Abstract
Retinoids are used in the treatment of inflammatory skin diseases and malignancies, but studies characterizing the in vivo actions of these drugs in humans are lacking. Isotretinoin is a pro-drug for all-trans retinoic acid that can induce long-term remissions of acne; however, its complete mechanism of action is unknown. We hypothesized that isotretinoin induces remission of acne by normalizing the innate immune response to the commensal bacterium P. acnes. Compared to normal subjects, peripheral blood monocytes from acne patients expressed significantly higher levels of TLR-2 and exhibited significantly greater induction of TLR-2 expression following P. acnes stimulation. Treatment of patients with isotretinoin significantly decreased monocyte TLR-2 expression and subsequent inflammatory cytokine response to P. acnes by one week of therapy. This effect was sustained six months following cessation of therapy, indicating that TLR-2 modulation may be involved in the durable therapeutic response to isotretinoin. This study demonstrates that isotretinoin exerts immunomodulatory effects in patients and sheds light on a potential mechanism for its long-term effects in acne. The modulation of TLR-2 expression on monocytes has important implications in other inflammatory disorders characterized by TLR-2 dysregulation.
Introduction
Acne is the most common inflammatory skin condition affecting millions of individuals. Its pathogenesis involves hormonally-induced production of sebum, altered keratinization of the hair follicle, and an inflammatory immune response to the Gram-positive Propionibacterium acnes bacterium that resides within the lipid-rich follicle. However, the specific trigger that initiates the development of acne is unknown. Although acne responds to antibiotics, it is not considered to be an infectious disease but may represent an exaggerated immune response to the commensal P. acnes bacteria. While not everyone gets acne, nearly all humans are colonized by P. acnes, and the density of P. acnes does not correlate with the severity of inflammation (Leyden et al., 1998). Acne patients’ immune responses to P. acnes may play a larger role in acne than the pathogenicity of P. acnes itself (Sugisaki et al., 2009).
Systemic retinoids are used to treat severe acne, inflammatory skin diseases, cutaneous T cell lymphoma, neuroblastoma and acute promyelocytic leukemia. Retinoids inhibit inflammatory TH17 T cell responses, promote regulatory T cell (Treg) responses, and regulate expression of toll-like receptors (TLRs) (Mucida et al., 2007; Xiao et al., 2008). In vitro treatment of normal human monocytes with all-trans retinoic acid (ATRA) down-regulates TLR-2 expression (Liu et al., 2005), but this effect has not been examined in patients treated with systemic retinoids. Isotretinoin (13-cis retinoic acid; 13-cis RA) is the only drug capable of inducing a long-term or permanent remission of acne; however, the mechanisms leading to this durable response are unknown. Given the role of TLR-2 in initiating immune responses to P. acnes, we hypothesized that down-regulation of TLR-2 on monocytes and/or the induction of Treg responses in vivo may represent mechanisms by which isotretinoin exerts its long-term effects. In this study, we document the time course of immunomodulation by isotretinoin in treated patients. These findings are relevant not only to treatment of acne, but also to disorders characterized by TLR-2 dysregulation and other retinoid-responsive conditions.
Results
Acne patients who were scheduled by their dermatologist to begin treatment with isotretinoin for severe acne were recruited. Normal healthy volunteers without acne or prior history of isotretinoin exposure served as controls. A total of 27 acne patients, 10 males and 17 females, with a mean (± SEM) age of 17.9 ± 1.4 years and 23 normal volunteers, 8 males and 15 females, with a mean (± SEM) age of 22.4 ± 1.5 years were enrolled. Of the 27 acne patients treated with isotretinoin, 10 completed the study through 20 weeks of isotretinoin therapy, eight of whom were reevaluated six months after completing therapy. Of the remaining patients, one discontinued isotretinoin at the direction of his dermatologist, two discontinued due to medication costs, one withdrew consent, and six were lost to follow-up between eight and 20 weeks.
Acne lesion counts were performed at all visits to assess treatment response (Figure S1). As an indicator of compliance, serum levels of isotretinoin and its isomers ATRA and 9-cis retinoic acid (9-cis RA) were assessed (Figure S2). Levels were also measured in normal volunteers for comparison. No differences in serum levels of isotretinoin, ATRA, or 9-cis RA were found between acne patients at baseline and normal volunteers.
Acne patients’ monocytes express significantly greater levels of TLR-2 and significantly lower levels of TLR-4 compared to normal volunteers
Monocytes from acne patients secrete significantly higher levels of cytokines when exposed to P. acnes compared to monocytes from normal subjects (Sugisaki et al., 2009). We sought to test the hypothesis that monocytes from acne patients express higher levels of TLR-2 when exposed to P. acnes, which may account for this observation. At baseline, acne patients’ unstimulated monocytes expressed higher levels of TLR-2 than normal volunteers (p = 0.036) (Figure 1). Treatment with P. acnes sonicate induced TLR-2 expression in both patients’ and volunteers’ monocytes, but expression was significantly greater in acne patients’ monocytes (p = 0.041). In contrast, P. acnes-stimulated monocytes from acne patients at baseline expressed significantly lower levels of TLR-4 compared to normal volunteers (p = 0.016) (Figure 2).
