Abstract
Background and Purpose
Increased levels of plasma troponins and natriuretic peptides are associated with increased risk of cardiovascular disease, but only limited information exists on these biomarkers and stroke occurrence. In a prospective epidemiological study, we tested the hypothesis that high-sensitivity troponin T (TnT) and N-terminal pro B-type natriuretic peptide (NT-proBNP) are associated positively with incidence of stroke.
Methods
The Atherosclerosis Risk in Communities (ARIC) Study measured plasma TnT and NT-proBNP in 10,902 men or women initially free of stroke and followed them for a mean of 11.3 years for stroke occurrence (n=507).
Results
Both biomarkers were associated positively with total stroke, nonlacunar ischemic, and especially, cardioembolic stroke, but not with lacunar or hemorrhagic stroke. For example, after adjustment for prevalent risk factors and cardiac diseases, the hazard ratios (95% confidence intervals) for jointly high values of TnT and NT-proBNP (versus neither biomarker high) were 2.70 (1.92, 3.79) for total stroke and 6.26 (3.40, 11.5) for cardioembolic stroke. Associations with stroke appeared somewhat stronger for NT-proBNP than TnT. Strikingly, approximately 58% of cardioembolic strokes occurred in the highest quintile of pre-stroke NT-proBNP, and 32% of cardioembolic strokes occurred in participants who had both NT-proBNP in the highest quintile and were known by ARIC to have atrial fibrillation sometime before their cardioembolic stroke occurrence.
Conclusions
In the general population, elevated plasma TnT and NT-proBNP concentrations are associated with increased risk of cardioembolic and other nonlacunar ischemic strokes.
Keywords: epidemiology, natriuretic peptides, risk factors, stroke, troponins
Troponins and natriuretic peptides, such as N-terminal pro B-type natriuretic peptide (NT-proBNP), are useful plasma diagnostic tests. They also are potent risk markers in the general population for the subsequent development of coronary heart disease (CHD) and heart failure events.1–5
In acute stroke, particularly in cardioembolic stroke, troponins and natriuretic peptides often are elevated and when elevated, are associated with a poorer prognosis after stroke.6,7 In a few studies, higher basal levels of natriuretic peptides also have been associated with increased future incidence of stroke.3,5,8–13 Much of this evidence on natriuretic peptides and incident stroke is from high-risk patient populations9,11,13; few prospective population-based studies exist and often did not study stroke subtypes or include African Americans.3,5,8,10,12 Information on the association of troponin levels and incident stroke occurrence is quite scant.13–15
We sought to provide new information on the association of these biomarkers with the incidence of stroke and stroke subtypes in the general population. We therefore tested the hypothesis that high-sensitivity troponin T (TnT) and NT-proBNP are associated positively with incidence of stroke over an average of 11 years of follow-up in the population-based Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study Population
In 1987–89, ARIC recruited and examined (visit 1) a cohort 15,792 men and women aged 45 to 64 years in 4 U.S. communities.16 ARIC performed follow-up visit 2 in 1990–92 (93% return rate), visit 3 in 1993–95 (86% return), visit 4 in 1996–98 (80% return, n=11,668). The present analysis used visit 4 as its start point for follow-up. The study protocol was approved by the Institutional Review Boards of the collaborating institutions and written informed consent was obtained from each participant.
Risk Factor Measurements
Participants were asked to fast for 12 hours before their morning visit 4 appointments, and plasma samples were stored at −70°C until assayed in 2009–2010. Troponin T levels were measured using the Elecys Troponin T, a high sensitivity assay (Roche Diagnostics, Indianapolis). The lower limit of detection is 0.003 µg/L, and values <0.003 µg/L, which were common, were grouped into a separate category that served as the reference group for hazard ratio estimation. The reliability coefficient for blinded quality control replicate measurements of troponin T from single blood draws was 0.98. Plasma NT-proBNP was measured on a Cobas e411 analyzer using the Elecys proBNP II immunoassay (Roche Diagnostics, Indianapolis). The reliability coefficient for blind replicate measurements was 0.99.
