Abstract
Objective
Psychological stress and sleep disturbances are highly prevalent and are both implicated in the etiology of cardiovascular diseases. Given the common co-occurrence of psychological distress and sleep disturbances including short sleep duration, this study examined the combined effects of these two factors on blood pressure reactivity to acute mental challenge tasks following well-rested and sleep deprived experimental conditions.
Methods
Participants (n=20) were healthy young adults free from current or past sleep, psychiatric, or major medical disorders. Using a within-subjects crossover design, we examined acute stress reactivity under two experimental conditions: following a night of normal sleep in the laboratory and following a night of total sleep deprivation. Two standardized psychological stress tasks were administered, a Stroop color-word naming interference task and a speech task, which were preceded by a pre-stress baseline and followed by a post-stress recovery period. Each period was 10 minutes in duration, and blood pressure recordings were collected every 2.5 minutes throughout each period. Mean blood pressure responses during the stress and recovery periods were examined with a mixed effects analysis of covariance, controlling for baseline blood pressure.
Results
There was a significant interaction between sleep deprivation and stress on systolic blood pressure (F2,82.7=4.05, p=0.02). Systolic blood pressure was higher in the sleep deprivation condition compared with the normal sleep condition during the speech task, as well as during two baseline periods.
Conclusions
Sleep deprivation amplified systolic blood pressure increases to psychological stress. Sleep loss may increase cardiovascular risk by dysregulating stress physiology.
Keywords: Sleep deprivation, stress, blood pressure, reactivity
Introduction
Psychological stress confers risk for many cardiovascular diseases, including hypertension and myocardial infarction (1). Sleep disturbances, like short sleep duration, insomnia and sleep apnea, are also now considered major risk factors for cardiovascular diseases, including hypertension and stroke (2, 3). Stress and sleep disturbances are prevalent conditions, and they often co-occur and influence one another. For instance, psychological stress contributes to sleep loss and insomnia (4). Conversely, sleep deprivation elicits psychological and physiological stress responses (5–8). This reciprocal relationship could suggest that stress and sleep loss interact to compound cardiovascular vulnerability. Given their prevalence and high co-occurrence, we know surprisingly little about how stress and sleep loss may interact to adversely affect cardiovascular function. The present study examined whether one night of total sleep deprivation results in increased blood pressure reactivity using a controlled experimental paradigm. Results from this study may have important implications for the predictive value that sleep problems in combination with psychological stress may have in increasing risk for cardiovascular disease.
Methods
Participants
Participants included 20 healthy young adults (11 female; 6 African American; 2 Asian; 12 white), ages 20–25 years old (mean=23.25), free from psychiatric, sleep, and major medical disorders. Smoking or consuming more than two caffeinated or alcoholic beverages per day was exclusionary. All participants provided written informed consent prior to study procedures, which were approved by the University of Pittsburgh Institutional Review Board. The study was conducted between April 2008 and November 2009.
Study Design
Using a within-subjects crossover design, we examined acute stress reactivity under two experimental conditions: following a night of normal sleep in the laboratory (rested wakefulness) and following a night of total sleep deprivation. Polysomnographic monitoring of sleep during an adaptation night preceded both experimental nights, which were presented in a counterbalanced order and separated by one week to allow for sufficient recovery following sleep deprivation. The stress protocol began in the morning, 1–2 hours following final awakening time during the rested wakefulness condition and following about 25–26 hours of wakefulness during the sleep deprivation condition. The stress protocol consisted of four 10-minute periods: (1) resting baseline; (2) Stroop color-word naming interference task; (3) speech preparation and delivery; and (4) a recovery period. Blood pressure and heart rate was collected with an automatic sphygmomanometer (Datascope ACCUTORR Plus, Paramus, NJ) every 2.5 minutes throughout each period. Subjective stress was rated on an 11-point Likert scale (0=not at all, 10=extremely) following each period. During the baseline and recovery periods, participants sat quietly reading magazines. Later that afternoon, we also collected 4 blood pressure recordings every 2.5 minutes under baseline (i.e., non-task related) conditions, which allowed us to examine the overall effect of sleep deprivation on blood pressure outside of the context of the stress reactivity protocol conducted in the morning. Participants were under constant behavioral and polysomnographic monitoring to ensure wakefulness. Participants abstained from caffeinated food (e.g., chocolate) and beverages for one day prior to and during their entire time in the laboratory.
