Introduction
Chronic kidney disease affects more than 50 million people in the U.S.1. The top three etiologies for end-stage kidney disease are diabetes mellitus (45% of patients), hypertension (29% of patients), and glomerulonephritis (19% of patients).2 While it is well established that chronic kidney diseases can result in decreased elimination of drugs via the kidneys, the effects of kidney disease on non-renal clearance processes, especially specific metabolic routes, are less well described. Experimental models of chronic kidney disease have reported reductions in hepatic cytochrome P450 (CYP) enzymes including 3A1, 3A2, 2C11, and N-acetyltransferases.3–5 Reductions in nonrenal clearance ranging from 30% to 67% have been reported for substrates of the CYP3A4, CYP2D6, CYP2B6, and CYP2C9 enzymes in patients with kidney disease.6 In order to fully understand the clinical significance of altered metabolic routes associated with kidney disease, it will be necessary to evaluate the effects that specific forms of kidney diseases have on these pathways and whether these processes effect drug disposition.
Numerous studies have reported various probe drugs or cocktail approaches to evaluate the in vivo function of various drug metabolizing enzymes and transporters in patients.7, 8 However, only a few studies9–12 were actually conducted in chronic kidney disease patients, and hence applicability of most of the published studies beyond the healthy control population remains to be established. As chronic kidney disease patients are commonly prescribed ~10–12 different daily medications13, studies that evaluate metabolic pathway alterations should address requirements for modifications in drug regimens as well as drug interaction potential.
While studies designed to assess and report the pharmacokinetics of bupropion exist14, 15, studies designed specifically to assess the influence of chronic kidney diseases on CYP2B6 activity are currently nonexistent. CYP2B6 is responsible for the metabolism of 3–8% of the currently marketed drugs16, 17 including bupropion, cyclophosphamide, efavirenz, selegiline, methadone, and sertraline18–22 and several drugs, including clopidrogrel, ticlopidine, clotrimazole, itraconazole, sertraline, and raloxifene have been purported to inhibit CYP2B6.23 Data from healthy subjects demonstrate that both the R and S enantiomers of bupropion and its hydroxy-metabolite are present in plasma, but only stereoselective S bupropion and (S,S) hydroxybupropion formation clearance have been shown to be a phenotypic probe for CYP2B624, complicating the assessment of in vivo activity. The purpose of the current study was to evaluate the pharmacokinetics of enantiomeric bupropion and its hydroxybupropion metabolite in patients with kidney diseases affecting the glomerulus in order to provide an assessment of CYP2B6 activity in this disease state.
Methods
Patients
Patients with biopsy confirmed lupus nephritis and ANCA-associated vasculitis were enrolled in a functional phenotyping study using oral bupropion as a CYP2B6 probe. Concomitant therapy with other immunosuppressants was allowed and recorded. Patients who were not able to abstain from ingestion of alcohol, orange and grapefruit juices for 14 days prior to and during the study were excluded from study participation. Clinical data including creatinine clearance (Clcr), urinary protein to creatinine ratio (UP:Cr), serum albumin, and serum creatinine were measured at the time of the study or abstracted from the medical record within 30 days of the study. The study and consent form were approved by the University’s Institutional Review Board and patient consent was required prior to participation.
Pharmacokinetic Study
Patients were admitted to the General Clinical Research Unit (GCRC) to participate in a 72-hour inpatient stay for pharmacokinetic analysis. Patients were fasting at study initiation and were fed a standard CYP diet in the research unit throughout the study period. This diet consisted of avoidance of foods that could interfere with cytochrome P450 metabolism (cruciferous vegetables, spinach, garlic, grapefruit, chargrilled meats, smoked meats). All patients received one bupropion 150mg sustained release tablet (Budeprion SR®, Teva Pharmaceutical Industries, LTD North Wales, PA) with 8 ounces water. Baseline blood was drawn for a trough plasma concentration and additional heparinized blood samples (7.5 mL) were obtained at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, 24, 36, 48, and 72 hours. Urine was collected during the following intervals: 0–6, 6–12, 12–24, 24–36, 36–48, 48–72 hours. Heparinized blood samples were centrifuged immediately for 10 minutes at 4°C, plasma was transferred to plastic screw top tubes and stored at −80°C until assay. Urine volume for each collection period was recorded, and 2 mL aliquots were stored at −80°C until analysis.