Figure 1. Isotretinoin therapy down-regulates surface expression of TLR-2 on patients’ monocytes in vivo.
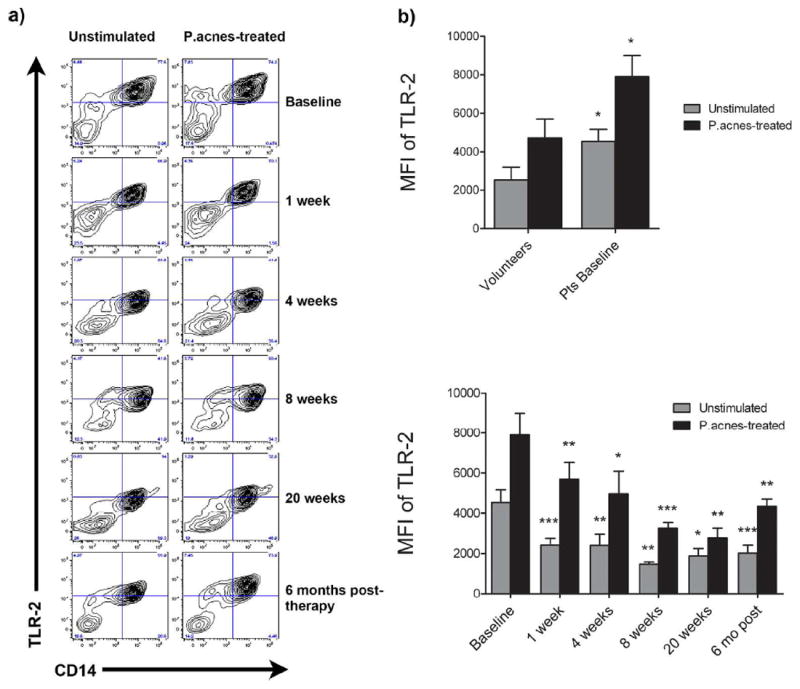
Peripheral blood was taken from normal volunteers (Vols; n = 23) and acne patients at baseline and 1, 4, 8, and 20 weeks of isotretinoin therapy and six months after cessation of therapy (n = 25, 19, 19, 17, 10, and 8, respectively). Isolated monocytes were treated with P. acnes sonicate or no antigen (unstimulated) and analyzed by flow cytometry. A) Dot plots of monocytes from one representative patient are shown. B) Displayed is the mean MFI ± SEM of TLR2 expression for patients at baseline versus volunteers (top) as well as patients’ TLR-2 levels over the course of isotretinoin therapy (bottom). *P<0.05, **P<0.01, and ***P<0.001.
Figure 2. Acne patients’ monocytes express lower levels of TLR-4 compared to normal volunteers.
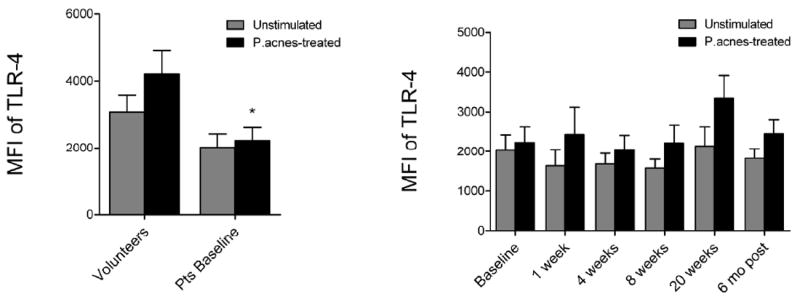
Mean MFI ± SEM of TLR-4 is shown for patients at baseline versus volunteers (left) as well as patients’ TLR-4 levels over the course of isotretinoin therapy (right). *P<0.05, ** P<0.01, and ***P<0.001.
Isotretinoin down-regulates cell surface expression of TLR-2, but not TLR-4, on patients’ monocytes in vivo
To assess the ability of systemic isotretinoin therapy to modulate TLR expression in vivo, peripheral blood samples were taken from acne patients prior to and during isotretinoin therapy for acne (Figure 1). Compared to baseline, unstimulated monocytes from patients treated with isotretinoin had significantly decreased TLR-2 expression as early as 1 week of treatment (p < 0.001), which continued to decrease at each time-point examined (4 weeks, p = 0.004; 8 weeks, p = 0.001; 20 weeks, p = 0.040) (Figure 1). Similarly, decreased TLR-2 expression was noted when monocytes from patients treated with isotretinoin were stimulated with P. acnes sonicate (1 week, p = 0.004; 4 weeks, p = 0.017; 8 weeks, p = 0.001; 20 weeks, p = 0.006) (Figure 1) or whole heat-killed P. acnes (data not shown). At the 6 month follow-up, the mean fluorescence intensity for TLR-2 expression in stimulated and unstimulated monocytes from the 8 subjects decreased by approximately 70% compared to baseline. In comparison, isotretinoin therapy did not affect TLR-4 expression on acne patients’ monocytes at any point during therapy (Figure 2).