Stress Tasks
Two standardized psychological stress tasks were administered, a Stroop color-word naming interference task and a speech task. The Stroop task was a modified version of a standardized stressor used in epidemiological and neuroimaging studies of cardiovascular reactivity (9, 10); the inter-trial interval was adaptive, maintaining around 60%. The speech task (11) required participants to give a speech defending themselves against running a stoplight or shoplifting from a store (in a counterbalanced order across test sessions). Participants were given 5 minutes to prepare a speech defending against the transgression. To increase the social-evaluative nature of the speech stressor, the speech delivery period was videotaped and participants were told to be as persuasive as possible and that their speech would be later rated by experts.
Data analysis
We employed a 2 (experimental condition: sleep deprivation, rested wakefulness) x 3 (stress: Stroop, speech, and recovery) mixed effects model with an autoregressive (ar1) covariance matrix on outcomes (i.e., mean systolic and diastolic blood pressure, heart rate, and subjective stress). Pre-stress baseline blood pressure was significantly higher during sleep deprivation, and was included as a covariate in the analyses. Interactions with p-values ≤0.1 were followed up with paired samples t-tests. Effect sizes were calculated as Cohen’s d (12) between the sleep deprivation and normal sleep condition means, correcting for dependence between means using Morris and DeShon’s (13) equation 8. Analyses were conducted on 19 of the participants, with one participant excluded for having consistent blood pressure readings in the borderline hypertension range (~140/90 mm Hg). Note that our primary outcome remained significant when all 20 participants were included in analyses.
Results
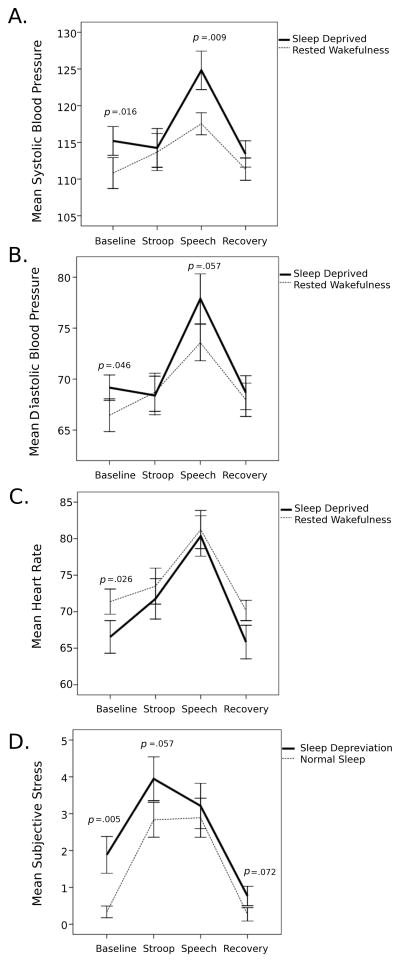
Results (Table 1) revealed a significant interaction between sleep deprivation and stress on systolic blood pressure (F2,82.7=4.05, p=0.02) (Figure 1). Systolic blood pressure was higher in the sleep deprivation compared with the normal sleep condition during the speech task (d=0.73). Blood pressure did not differ among sleep conditions during the Stroop task or the post-stress recovery period. Subjective stress was greater in sleep-deprived participants during baseline, with a trend toward greater stress ratings during the Stroop and recovery periods. The interaction of sleep and task-induced changes on diastolic blood pressure was not significant (p=0.13); however, the strongest effect was found for the comparison during the Speech task, which was marginally significant when comparing sleep deprivation versus the normal sleep condition (t(18)=2.034, p=0.06, d=0.49).