Analytical Methods
Plasma and urine samples were assayed for R- and S- bupropion and (R,R) and (S,S) hydroxybupropion by high-performance liquid chromatography (HPLC) tandem mass spectrometry as described previously.25 The bupropion enantiomer assays were linear from 0.5–200 ng/mL plasma and 5–2000 ng/mL urine, and the hydroxybupropion stereoisomer assays were linear from 2.5–1000 ng/mL in plasma and 25–10,000 ng/mL in urine. Interday coefficients of variations were 6% (S-bupropion), 6% (R-bupropion), 7% ((S,S) hydroxybupropion), and 5% ((R,R) hydroxybupropion), respectively.
Pharmacokinetic Analysis
Noncompartmental pharmacokinetic analyses of (R) and (S) bupropion and (R,R) and (S,S) hydroxybupropion were conducted using WinNonlin v4.1 (Pharsight, Mountain View CA) with the linear up-log down method for AUC determination. The following parameters were reported: observed concentration maximum (Cmax), time to maximum concentration (Tmax), half-life (T1/2), area under the plasma concentration time curve from 0–∞ hours (AUC0–∞) and 0–72 hours (AUC0–72), apparent oral clearance (Cl/F), and renal clearance (Clr). Amount of drug/metabolite in the urine (Ae) was calculated by multiplying the assayed concentration by the total urine volume for each collection period (0–6, 6–12, 12–18, 18–24, 24–36, 36–48, 48–72 hours). The Ae over the 72 hour study period was computed by adding the Ae for the seven collection intervals. Clr for the 0–72 hour time frame was calculated by Ae0–72/ AUC0–72. The percentage of the bupropion dose excreted in urine as the R and S bupropion and the R,R and S,S hydroxybupropion were calculated as the amount of enantiomer in the urine in the 0–72 hour time frame divided by the bupropion dose. Hydroxybupropion formation clearance (Clformation) for the 0–72 hour time frame was calculated as hydroxybupropion Ae0–72/ buproprion AUC0–72.
Statistics
Descriptive analyses for pharmacokinetic parameters, demographic variables and clinical laboratories included mean values and standard deviations, as appropriate. Paired t-tests or nonparametric equivalent were used to assess the significance of differences between enantiomeric parent and enantiomeric metabolite pharmacokinetic parameters, respectively. (Instat v3.0 GraphPad, Inc, La Jolla, CA) Relationships between pharmacokinetic variables and clinical laboratories were assessed by Pearson Correlation Coefficients. P values <0.05 were considered statistically significant.
Results
A total of 10 biopsy-confirmed lupus nephritis (n=3) and ANCA vasculitis (n=7) patients completed the bupropion and hydroxybupropion pharmacokinetic study. The patient demographic composition included: age 43 ± 18 years, 7 female/3 male, 50% Caucasian, weight 90.4 ± 14.3 kg. The non-Caucasian patients included 3 African Americans, 1 Hispanic, and 1 “other” patient. Clcr was used as the assessment of GFR in this study.26 The mean (± standard deviation) clinical laboratory data at baseline included: serum creatinine, 1.2 ± 0.4 mg/dL; UP:Cr, 1.1 ± 0.9; Clcr, 102 ± 33 mL/min; serum albumin, 3.5 ± 0.6 g/dL. All patients received intravenous cyclophosphamide treatment during the bupropion pharmacokinetics study. Seventy percent (n = 7) of patients were receiving concomitant chronic glucocorticoids, with a (mean ± SD) daily prednisone dose of 21.4 ± 18.9 mg. No other immunosuppressants were prescribed. Most patients received a pre-cyclophosphamide anti-emetic regimen consisting of dexamethasone and ondansetron.
Pharmacokinetics
Single dose concentration vs time profiles for bupropion/hydroxybupropion, R-bupropion/(R,R) hydroxybupropion, and S-bupropion/(S,S) hydroxybupropion concentrations are shown in Figure 1. The mean (± standard deviation) bupropion and hydroxybupropion pharmacokinetic parameters for the 10 study patients are provided in Table 1.