Since the majority of patients treated with a full course of isotretinoin (approximately 20 weeks) have a permanent remission of their acne (Liu et al., 2008a), we sought to test the hypothesis that the dramatic reduction in monocyte TLR-2 expression persists following cessation of isotretinoin therapy. To do this, we modified our clinical protocol and were able to reexamine 8 of the 10 patients that completed 20 weeks of therapy. Six months after stopping isotretinoin, TLR-2 expression in unstimulated and P. acnes-stimulated monocytes was still significantly decreased compared to baseline (p = 0.004 and 0.0004, respectively), and not significantly different from normal volunteers (Figure 1). Additionally, TLR-4 expression in monocytes from acne patients six months after treatment was no longer significantly lower than normal volunteers.
Isotretinoin therapy decreases inflammatory cytokine production by patients’ monocytes in response to P. acnes
To determine if isotretinoin altered monocyte cytokine response to P. acnes stimulation, supernatants from cultured monocytes were assayed for inflammatory cytokines using bead arrays. Prior to isotretinoin therapy, acne patients’ unstimulated monocytes secreted more TNFα than monocytes from normal volunteers, but due to inter-patient variability, did not differ in secretion of other inflammatory cytokines (Figure 3). In response to stimulation with P. acnes sonicate, acne patients’ monocytes at baseline secreted more IL-1β, IL-6, IL-10, and IL-12p70 than monocytes from normal volunteers. Isotretinoin therapy significantly decreased the secretion of IL-1β, IL-6, IL-10, and IL-12p70 by acne patients’ monocytes in response to P. acnes compared to pretreatment levels. Cytokine secretion decreased as early as one week of isotretinoin therapy and continued to decrease through 20 weeks of therapy. Additionally, this decrease was sustained at six months after the cessation of therapy. Pearson correlation analysis showed high correlations between TLR-2 expression and secretion of IL-1β (p < 0.000), IL-6 (p < 0.000), IL-8 (p = 0.001), and IL-10 (p < 0.000), but not IL-12p70 (p > 0.1) in P. acnes-stimulated monocytes from volunteers and acne patients during isotretinoin therapy. Similar results were observed in monocytes treated with whole heat-killed P. acnes (data not shown).
Figure 3. Isotretinoin therapy decreases inflammatory cytokine production by acne patients’ monocytes in response to P. acnes.
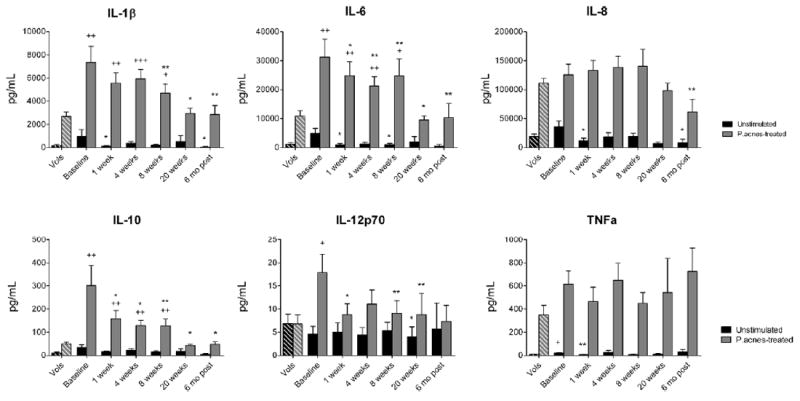
Media supernatants from treated monocytes were analyzed for inflammatory cytokine concentrations using bead arrays. Basal cytokine concentrations in the serum (at 10% final concentration) were subtracted from levels found in the media supernatants to calculate the amounts produced de novo by the monocytes. Concentrations of cytokines ± SEM are displayed for unstimulated and P. acnes-treated monocytes from normal volunteers (Vols) (n = 22) and from patients at baseline (n = 25), 1 week (n = 19), 4 weeks (n = 19), 8 weeks (n = 17), and 20 weeks (n = 10) of isotretinoin therapy as well as six months after the cessation of therapy (n = 8). *P<0.05, **P<0.01, and ***P<0.001 compared with patients’ baseline. +P<0.05, ++P<0.01, and +++P<0.001 compared with normal volunteers.