Table 1.
Mixed effects model results comparing blood pressure, heart rate, and subject stress responses during the stress and recovery periods in healthy young adults.
| Systolic Blood Pressure | Diastolic Blood Pressure | Heart Rate | Subjective Stress | |
|---|---|---|---|---|
| Main Effect of Experimental Condition | F(1,71.9)=0.23, p=0.64 | F(1,66.8)=0.18, p=0.67 | F(1,73.6)=1.8, p=0.18 | F(3,17.5)=29.16, p<0.001 |
| Main Effect of Stress Task | F(2,78.5)=36.43, p<0.001 | F(2,75.2)=27.8, p<0.001 | F(2,78.3)=65.83, p<0.001 | F(1,19.2)=5.53, p=0.03 |
| Sleep by Stress Interaction | F(2,82.7)=4.05, p=0.02 | F(2,82.5)=2.09, p=0.13 | F(2,81.7)=0.49, p=0.62 | F(3,17.3)=2.44, p=0.10 |
| Baseline Covariate | F(1,40.8)=32.24, p<0.001 | F(1,41.7)=53.81, p<0.001 | F(1,46.5)=52.99, p<0.001 | - |
Figure 1.
Means (error bars are the standard error of the mean) of (a) systolic and (b) diastolic blood pressure (mm Hg), (c) heart rate (beats per minute), and (d) subjective stress (rated on a Likert scale from 0=not at all to 10=extremely) during baseline, stress, and recovery periods tested under sleep deprived (solid line) and rested wakefulness (dotted line) experimental conditions.
We also examined models in which mean blood pressure responses during the speech preparation and delivery periods were separately included, as the act of speaking during a speech task can itself increase blood pressure, but the results from these models did not qualitatively differ from those reported above (i.e., systolic blood pressure was significantly larger when participants were sleep deprived compared to rested conditions during both speech preparation and delivery).
To examine whether an overall main effect of sleep deprivation on elevated blood pressure was present (outside of the context of anticipatory reactivity to the stress protocol or the stress reactivity effects), a paired sample t-tests of mean blood pressure during an afternoon baseline period revealed significantly higher systolic (sleep deprivation M ± SD=110.9 ± 7.2 mm Hg; normal sleep M ± SD=106.4 ± mm Hg; t(16)=2.50, p=0.03) but not diastolic blood pressure (sleep deprivation M ± SD=67.0 ± 6.0 mm Hg; normal sleep M ± SD=66.3 ± 5.2 mm Hg; t(16)=0.49, p=0.6).
Discussion
Acute psychological stress involving social-evaluative threat (i.e., the speech task) had a powerful effect on blood pressure reactivity in these healthy young adults, and this effect was amplified after a night of total sleep deprivation. Subjective stress ratings to the speech task were of similar magnitude across the sleep deprived and rested conditions, suggesting that sleep deprivation is more than psychologically stressful, and raises mechanistic questions of the synergy between stressors and sleep on cardiovascular function. It seems plausible that chronic sleep loss and psychological stress may interact to increase the risk for preclinical and clinical cardiovascular morbidity. Whether these results generalize to older or younger samples, chronically stressed and/or ill populations, or to other types of stressors and doses of sleep deprivation requires careful study. Stressor-evoked blood pressure reactivity is typically greater in men and older adults (10), the populations at the greatest risk for hypertension and cardiovascular disease. Chronic stressors encountered during daily life might have stronger effects on blood pressure than those observed in response to the laboratory stressors employed in this experiment.
In our analyses, we controlled for baseline systolic blood pressure which was significantly larger during the sleep deprived compared to the rested condition. One plausible explanation for our findings of elevated systolic blood pressure during the pre-stress baseline was due to an anticipatory reaction to the stress protocol, which may be consistent with the finding of increased stress ratings during the baseline when participants were sleep deprived. A control condition in which individuals were sleep deprived but do not undergo the stress protocol would clarify these potential effects. Nevertheless, we also observed greater systolic blood pressure in the sleep deprived participants when collected under baseline conditions in the afternoon, suggesting a main effect of sleep deprivation in elevating blood pressure.