ARIC also measured plasma total and HDL cholesterol,2 C-reactive protein (CRP) (Roche Diagnostics, Indianapolis), lipoprotein-associated phospholipase A2 (Lp-PLA2) activity (PLAC; diaDexus Inc., South San Francisco, CA), and estimated glomerular filtration rate (eGFR).17 Sitting blood pressures, body mass index, waist-to-hip ratio (WHR), and diabetes were assessed by published methods.2,16 The Baecke sport index (physical activity)18 was completed at visit 3.
Prevalent atrial fibrillation was determined from electrocardiograms at ARIC visits 1 to 4 or from hospitalizations with a discharge diagnosis including atrial fibrillation between visits 1 and 4. Incident atrial fibrillation after visit 4 was based on hospital discharge codes between ARIC visit 4 and the end of follow-up. The completeness of ARIC atrial fibrillation ascertainment is quite good.19 We defined prevalent and incident CHD and HF as previously described.2 We defined prevalent stroke, which was used for exclusion, as (1) a history of stroke (unvalidated) at visit 1 or (2) an incident definite or probable stroke (validated) between visit 1 and visit 4.
Incident Stroke
We identified incident stroke events occurring between ARIC visit 4 (1996–1998) and December 31, 2009. Transient ischemic attacks were not ascertained. ARIC participants were contacted annually by telephone, and reported hospitalizations and deaths related to possible strokes in the previous year were identified. We also surveyed lists of discharges from local hospitals and death certificates from state vital statistics offices for potential cerebrovascular events. Abstractors recorded stroke signs and symptoms and photocopied neuroimaging (CT or MRI) and other diagnostic reports if the list of discharge diagnoses included a cerebrovascular disease code (International Classification of Diseases, 9th Revision, code 430–437), if a cerebrovascular condition or procedure was mentioned in the discharge summary, or if a cerebrovascular finding was noted on a CT or MRI report. Each eligible case was classified by computer algorithm and by a physician reviewer,20 according to criteria adapted from the National Survey of Stroke.21 Disagreements were adjudicated by another reviewer. Qualifying strokes were further classified into definite or probable hospitalized ischemic stroke (neuroimaging showed acute infarction or no hemorrhage) or hemorrhagic (intraparenchymal or subarachnoid) stroke on the basis of neuroimaging studies or autopsy, when available. All definite ischemic strokes were further classified as either lacunar, nonlacunar, or cardioembolic on the basis of the recorded neuroimaging and medical record findings.22 A stroke was classified as lacunar if 2 criteria were met: (1) typical location of the infarct (basal ganglia, brain stem, thalamus, internal capsule, or cerebral white matter) and (2) infarct size of ≤2 cm or unstated size. Cardioembolic ischemic stroke required (1) autopsy evidence of an infarcted area in the brain and a source of possible cerebral emboli in a vessel or presence of an embolus in the brain or (2) medical record evidence at the time of stroke of a possible source of embolus from echocardiograms, ECG, or medical history, such as moderate or greater valvular heart disease, dilated cardiomyopathy, atrial fibrillation, cardiac or arterial procedure, or intracardiac thrombus. The remaining ischemic strokes were categorized as non-lacunar. Cryptogenic ischemic strokes were not separately categorized and thus mostly would be in the non-lacunar category.
Statistical Analysis
Of the original 15,792 ARIC participants, we excluded those not attending ARIC visit 4 (n=4,136), those with a stroke prior to visit 4 (n=271), those with missing follow-up information after visit 4 (n=22), and, due to small numbers, a few minority participants in two field centers (n=67). Finally, we excluded the remaining participants without measurements of TnT or NT-proBNP (n=394), leaving a maximum of 10,902 participants for the present analyses. Time at risk was computed from the date of visit 4 to the earliest of the following: date of hospital admission for incident definite or probable stroke, date of death, date of last follow-up contact, or December 31, 2009.