Isotretinoin therapy does not alter the proportion or function of circulating Treg
Treg suppress inflammatory responses and are of great interest as potential therapies for autoimmune and chronic inflammatory diseases. Because the peripheral conversion of undifferentiated T cells into Treg may involve ATRA (Mucida et al., 2007; Wang et al., 2009; Xiao et al., 2008), as part of the original study we also sought to test the hypothesis that isotretinoin might increase the proportion of circulating Treg in treated patients. Peripheral proportions of CD4+CD25+Foxp3+ Treg were not significantly different in acne patients during isotretinoin therapy compared to baseline (Figure S3). Additionally, we found no differences between acne patients and normal volunteers in peripheral proportions of Treg. To investigate whether in vivo priming with isotretinoin renders peripheral naïve T cells more likely to differentiate into Treg upon activation, lymphocytes were also stimulated for five days with anti-CD3 and anti-CD28 antibodies, P. acnes sonicate, or vehicle. Acne patients’ T cells during isotretinoin therapy were not more likely to differentiate into Treg upon stimulation compared to baseline (data not shown). These data suggest that systemic isotretinoin therapy does not necessarily impart Treg-differentiating signals on naïve T cells in the periphery in vivo.
To determine if isotretinoin affects peripheral Treg suppressive function, peripheral lymphocyte proliferation was measured using 3H-thymidine incorporation assays. Isotretinoin therapy did not affect proliferation of acne patients’ lymphocytes at either an unstimulated basal level or in response to activation by anti-CD3/anti-CD28 antibodies or P. acnes sonicate (Figure 4). Interestingly, in response to P. acnes antigen, acne patients had significantly suppressed lymphocyte proliferation compared to normal volunteers (p= 0.009). Taken together, these data suggest that acne patients have a blunted lymphocyte proliferative response to P. acnes and that treatment with isotretinoin does not alter the proportion or function of circulating Treg in acne patients.
Figure 4. Lymphocyte proliferative response to P.acnes is blunted in acne patients and is unaffected by isotretinoin.
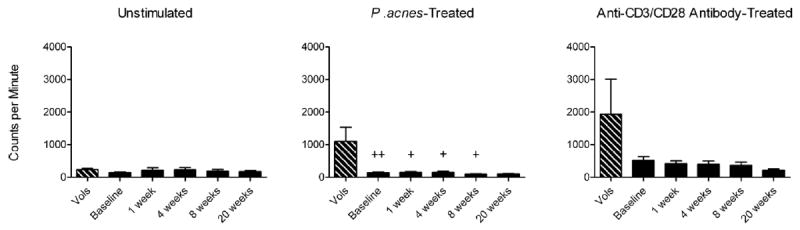
Lymphocytes from normal volunteers (Vols) (n = 11) and from acne patients at baseline (n = 14), 1 week (n = 13), 4 weeks (n = 11), 8 weeks (n = 10), and 20 weeks (n = 5) of isotretinoin therapy were treated with vehicle (no antigen), 1 μM P. acnes sonicate, or 1 μg/mL of each anti-CD3 and anti-CD28 antibodies for five days. For the last four hours of incubation, cells were pulsed with 1 μCi of 3H-thymidine, and samples were then analyzed by scintillation counting. Mean counts per minute (CPM) ± SEM are shown. +P<0.05 and ++P<0.01 compared with normal volunteers.
Acne patients have higher serum concentrations of IL-10, but not inflammatory cytokines, compared to healthy volunteers
Aside from suppression by Treg, lymphocyte proliferation can also be inhibited by immunosuppressive cytokines such as IL-10. Serum was collected from all subjects during the study for assay of cytokine levels. At baseline, acne patients had significantly higher levels of serum IL-10 compared to normal volunteers (P = 0.002) (Figure 5). Interestingly, isotretinoin did not affect serum IL-10 levels at any time-point, and serum IL-10 remained elevated throughout therapy. Of note, these levels are low and their clinical significance is unknown. In contrast, acne patients did not differ from normal volunteers in serum concentrations of IL-1β, IL-6, IL-8, or IL-12p70, nor did isotretinoin therapy significantly affect serum concentrations of these cytokines.
Figure 5. Acne patients have higher serum levels of IL-10 compared to normal subjects.