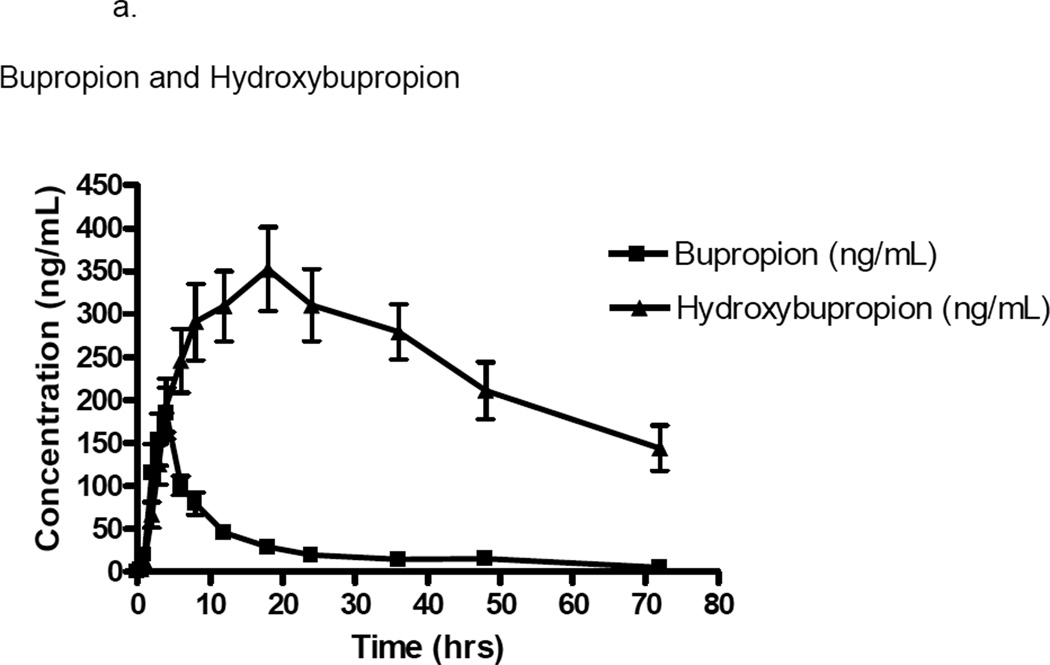
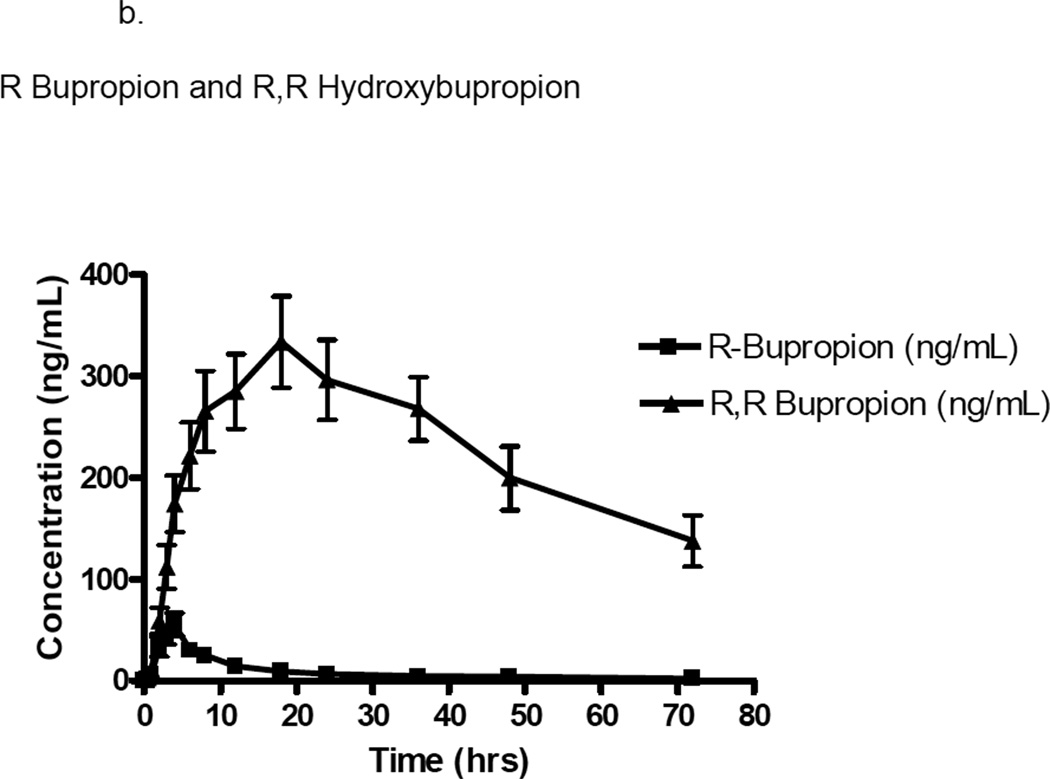
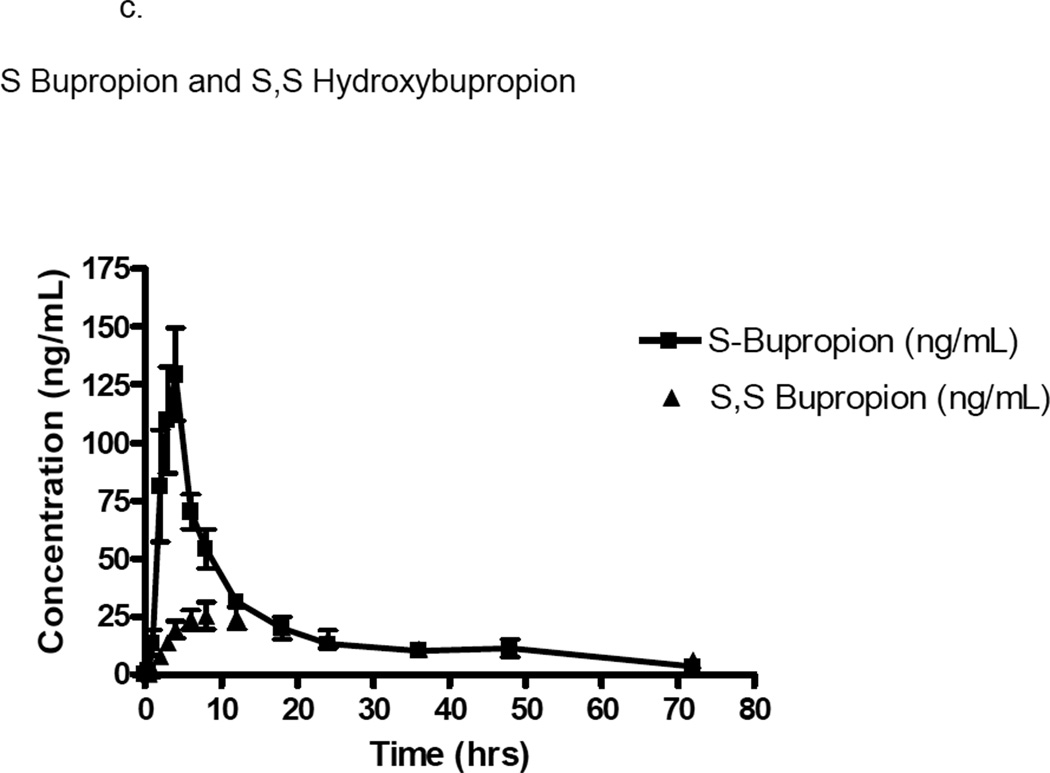
Figure 1.
a. Plasma concentration (ng/mL) versus time (hours) curve of bupropion and hydroxybuproprion in ten patients with glomerulonephritis. Error bars represent mean ± standard deviation.
b. Plasma concentration (ng/mL) versus time (hours) curve of R bupropion and R,R hydroxybupropion in ten patients with glomerulonephritis. Error bars represent mean ± standard deviation.
c. Plasma concentration (ng/mL) versus time (hours) curve of S bupropion and S,S hydroxybupropion in ten patients with glomerulonephritis. Error bars represent mean ± standard deviation.
Table 1.
Pharmacokinetics of Bupropion, Hydroxybupropion and Enantiomers
| Bupropion | R Bupropion | S Bupropion | Hydroxybupropion | R,R Hydroxybupropion | S,S Hydroxybupropion | |