Our main hypothesis was that the plasma biomarkers, TnT and NT-proBNP, would be associated positively with stroke incidence. For most analyses, we categorized TnT (using published cutpoints2) and NT-proBNP (into quintiles), but some models employed natural log-transformed TnT and NT-proBNP as continuous variables. Poisson regression was used to compute stroke incidence rates. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals of incident stroke and its subtypes. We verified the proportional hazards assumption of the Cox models by inspection of ln(-ln) survival curves for TnT and NT-proBNP categories. We tested trends in stroke HRs across risk factor categories by including an ordinal variable for each category in the Cox models. We also used restricted cubic splines with 3 knots, located at the 5th, 50th, and 95th percentiles, to graphically characterize the dose-relation between total stroke and each biomarker.23 We tested multiplicative interactions of TnT and NT-proBNP with sex, race, and hypertension status, but none was significant in the Cox models (p>0.30); thus, analyses pooled men and women, whites and African Americans. We selected possible confounding variables for regression models based on previous prospective findings for stroke in ARIC.22 CHD, heart failure, and atrial fibrillation were adjusted for initially as visit 4 prevalence variables (Model 2), but also as time-dependent variables as these events occurred during follow-up (Model 3).
We assessed prediction parameters (area under receiver operater characteristics curve (AUC), net reclassification index, integrated discrimination index) for 10-year risk of stroke using Cox proportional hazards regression models24,25 and TnT and NT-proBNP as continuous variables.
Results
In the 10,902 participants at baseline who were free of stroke at visit 4, plasma TnT and NT-proBNP were associated positively with most other stroke risk factors and prevalent cardiovascular conditions (Supplemental Tables 1 and 2). Stroke risk factors were particularly prevalent among participants in the highest TnT or NT-proBNP categories.
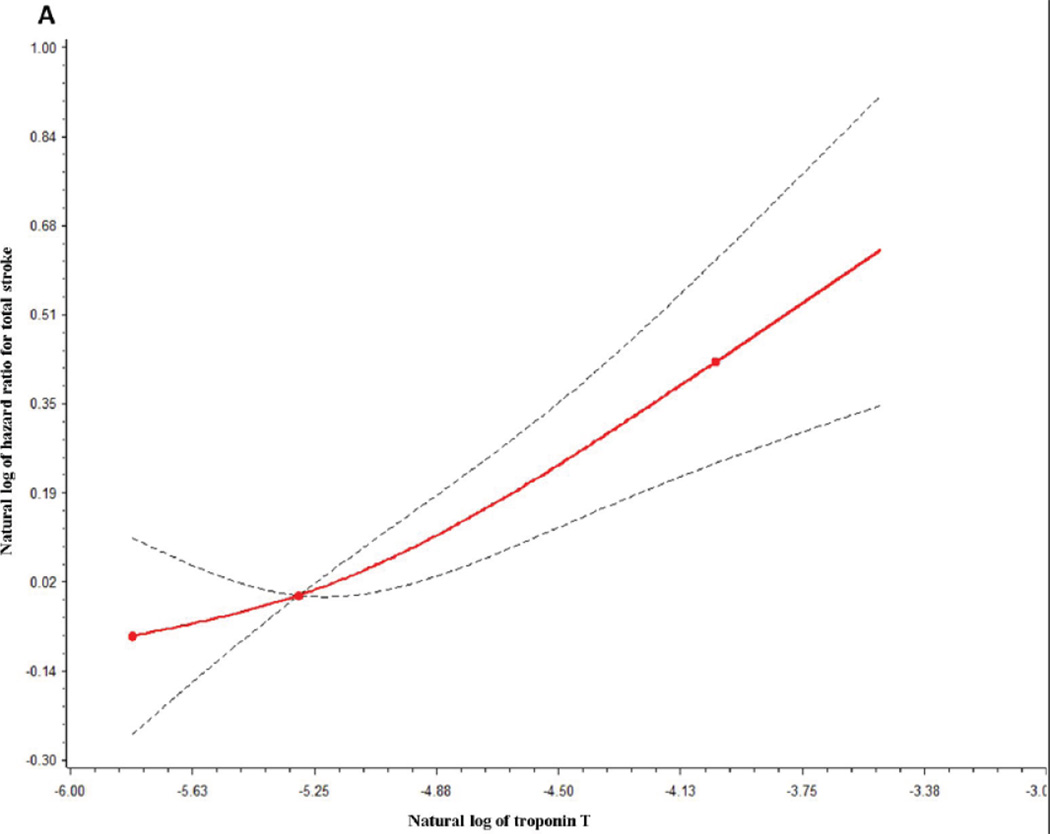