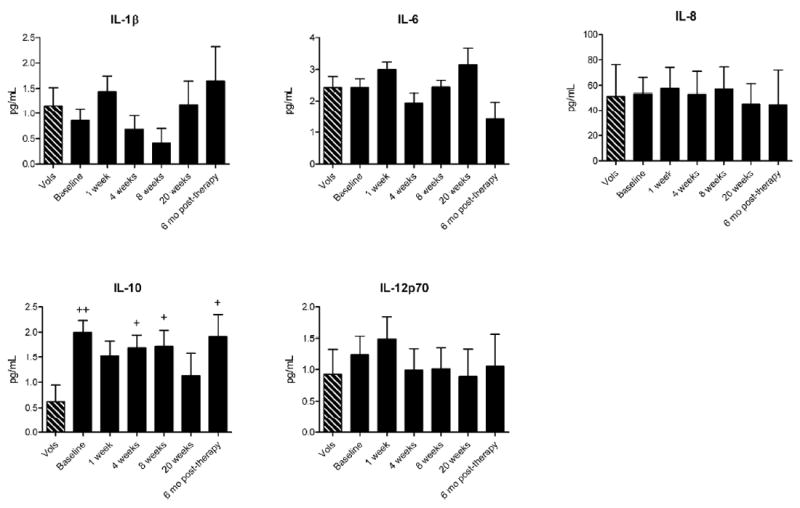
Concentrations of cytokines in the serum of normal volunteers (Vols) (n = 13) and acne patients at baseline (n = 26), 1 week (n = 20), 4 weeks (n = 18), 8 weeks (n = 16), and 20 weeks (n = 9) of isotretinoin therapy as well as six months after the cessation of therapy (n = 8) were analyzed using bead arrays. Mean concentrations ± SEM are shown. +P<0.05 and ++P<0.01 compared with normal volunteers.
Isotretinoin therapy does not affect cytokine secretion by peripheral blood lymphocytes
Retinoid treatment of T cells can skew helper T cell differentiation and responses. To investigate the effects of systemic isotretinoin therapy on the secretion of TH1, TH2, and TH17-associated cytokines by peripheral lymphocytes, media supernatants from P. acnes- and anti-CD3/anti-CD28-stimulated lymphocytes were analyzed at 20 hours and five days of treatment as part of the original study. Isotretinoin did not significantly affect IL-2, IL-4, IL-5, IL-10, IL-17A, TNFα, or IFNγ secretion by lymphocytes in any treatment group (data not shown), suggesting that T cell differentiation was unaffected. Additionally, we did not find any difference in proportions of Treg, Th2, or Th17 cells as assessed by flow cytometry of patients’ lymphocytes (data not shown).
Discussion
Disorders characterized by inflammation such as acne, rosacea, psoriasis, psoriatic arthritis, and Behcet’s disease have been linked to dysregulation of innate immune signaling (Candia et al., 2007; Do et al., 2008; Yamasaki et al., 2011). Acne, psoriasis, and rosacea are thought to represent disorders of dysbiosis characterized by an imbalance of microorganisms within the cutaneous microbiome that leads to altered innate immune responses and noninfectious skin inflammation (Gallo and Nakatsuji). Additionally, patients with acne, psoriatic arthritis and Behcet’s disease have increased expression of TLR-2 on circulating monocytes, implying that the dysregulation of innate immune signaling extends beyond the skin (Candia et al., 2007; Do et al., 2008; Fathy et al., 2009). The increased expression of TLR-2 on acne patients’ peripheral monocytes suggests that acne is one such disorder characterized by dysregulation of innate immune signaling in response to P. acnes.
Isotretinoin’s mechanism of action in inducing long-term remissions of acne is unknown. We sought to test the hypothesis that the systemic administration of isotretinoin, a pro-drug for ATRA would ‘normalize’ the innate immune response to the P. acnes bacteria in patients with severe acne. To our knowledge, this study is the first to demonstrate a durable down-regulation of TLR-2 and down-stream cytokines from systemic administration of a retinoid. Treatment with oral isotretinoin not only decreased TLR-2 expression beginning as early as one week of therapy, but also blunted the rise in TLR-2 expression when monocytes were exposed to P. acnes. Most interestingly, the suppression of TLR-2 expression persisted at least six months following completion of therapy, suggesting that this may play a role in the long-term remission of acne following isotretinoin. We did not observe a significant correlation between age and monocyte TLR-2 expression in either acne patients or normal volunteers within the age range of this study, suggesting that the down-regulation of TLR-2 does not represent a physiologic mechanism for the resolution of acne.
The mechanism by which retinoids affect TLR expression is unknown. In vitro treatment of normal human monocytes with ATRA down-regulates TLR-2 mRNA (Liu et al., 2005), suggesting that isotretinoin operates by a similar mechanism in acne patients’ monocytes in vivo. Though retinoic acid receptor-binding elements (RAREs) are notably absent from the human TLR-2 gene promoter, it is possible that isotretinoin may be acting through retinoid-X-receptor (RXR) activation via 9-cis RA. RXRs dimerize with multiple different nuclear receptors such as the vitamin D receptor (VDR). Activation of VDR can down-regulate TLR-2 expression on normal human monocytes and monocytes from Bechet’s patients (Do et al., 2008; Sadeghi et al., 2006); however, vitamin D did not affect TLR-2 expression in normal monocytes in our system when incubated with either FBS or human serum (data not shown).