|---|---|---|---|---|---|---|
| Cmax (ng/mL)a,b | 213±93.7 | 66.2±33.9 | 147±59.8 | 388±135 | 356±118 | 31.5±17.0 |
| Tmax (hrs)b | 4.0±1.8 | 4.2±2.1 | 4.0±1.8 | 16.6±4.9 | 16.6±4.9 | 8.8±5.8 |
| T1/2 (hrs) | 22.9±18.0 | 34.6±52.1 | 19.9±13.3 | 43.0±29.7 | 48.7±29.4 | 34.8±14.4 |
| AUC 0–72 (µg hr/L)a,b | 2021±592 | 617±169 | 1404±423 | 16400±6698 | 15800±5828 | 912±487 |
| AUC 0–∞ (µg hr/L) a,b | 2329±953 | 872±695 | 1549±607 | 31600±14800 | 30300±14300 | 1297±719 |
| Cl/F (L/hr)a | 73.5±27.2 | 113±44.6 | 55.8±22.6 | N/A | N/A | N/A |
| Clr L/hr)a, b | 1.3±0.6 | 0.7±0.4 | 1.5±0.7 | 0.4±0.2 | 0.45±0.2 | 1.8±0.9 |
| Vz/F (L)a | 2108±1039 | 7192±3606 | 2852±1421 | N/A | N/A | N/A |
– p<0.05 R bupropion vs S bupropion
– p<0.05 R,R bupropion vs S,S bupropion
Data presented as mean ± standard deviation.