As reviewed below, these findings are consistent with some laboratory and ambulatory findings, but not all. There are several possible reasons for divergent findings, including age and clinical status of participants, as well as study designs. In addition, experiencing sleep deprivation is in itself a stressor—how stressful is likely related to the overall experimental setting and context, demands placed on participants (e.g., other testing procedures), and other environmental factors. Pagnini and colleagues (14) failed to detect systolic arterial pressure differences in the morning following carefully controlled normal sleep and sleep deprived experimental conditions in 24 healthy adults. Two partial sleep restriction studies reported elevated ambulatory systolic blood pressure during the day following a night of partial sleep restriction compared to a night of normal sleep (15, 16), one of which (16) was conducted in the workplace and sleep restriction occurred as a result of working overtime. In such uncontrolled settings, it is difficult to tease apart the impact of sleep loss versus stressful events encountered in the environment. Sleep loss may have even greater consequences on blood pressure with aging. Following a night of total sleep deprivation in 8 middle-age adults, supine systolic blood was significantly elevated and diastolic blood pressure was marginally elevated (17). That study also recorded blood pressure during two brief stressors (i.e., serial subtraction for two minutes and a two-minute cold pressor test), however responses did not differ across the experimental sleep conditions. It may be that these stressors were of insufficient duration or magnitude to reveal sleep deprivation-enhanced cardiovascular responses. In a recent report (18), elevated systolic and diastolic blood pressure in the morning following a night of sleep deprivation were detected in a sample of 8 elderly adults, but not in 8 younger adults. Finally, studies in clinical populations suggest that even brief amounts of sleep loss may result in blood pressure elevations. In a sample of newly diagnosed medication-free participants with mild to moderate essential hypertension (19), both systolic and diastolic blood pressure was higher in the morning following a night of 4 hours of sleep restriction then when tested following a night of normal sleep. Diastolic but not systolic blood pressure increased across a simulated night shift only in individuals at familial risk for hypertension (i.e., having a biological parent diagnosed with hypertension) (20). Thus, the impact of sleep deprivation on diastolic blood pressure maybe more pronounced in preclinical or clinical samples, and in older adults.
Our findings were also limited to amplified blood pressure responses to the speech but not the Stroop task, despite marginally elevated subjective stress ratings following sleep deprivation. Findings of blood pressure reactivity for versions of this particular task are generally modest in other studies (e.g., 21). Thus, given the modest effect size and individual variability in blood pressure reactivity to this task, it is likely that larger samples may be needed to detect statistically significant reactivity effects. Alternatively, synergistic increases in blood pressure following sleep deprivation may require tasks that are more stressful. Compared to the Stroop task, systolic blood pressure increased the most during the speech task across both experimental sleep conditions. Stress tasks with a social evaluative component, such as the speech task used in this study, pose a clear threat to self-esteem, and elicit larger cortisol responses than mental stressors (22). By contrast, the Stroop task can be considered a mental stressor that taxes executive function and engages conflict monitoring and resolution processes. Future studies that measure different magnitudes and types of stressors, as well as other doses of sleep deprivation, will improve our understanding of how sleep loss may interact with stress in contributing to dysregulated cardiovascular responses.
We conclude that sleep loss may increase risk for some cardiovascular diseases, at least partly by affecting blood pressure reactions to stress. Prospective studies have provided some evidence that exaggerated stressor-evoked blood pressure reactivity increases risk for hypertension and premature cardiovascular disease (23–25). Our results suggest that sleep loss may moderate this relationship, which has appreciable public health significance given the prevalence of both chronic sleep loss (26) and stress. Sleep loss is also a potentially modifiable risk factor. Stress reduction interventions designed to reduce hypertension and cardiovascular disease may need to additionally target sleep duration and quality to provide maximal benefits. Determining the long-term synergistic consequences of sleep loss and stress, and the mechanisms by which they contribute to the etiology of hypertension and cardiovascular disease, may help reduce cardiovascular morbidity.