Our study also shows that systemic isotretinoin decreases secretion of inflammatory cytokines by patients’ peripheral monocytes to ‘normal’ levels for at least six months after the cessation of therapy. These results are in accordance with in vitro data on the effect of ATRA on monocyte cytokine response to P. acnes (Liu et al., 2005). Although a minor point of our paper, it is interesting to note that the secretion of IL-10 was higher in acne patients’ monocytes at baseline compared to healthy volunteers’ in response to P. acnes. IL-10 is an anti-inflammatory cytokine that is produced by multiple cell types including Treg, TH2 helper T cells, and monocytes. Our findings differ from reports that acne patients’ PBMC secrete less IL-10 in response to P. acnes than PBMC from healthy volunteers. These differences may be due to the increased severity of acne in our patient population. The inflammatory signature of acne lesions has been examined (Kang et al., 2005; Trivedi et al., 2006a). Acne lesions express 46-fold higher IL-10 mRNA compared to normal skin (Kang et al., 2005), and increased lesional expression of IL-10 has been recently reported in hidradenitis suppuritiva (van der Zee et al., 2011; Wolk et al., 2011). This increase in IL-10 is thought to represent a compensatory response to intense inflammation (Kang et al., 2005). In terms of a putative role of IL-10 in acne, IL-10 induces differentiation of monocytes into CD209+ CD163+ macrophages (Montoya et al., 2009). CD209+ macrophages have been shown to phagocytose P. acnes, and CD163 is a scavenger receptor for heme (Liu et al., 2008b). In a previous study of gene expression in acne lesions, CD163 was significantly up-regulated in acne lesions compared to normal skin (Trivedi et al., 2006b). Interestingly, ATRA has been shown to induce differentiation of CD209+ monocytes in vitro (Liu et al., 2008b). These data would suggest a model wherein IL-10 may in part suppress inflammation in acne lesions by inducing differentiation of monocytes into macrophages that scavenge both P. acnes and cellular debris, and retinoic acid may augment this endogenous response. Although isotretinoin decreases IL-10 secretion in monocytes (most likely via TLR-2 down-regulation), it has no effect on the low serum levels of IL-10, possibly representing the contributions of other cell types.
We also present evidence that oral retinoid therapy for up to 20 weeks does not affect peripheral proportions of Treg. Although ATRA concentrations of 10 nM are sufficient for promoting naïve T cell differentiation into Treg in vitro (Wang et al., 2009), the serum levels of ATRA obtained with therapeutic administration of isotretinoin (in the 200 nM range) had no effect on Treg. This is particularly relevant to the use of retinoids in chemotherapy or chemoprevention where there is a delicate balance between cytotoxicity and preservation of immune surveillance. Indeed, in multiple cancers the numbers or activity of Treg are increased in the periphery and the tumor microenvironment, and higher numbers of Treg correlate with a worse prognosis (Curiel et al., 2004; Liyanage et al., 2002; Motta et al., 2005; Sasada et al., 2003; Woo et al., 2001; Yokokawa et al., 2008).
In conclusion, our data suggest that isotretinoin induces remission in acne in part by ‘normalizing’ acne patients’ immune response to P. acnes through down-regulation of TLR-2 expression on monocytes. These changes persist for at least six months after the cessation of therapy, suggesting that this effect may represent a long-term action of isotretinoin. Future studies could determine the duration of these effects as well as the effects of isotretinoin on the inflammatory signature of acne microenvironment. TLR-2 down-regulation may not be permanent, but may be maintained long enough to either modulate the adaptive immune response or to merely keep acne in remission until other age-related factors affecting the skin microbiome or sebaceous gland subside.
Methods
Human Subjects
Human studies were conducted in accordance with principles of the Declaration of Helsinki as approved by Penn State’s Institutional Review Board. All subjects gave written, informed consent. Subjects included males and females ages 12 to 40 who were scheduled to begin treatment with isotretinoin for their moderately severe or severe acne. Patients were not using other acne medications during their isotretinoin therapy. One patient was using topical tretinoin cream at the six month post-isotretinoin visit which has not been shown to alter serum retinoids more than dietary fluctuations (Buchan et al., 1994). Facial acne was assessed by visual counting of the numbers of inflammatory and noninflammatory lesions at each visit. A 30 mL peripheral blood sample was obtained before starting isotretinoin therapy (baseline) and at 1, 4, 8, and 20 weeks after starting their isotretinoin therapy (0.5 to 1 mg/kg/day) and when possible, six months after the cessation of their isotretinoin therapy. Samples were assessed for retinoid levels and isolation of PBMC. A single 30 mL peripheral blood sample was obtained from healthy volunteers ages 12 to 40 for the same analyses. All blood samples were drawn into sodium-heparin tubes (BD Biosciences).
High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry
Concentrations of the three retinoic acid isomers in patients’ serum samples were assayed by HPLC and mass spectrometry. Revisions were made to an established method of sample extraction (Qu et al., 2007), and liquid chromatography conditions were modified from the literature (Kane et al., 2005). Selective solid-phase extraction was employed varying the wash to 3 mL of 50% methanol and elution to 3 mL of 90% acetonitrile. Run time for the liquid chromatography protocol was reduced from 30 minutes to 20 minutes via gradient, internal standard, and MRM modifications. Analyst software version 1.5.1 (AB Sciex) was used to calibrate curves for the internal standard (acitretin).