In our population of patients with glomerulonephritis, the half-life was ~2-fold greater (43.0±29.7 hr vs 22.9±18.0 hr) and exposure (AUC0–∞) was ~13-fold greater (31600±14800 µg hr/L vs 2329±593 µg hr/L) for the hydroxybupropion metabolite as compared to the parent drug. Renal clearance estimates demonstrated a 3-fold greater elimination rate of parent drug as compared to metabolite (1.3±0.6 L/hr vs 0.4±0.2 L/hr). Formation clearance of bupropion to hydroxybupropion was 3.8±3.0 L/hr.
The pharmacokinetics of the enantiomers of bupropion and hydroxybupropion were significantly different. For S-bupropion, the Cmax was 3-fold greater (p=0.0015) and the AUC0–∞ (p=0.0003) was ~2-fold greater than R-bupropion. The apparent oral clearance was 2-fold greater for R-bupropion than S-bupropion (p=0.002). Exposure (AUC0–∞) to the (R,R) hydroxybupropion was 17-fold greater (p=0.0005), Cmax was 11-fold greater (p<0.0001), and Tmax was 2-fold greater (p=0.0215) than the (S,S) hydroxybupropion. The (R,R) hydroxybupropion plasma half-life was non-significantly greater than the (S,S) hydroxybupropion. The metabolic ratio, as defined by the metabolite AUC/parent AUC, was significantly greater for the R vs S enantiomer (25.6±34.5 vs 0.7±1.2 respectively). Assessment of correlations between albumin or creatinine clearance and apparent oral clearance, renal clearance, formation clearance, or metabolic ratios of the parent or metabolite failed to show any relevant trends. Urinary protein to creatinine ratio, the clinical laboratory measure demonstrating the largest degree of abnormality in this study population, showed a significant negative relationship with buproprion apparent oral clearance (r=−0.6848, p=0.0347), and a trend toward significance in the relationship with metabolic ratio (r=−0.6121, p=0.0667). Additionally, urinary protein to creatinine ratio showed a trend toward a significant negative relationship with S buproprion apparent oral clearance (r=−0.6121, p=0.0667), while R bupropropion showed a trend in the relationship between urinary protein to creatinine ratio and metabolic ratio (r=−0.6242, p=0.0603).
S-bupropion and (S,S) hydroxybupropion exhibited 2-fold (p=0.0079) and 5-fold (p=0.0001) greater renal clearance values than the R-bupropion and (R,R) hydroxybupropion enantiomers. The parent and metabolite enantiomers all exhibited a fairly low fraction of the dose in the urine. The fraction of the dose excreted in urine as S-bupropion was 3-fold greater than the R- bupropion; the fraction of the dose excreted in urine as the (R,R) hydroxymetabolite was 4-fold greater than the (S,S) hydroxymetabolite. Overall, ~2% of the bupropion dose was recovered unchanged in the urine, and 4% was excreted in urine as hydroxybupropion. Hence, the primary elimination route for bupropion was through a nonrenal clearance pathway. Assessment of potential correlations between albumin or creatinine clearance and apparent oral clearance, renal clearance, formation clearance, or metabolic ratios of the parent or metabolite enantiomers failed to show any relevant trends.
Discussion
The current study enhances the understanding of enantiomeric bupropion and hydroxybupropion pharmacokinetics and CYP2B6 activity in patients with chronic kidney diseases due to glomerulonephritis, e.g. ANCA-associated vasculitis and lupus nephritis. These diseases typically disrupt the normal filtration barrier and result in enhanced filtration of larger molecular weight substances including plasma proteins and effect creatinine clearance to various extents. Our study suggested a reduction in buproprion apparent oral clearance and resultant metabolic ratios in glomerulonephritis patients with higher urinary protein to creatinine ratios. A potential mechanism behind this finding may be related to disease severity and/or activity; patients with enhanced urinary protein to creatinine ratios have reduced metabolic capabilities of the CYP2B6 enzyme.
Reductions in drug binding proteins such as albumin, occur in glomerular diseases, and result in the potential for alterations in unbound drug pharmacokinetics.27,38 The protein binding of bupropion to albumin is reported to be 84%,28 hence, extremely large changes in plasma albumin would be necessary to affect the unbound concentration. In the present study, patients exhibited only mild to moderate changes in serum albumin (2.7 g/dL to 4.4 g/dL) and analyses did not support correlation trends between serum albumin and clearance or metabolism. A reduction in kidney function as measured by the glomerular filtration rate or creatinine clearance can also occur and result in reduced clearance of small molecules through the kidneys requiring decreases in drug dosages. Creatinine clearance measures in the glomerular disease patients were reasonably preserved in our study population and we did not detect any relationships between creatinine clearance and metabolism.
