G. Monoclonal antibodies to three phospholipase C isozymes from bovine brain. J Biol Chem. 1988 Oct 5;263(28):14497–14504. [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Wahl M. I., Nishibe S., Suh P. G., Rhee S. G., Carpenter G. Epidermal growth factor stimulates tyrosine phosphorylation of phospholipase C-II independently of receptor internalization and extracellular calcium. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1568–1572. doi: 10.1073/pnas.86.5.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M., Cantley L. Phosphoinositide metabolism and the control of cell proliferation. Biochim Biophys Acta. 1989 Feb;948(3):327–344. doi: 10.1016/0304-419x(89)90005-x. [DOI] [PubMed] [Google Scholar]