P. acnes Cultures
Reinforced Clostridal Broth (Difco, BD Biosciences) was inoculated with P. acnes (ATCC strain 6919), overlaid with N2 gas, and grown overnight at 37° C in a shaker until bacteria reached their log growth phase. Bacteria were then washed twice with PBS and resuspended in sterile PBS. Sonicates were made by sonicating suspensions three times for 30 seconds each. The sonicates and the whole heat-killed P. acnes were completely non-viable as tested by plating on Brucella agar in described conditions. Weights were measured using wet conditions. All sonicate preparations were stored at -20° C until use.
Stimulation of Monocytes and Lymphocytes
All sample processing took place in a sterile tissue culture hood under yellow light. Blood samples were processed using a Ficoll-Paque PLUS (GE Healthcare) density gradient according to manufacturer’s guidelines. PBMC were separated into lymphocytes and monocytes using the standard adherence method (Jones et al., 1989). Samples consistently achieved at least 95% purity with minimal contamination by other cell types as assessed by flow cytometry. Monocytes were treated with vehicle or 1 μg/mL P. acnes sonicate in RPMI/10% (v/v) patient serum/antibiotics for 20 hours. Lymphocytes were treated with vehicle, 1 μg/mL P. acnes sonicate, or 1 μg/mL anti-CD3 antibody plus 1 μg/mL anti-CD28 antibody for 20 hours or five days. No changes in PBMC viability were observed due to isotretinoin therapy or in vitro stimulation.
Flow Cytometry
Anti-human fluorochrome-tagged antibodies were all purchased from BD Biosciences. Single cell suspensions of monocytes and lymphocytes were incubated with the appropriate antibodies in PBS containing 0.1% (w/v) NaN3 and 2% (v/v) FBS. For intracellular staining for Foxp3 in lymphocytes, the Foxp3 Staining Buffer Set (eBiosciences) was used according to the manufacturer’s directions. All samples were analyzed using an LSR II flow cytometer with FACSDiva Software (both BD Biosciences) and FlowJo Flow Cytometry Analysis Software (Tree Star, Inc.). All samples were normalized to corresponding negative controls.
Cytokine Detection Using Bead Arrays
Supernatants from treated monocytes and lymphocytes and patient serum were assayed for cytokines using Cytometric Bead Array kits (BD Biosciences) according to manufacturer’s directions. Samples were run on a LSR II flow cytometer using FACSDiva Software and analyzed using FCAP Analysis Software (both BD Biosciences). The theoretical minimum limit of detection for each cytokine in samples was 0.25 to 0.5 pg/mL.
Lymphocyte Proliferation Assays
Patients’ lymphocytes were stimulated with anti-CD3 and anti-CD28 antibodies or P. acnes sonicate in RPMI/10% (v/v) patient serum/antibiotics as described above. Cells were plated in triplicate for five days. For the last four hours of incubation, cells were pulsed with 1 μCi/well of tritiated thymidine (Perkin-Elmer). At the end of the incubation, cells were collected and the degree of 3H-thymidine incorporation was determined by liquid scintillation analysis.
Statistical Analyses
For all assays, each patient served as his/her own control, with data from each time-point during isotretinoin treatment being compared back to his/her own baseline value. All patient data was analyzed using paired Student’s t-tests for comparison of patient data across isotretinoin therapy, and unpaired t-tests for comparison between patients and healthy volunteers.
Supplementary Material
Acknowledgments
We would like to thank Dr. David Stanford and Nate Sheaffer in the PSU Hershey Flow Cytometry Core for their technical assistance. This work was supported by the American Acne and Rosacea Society Clinical Research Grant, the Penn State University Finkelstein Memorial Student Research Award, the Penn State Hershey Department of Dermatology, NIH grant RO1 AR047820 to DMT, and the Jake Gittlen Cancer Research Foundation. Core Facility services and instruments used in this project were funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Conflict of Interest Statement
The authors state no conflict of interest.