Our glomerulonephritis patients had apparent differences in bupropion and hydroxybupropion pharmacokinetics than previously reported in healthy control subjects.24, 29–31 The glomerulonephritis patients exhibited greater (R+S) bupropion AUC0-∞ (2329±953 vs 1240±395 µg hr/L) and lower bupropion apparent oral clearance (73.5±27.2 vs 137±52.2 L/hr) values than in healthy controls.24,29–31 Historical bupropion pharmacokinetic data from patients with reduced creatinine clearance, however, were more consistent with results from healthy control patients,14, 15 suggesting a mechanism other than creatinine clearance reductions for altered pharmacokinetics in the glomerulonephritis population. For the hydroxybupropion metabolite, which exhibits ~50% of the pharmacologic activity of the parent, the trend in half-life was longer for glomerular disease patients (43.0±29.7 vs 28.2±9.2 hrs) than healthy controls24 and impaired kidney disease patients14. The metabolic ratio in patients with glomerular disease was considerably lower (~8.3) compared to values previously reported in healthy subjects (~14)24 and patients with impaired kidney function (12 and 26).14, 15 These results suggest a reduced metabolic capability in patients with glomerulonephritis.
Enantiomers of bupropion and hydroxybupropion have different pharmacokinetic profiles per in vitro and in vivo studies employing healthy control subjects.24, 32 A previous in vitro study using recombinant CYP2B6 reported a 3-fold greater rate of (S,S) vs (R,R) hydroxybupropion formation (0.65 µl/min/pmol 2B6 vs 0.21 µl/min/pmol 2B6).33 Higher rates of formation of the (S,S) hydroxybupropion enantiomer may be relevant pharmacologically as it is more potent at nicotinic receptors, as well as noradrenergic and dopaminergic transporters.34, 35 Our glomerulonephritis patient data supported a higher formation clearance of (R,R) hydroxybupropion (9.3±6.3 L/hr) versus (S,S) hydroxybupropion (1.2±1.1 L/hr).
The S-bupropion and (R,R) hydroxybupropion enantiomers contributed predominantly to bupropion and hydroxybupropion concentrations, respectively, in the glomerulonephritis population. However, previous data in healthy subjects suggested that the R-bupropion enantiomer was in excess (64% of total bupropion) to the S bupropion33, conflicting with our data, while other reports indicate that (R,R) hydroxybupropion represented 83%–94% of total hydroxybupropion, consistent with our data.32, 33 As bupropion enantiomers are known to rapidly racemize36, it is unknown whether clinical assay results actually reflect what was present in the blood at the time of sample collection versus conditions at the time of storage or analysis, and this is a potential confounding factor in all studies.
Since it is plausible that kidney diseases may alter the disposition of bupropion and hydroxybupropion, we collected and assayed the urine. Our current results showed that although bupropion and hydroxybupropion exhibited a fairly low fraction of the total dose excreted in the urine (e.g., 2 to 4%), a relatively larger percentage of the total dose was excreted in the urine as the S- enantiomer of the parent and the (S,S) enantiomer of the hydroxy-metabolite. This contrasts with healthy control data that demonstrated a greater urinary recovery of the R bupropion.24 Renal clearance assessments in glomerulonephritis patients, in fact, showed that the S-bupropion and (S,S) hydroxybupropion were 2- and 5-fold increased, respectively, over the R- bupropion and (R,R) hydroxybupropion. Previous data have shown stereoselective pharmacokinetics for nonsteroidal anti-inflammatory drugs in patients with diminished kidney function.37–39
The current study adds to the existing literature of drug metabolizing enzyme assessments in kidney disease, but contributes a new dimension into a specific group of patients with glomerulonephrits. The current study does not support applying bupropion pharmacokinetic data derived from healthy controls and general kidney diseases to patients with diseases such as lupus or ANCA vasculitis. Ongoing studies in these patients will help to identify alterations in other metabolic pathways.
Conclusions
The pharmacokinetics of bupropion and hydroxybupropion are altered in patients with glomerulonephritis. Bupropion exposure (AUC0–∞) was 2-fold greater and apparent clearance was ~one-half what has been reported previously in healthy controls and chronic kidney disease patients in general. However, the hydroxy-metabolite of bupropion, with 50% of the pharmacological activity, appears to exhibit a longer half-life and AUC in glomerulonephritis patients, with possible pharmacological implications for drugs whose metabolites harbor some activity. Stereoselectivity in drug and metabolite disposition is apparent from differences in measured pharmacokinetic parameters between the enantiomers of bupropion and hydroxybupropion, with the S- and (R,R) enantiomers most contributory toward overall patient exposure.