Acknowledgments
We thank Denise Duryea, Annette Wood, and the N-CTRC staff for their excellent contribution to data collection and management. Funding for this study was provided in part by the National Institute of Health (NIH) grants UL1 RR024153, K01 MH077106, the Commonwealth of Pennsylvania Department of Health, and the Pittsburgh Mind-Body Center (NIH grants HL076852/076858). The authors have no perceived conflicts of interest with the current manuscript; Dr. Buysse serves as a consultant/speaker for the following: Actelion, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, sanofi-aventis, Sepracor, Servier and Takeda.
Abbreviations
- mm Hg
millimeters of mercury
- M
mean
- SD
standard deviation
References
- 1.Figueredo VM. The time has come for physicians to take notice: the impact of psychosocial stressors on the heart. Am J Med. 2009;122:704–12. doi: 10.1016/j.amjmed.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Quan SF. Sleep Disturbances and their Relationship to Cardiovascular Disease. Am J Lifestyle Med. 2009;3:55s–9s. doi: 10.1177/1559827609331709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 4.Akerstedt T. Psychosocial stress and impaired sleep. Scand J Work Environ Health. 2006;32:493–501. [PubMed] [Google Scholar]
- 5.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55:S20–S3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P, Bourrilhon C, Florence G, Chennaoui M. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 9.Jennings PJ, JR, Sheu LK, Derbyshire SW, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–40. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamarck TW, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy MJ, Manuck SB. Reliable measures of behaviorally-evoked cardiovascular reactivity from a PC-based test battery: results from student and community samples. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 11.Marsland AL, Manuck SB, Fazzari TV, Stewart CJ, Rabin BS. Stability of individual differences in cellular immune responses to acute psychological stress. Psychosom Med. 1995;57:295–8. doi: 10.1097/00006842-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 13.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeatedmeasures and independent-groups designs. Psychol Methods. 2002;7:105–25. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 14.Pagani M, Pizzinelli P, Traon AP, Ferreri C, Beltrami S, Bareille MP, Costes-Salon MC, Beroud S, Blin O, Lucini D, Philip P. Hemodynamic, autonomic and baroreflex changes after one night sleep deprivation in healthy volunteers. Auton Neurosci. 2009;145:76–80. doi: 10.1016/j.autneu.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9:503–5. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- 16.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–24. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 17.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 18.Robillard R, Lanfranchi PA, Prince F, Filipini D, Carrier J. Sleep deprivation increases blood pressure in healthy normotensive elderly and attenuates the blood pressure response to orthostatic challenge. Sleep. 2011;34:335–9. doi: 10.1093/sleep/34.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12:63–8. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 20.McCubbin JA, Pilcher JJ, Moore DD. Blood pressure increases during a simulated night shift inpersons at risk for hypertension. Int J Behav Med. 2010;17:314–20. doi: 10.1007/s12529-010-9117-6. [DOI] [PubMed] [Google Scholar]
- 21.Gianaros PJ, Sheu LK, Remo AM, Christie IC, Crtichley HD, Wang J. Heightened resting neural activity predicts exaggerated stressor-evoked blood pressure reactivity. Hypertension. 2009;53:819–25. doi: 10.1161/HYPERTENSIONAHA.108.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 23.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–8. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 24.Carroll D, Smith GD, Shipley MJ, Steptoe A, Brunner EJ, Marmot MG. Blood pressure reactions to acute psychological stress and future blood pressure status: a 10-year follow-up of men in the Whitehall II study. Psychosom Med. 2001;63:737–43. doi: 10.1097/00006842-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Bliwise DL. Historical change in the report of daytime fatigue. Sleep. 1996;19:462–4. doi: 10.1093/sleep/19.6.462. [DOI] [PubMed] [Google Scholar]