References
- Buchan P, Eckhoff C, Caron D, Nau H, Shroot B, Schaefer H. Repeated topical administration of all-trans-retinoic acid and plasma levels of retinoic acids in humans. Journal of the American Academy of Dermatology. 1994;30:428–434. doi: 10.1016/s0190-9622(94)70051-6. [DOI] [PubMed] [Google Scholar]
- Candia L, Marquez J, Hernandez C, Zea AH, Espinoza LR. Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: a pathogenic role for innate immunity? The Journal of rheumatology. 2007;34:374–379. [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Do JE, Kwon SY, Park S, Lee ES. Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behcet’s disease. Rheumatology (Oxford, England) 2008;47:840–848. doi: 10.1093/rheumatology/ken109. [DOI] [PubMed] [Google Scholar]
- Fathy A, Mohamed RW, Ismael NA, El-Akhras MA. Expression of toll-like receptor 2 on peripheral blood monocytes of patients with inflammatory and noninflammatory acne vulgaris. Egypt J of Immun. 2009;16:127–134. [PubMed] [Google Scholar]
- Gallo RL, Nakatsuji T. Microbial Symbiosis with the Innate Immune Defense System of the Skin. The Journal of investigative dermatology. doi: 10.1038/jid.2011.1182. Published online ahead of print June 23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BM, Nicholson JKA, Holman RC, Hubbard M. Comparison of monocyte separation methods using flow cytometric analysis. J of Immun Meth. 1989;125:41–47. doi: 10.1016/0022-1759(89)90076-8. [DOI] [PubMed] [Google Scholar]
- Kane MA, Chen N, Sparks S, Napoli JL. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J. 2005;388:363–369. doi: 10.1042/BJ20041867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–1699. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden JJ, McGinley KJ, Vowels B. Propionibacterium acnes colonization in acne and nonacne. Dermatology. 1998;196:55–58. doi: 10.1159/000017868. [DOI] [PubMed] [Google Scholar]
- Liu A, Yang DJ, Gerhardstein PC, Hsu S. Relapse of acne following isotretinoin treatment: a retrospective study of 405 patients. J Drugs Dermatol. 2008a;7:963–966. [PubMed] [Google Scholar]
- Liu PT, Krutzik SR, Kim J, Modlin RL. Cutting edge: all-trans retinoic acid down-regulates TLR2 expression and function. J Immunol. 2005;174:2467–2470. doi: 10.4049/jimmunol.174.5.2467. [DOI] [PubMed] [Google Scholar]
- Liu PT, Phan J, Tang D, Kanchanapoomi M, Hall B, Krutzik SR, et al. CD209(+) macrophages mediate host defense against Propionibacterium acnes. J Immunol. 2008b;180:4919–4923. doi: 10.4049/jimmunol.180.7.4919. [DOI] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- Montoya D, Cruz D, Teles RM, Lee DJ, Ochoa MT, Krutzik SR, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell host & microbe. 2009;6:343–353. doi: 10.1016/j.chom.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M, Rassenti L, Shelvin BJ, Lerner S, Kipps TJ, Keating MJ, et al. Increased expression of CD152 (CTLA-4) by normal T lymphocytes in untreated patients with B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1788–1793. doi: 10.1038/sj.leu.2403907. [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science (New York, NY. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Qu J, Qu Y, Straubinger RM. Ultra-sensitive quantification of corticosteroids in plasma samples using selective solid-phase extraction and reversed-phase capillary high-performance liquid chromatography/tandem mass spectrometry. Analytical chemistry. 2007;79:3786–3793. doi: 10.1021/ac062184r. [DOI] [PubMed] [Google Scholar]
- Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. European journal of immunology. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- Sugisaki H, Yamanaka K, Kakeda M, Kitagawa H, Tanaka K, Watanabe K, et al. Increased interferon-gamma, interleukin-12p40 and IL-8 production in Propionibacterium acnes-treated peripheral blood mononuclear cells from patient with acne vulgaris: host response but not bacterial species is the determinant factor of the disease. J Dermatol Sci. 2009;55:47–52. doi: 10.1016/j.jdermsci.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Trivedi NR, Cong Z, Nelson AM, Albert AJ, Rosamilia LL, Sivarajah S, et al. Peroxisome proliferator-activated receptors increase human sebum production. The Journal of investigative dermatology. 2006a;126:2002–2009. doi: 10.1038/sj.jid.5700336. [DOI] [PubMed] [Google Scholar]
- Trivedi NR, Gilliland KL, Zhao W, Liu W, Thiboutot DM. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. The Journal of investigative dermatology. 2006b;126:1071–1079. doi: 10.1038/sj.jid.5700213. [DOI] [PubMed] [Google Scholar]
- van der Zee HH, de Ruiter L, van den Broecke DG, Dik WA, Laman JD, Prens EP. Elevated levels of tumour necrosis factor (TNF)-alpha, interleukin (IL)-1beta and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-alpha and IL-1beta. The British journal of dermatology. 2011;164:1292–1298. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol. 2009;183:4119–4126. doi: 10.4049/jimmunol.0901065. [DOI] [PubMed] [Google Scholar]
- Wolk K, Warszawska K, Hoeflich C, Witte E, Schneider-Burrus S, Witte K, et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186:1228–1239. doi: 10.4049/jimmunol.0903907. [DOI] [PubMed] [Google Scholar]
- Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer research. 2001;61:4766–4772. [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. The Journal of investigative dermatology. 2011;131:688–697. doi: 10.1038/jid.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, et al. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