Acknowledgments
This research was funded by the National Institutes of Health K23DK64888 (MSJ), General Clinical Research Centers program of the Division of Research Resources, National Institutes of Health RR00046 (MSJ), Clinical and Translational Science Award U54RR024383 (MSJ), American College of Clinical Pharmacy Research Institute’s Frontier’s Award (MSJ), and K24DA00417 (EDK).
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.System USRD. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 3.Guevin C, Michaud J, Naud J, Leblond FA, Pichette V. Down-regulation of hepatic cytochrome p450 in chronic renal failure: role of uremic mediators. Br J Pharmacol. 2002 Dec;137(7):1039–1046. doi: 10.1038/sj.bjp.0704951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naud J, Michaud J, Leblond FA, Lefrancois S, Bonnardeaux A, Pichette V. Effects of chronic renal failure on liver drug transporters. Drug Metab Dispos. 2008 Jan;36(1):124–128. doi: 10.1124/dmd.107.018192. [DOI] [PubMed] [Google Scholar]
- 5.Simard E, Naud J, Michaud J, et al. Downregulation of hepatic acetylation of drugs in chronic renal failure. J Am Soc Nephrol. 2008 Jul;19(7):1352–1359. doi: 10.1681/ASN.2007090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolin TD, Naud J, Leblond FA, Pichette V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin Pharmacol Ther. 2008 Jun;83(6):898–903. doi: 10.1038/clpt.2008.59. [DOI] [PubMed] [Google Scholar]
- 7.Chainuvati S, Nafziger AN, Leeder JS, et al. Combined phenotypic assessment of cytochrome p450 1A2, 2C9, 2C19, 2D6, and 3A, N-acetyltransferase-2, and xanthine oxidase activities with the "Cooperstown 5+1 cocktail". Clin Pharmacol Ther. 2003 Nov;74(5):437–447. doi: 10.1016/S0009-9236(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 8.Zgheib NK, Frye RF, Tracy TS, Romkes M, Branch RA. Validation of incorporating flurbiprofen into the Pittsburgh cocktail. Clin Pharmacol Ther. 2006 Sep;80(3):257–263. doi: 10.1016/j.clpt.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Nolin TD, Appiah K, Kendrick SA, Le P, McMonagle E, Himmelfarb J. Hemodialysis acutely improves hepatic CYP3A4 metabolic activity. J Am Soc Nephrol. 2006 Sep;17(9):2363–2367. doi: 10.1681/ASN.2006060610. [DOI] [PubMed] [Google Scholar]
- 10.Dreisbach AW, Japa S, Gebrekal AB, et al. Cytochrome P4502C9 activity in end-stage renal disease. Clin Pharmacol Ther. 2003 May;73(5):475–477. doi: 10.1016/s0009-9236(03)00015-8. [DOI] [PubMed] [Google Scholar]
- 11.Dowling TC, Briglia AE, Fink JC, et al. Characterization of hepatic cytochrome p4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther. 2003 May;73(5):427–434. doi: 10.1016/s0009-9236(03)00056-0. [DOI] [PubMed] [Google Scholar]
- 12.Kim YG, Shin JG, Shin SG, et al. Decreased acetylation of isoniazid in chronic renal failure. Clin Pharmacol Ther. 1993 Dec;54(6):612–620. doi: 10.1038/clpt.1993.198. [DOI] [PubMed] [Google Scholar]
- 13.Manley HJ, Garvin CG, Drayer DK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004 Jul;19(7):1842–1848. doi: 10.1093/ndt/gfh280. [DOI] [PubMed] [Google Scholar]
- 14.Turpeinen M, Koivuviita N, Tolonen A, et al. Effect of renal impairment on the pharmacokinetics of bupropion and its metabolites. Br J Clin Pharmacol. 2007 Aug;64(2):165–173. doi: 10.1111/j.1365-2125.2007.02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worrall SP, Almond MK, Dhillon S. Pharmacokinetics of bupropion and its metabolites in haemodialysis patients who smoke. A single dose study. Nephron Clin Pract. 2004;97(3):c83–c89. doi: 10.1159/000078635. [DOI] [PubMed] [Google Scholar]
- 16.Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 1997 Feb-May;29(1–2):413–580. doi: 10.3109/03602539709037591. [DOI] [PubMed] [Google Scholar]
- 17.Nolan D, Phillips E, Mallal S. Efavirenz and CYP2B6 polymorphism: implications for drug toxicity and resistance. Clin Infect Dis. 2006 Feb 1;42(3):408–410. doi: 10.1086/499369. [DOI] [PubMed] [Google Scholar]
- 18.Hesse LM, Venkatakrishnan K, Court MH, et al. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos. 2000 Oct;28(10):1176–1183. [PubMed] [Google Scholar]
- 19.Roy P, Yu LJ, Crespi CL, Waxman DJ. Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos. 1999 Jun;27(6):655–666. [PubMed] [Google Scholar]
- 20.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003 Jul;306(1):287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 21.Hidestrand M, Oscarson M, Salonen JS, et al. CYP2B6 and CYP2C19 as the major enzymes responsible for the metabolism of selegiline, a drug used in the treatment of Parkinson's disease, as revealed from experiments with recombinant enzymes. Drug Metab Dispos. 2001 Nov;29(11):1480–1484. [PubMed] [Google Scholar]
- 22.Obach RS, Cox LM, Tremaine LM. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos. 2005 Feb;33(2):262–270. doi: 10.1124/dmd.104.002428. [DOI] [PubMed] [Google Scholar]
- 23.Walsky RL, Astuccio AV, Obach RS. Evaluation of 227 drugs for in vitro inhibition of cytochrome P450 2B6. J Clin Pharmacol. 2006 Dec;46(12):1426–1438. doi: 10.1177/0091270006293753. [DOI] [PubMed] [Google Scholar]
- 24.Kharasch ED, Mitchell D, Coles R. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J Clin Pharmacol. 2008 Apr;48(4):464–474. doi: 10.1177/0091270008314254. [DOI] [PubMed] [Google Scholar]
- 25.Coles R, Kharasch ED. Stereoselective analysis of bupropion and hydroxybupropion in human plasma and urine by LC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Sep 15;857(1):67–75. doi: 10.1016/j.jchromb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 27.Joy MS, Hilliard T, Hu Y, et al. Pharmacokinetics of mycophenolic acid in patients with lupus nephritis. Pharmacotherapy. 2009 Jan;29(1):7–16. doi: 10.1592/phco.29.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GlaxoSmithKline. Zyban SR Product Information. 2006 [Google Scholar]
- 29.Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2005 Jun;77(6):553–559. doi: 10.1016/j.clpt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Fan L, Wang JC, Jiang F, et al. Induction of cytochrome P450 2B6 activity by the herbal medicine baicalin as measured by bupropion hydroxylation. Eur J Clin Pharmacol. 2009 Apr;65(4):403–409. doi: 10.1007/s00228-008-0594-3. [DOI] [PubMed] [Google Scholar]
- 31.Loboz KK, Gross AS, Williams KM, et al. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin Pharmacol Ther. 2006 Jul;80(1):75–84. doi: 10.1016/j.clpt.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Loboz KK, Gross AS, McLachlan AJ. Stereoselective analysis of hydroxybupropion and application to drug interaction studies. Chirality. 2007 Mar;19(3):163–170. doi: 10.1002/chir.20356. [DOI] [PubMed] [Google Scholar]
- 33.Coles R, Kharasch ED. Stereoselective metabolism of bupropion by cytochrome P4502B6 (CYP2B6) and human liver microsomes. Pharm Res. 2008 Jun;25(6):1405–1411. doi: 10.1007/s11095-008-9535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003 Aug 1;474(1):85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- 35.Damaj MI, Carroll FI, Eaton JB, et al. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004 Sep;66(3):675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- 36.Fang QK, Han Z, Grover P, Kessler D, Senanayake CH, Wald SA. Rapid access to enantiopure bupropion and its major metabolite by stereospecific nucleosphilic substitution on an [alpha]-ketogriflate. Tetrahedron. 2000;11:3635–3663. [Google Scholar]
- 37.Grubb NG, Rudy DW, Brater DC, Hall SD. Stereoselective pharmacokinetics of ketoprofen and ketoprofen glucuronide in end-stage renal disease: evidence for a 'futile cycle' of elimination. Br J Clin Pharmacol. 1999 Oct;48(4):494–500. doi: 10.1046/j.1365-2125.1999.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudy AC, Knight PM, Brater DC, Hall SD. Enantioselective disposition of ibuprofen in elderly persons with and without renal impairment. J Pharmacol Exp Ther. 1995 Apr;273(1):88–93. [PubMed] [Google Scholar]
- 39.Chen CY, Chen CS. Stereoselective disposition of ibuprofen in patients with compromised renal haemodynamics. Br J Clin Pharmacol. 1995 Jul;40(1):67–72. doi: 10.1111/j.1365-2125.1995.tb04536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]