Over a mean of 11.3 years of follow-up, 507 incident strokes were identified, of which 444 were ischemic (87 lacunar; 125 cardioembolic; 232 non-lacunar, of which 26 were judged to be clearly carotid atheroembolic) and 63 were hemorrhagic. TnT was a risk marker for incident stroke (Table 1). After adjustment for age, race, and sex (Model 1), total stroke risk was increased 1.80-fold for those with TnT of 0.009–0.013 µg/L and 2.87-fold for TnT ≥ 0.014 µg/L, compared with TnT <0.003 µg/L. Adjustment for other stroke risk factors and prevalent cardiovascular diseases (Model 2) moderately attenuated the HRs. Additional adjustment for incident CHD, heart failure, and atrial fibrillation occurring during follow-up (Model 3) attenuated the total stroke HRs further, but the association between TnT and total stroke remained statistically significant. TnT was a similarly strong risk marker for total ischemic stroke and its largest subtype -- nonlacunar ischemic stroke (Table 1). TnT HRs were even stronger for cardioembolic stroke, but they were weak for lacunar stroke. There were relatively few hemorrhagic strokes, and their occurrence was not associated with TnT. The dose response relation between TnT and total stroke is further depicted in Figure 1A.
Table 1.
Adjusted hazard ratios and 95% confidence intervals (HR, 95% CI) for incident stroke by troponin T group, ARIC, 1996–2009.
| Troponin T group (µg/L) | ||||||
|---|---|---|---|---|---|---|
| <0.003 µg/L | 0.003–0.005 µg/L | 0.006–0.008 µg/L | 0.009–0.013 µg/L | ≥0.014 µg/L | ||
| Stroke Model | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | P trend |
| N for Model 1* | 3,492 | 2,715 | 2,214 | 1,493 | 988 | |
| N for Models 2† and 3‡ | 3,317 | 2,605 | 2,105 | 1,411 | 912 | |
| Total stroke | ||||||
| Rate/1000 person-years* | 2.79 | 3.18 | 3.20 | 4.93 | 7.71 | |
| n of strokes | 109 | 106 | 95 | 103 | 94 | |
| Model 1a | 1 (reference) | 1.14 (0.87, 1.50) | 1.16 (0.87, 1.54) | 1.80 (1.34, 2.40) | 2.87 (2.11, 3.91) | <.0001 |
| Model 2† | 1 (reference) | 1.25 (0.94, 1.65) | 1.13 (0.84, 1.53) | 1.60 (1.17, 2.18) | 2.04 (1.45, 2.87) | <.0001 |
| Model 3‡ | 1 (reference) | 1.23 (0.93, 1.63) | 1.09 (0.81, 1.48) | 1.51 (1.11, 2.06) | 1.85 (1.31, 2.61) | 0.001 |
| Ischemic stroke | ||||||
| n of strokes | 88 | 93 | 80 | 92 | 91 | |
| Model 2† | 1 (reference) | 1.35 (0.99, 1.83) | 1.16 (0.84, 1.62) | 1.68 (1.20, 2.35) | 2.30 (1.60, 3.31) | <.0001 |
| Model 3‡ | 1 (reference) | 1.32 (0.97, 1.80) | 1.12 (0.80, 1.55) | 1.57 (1.12, 2.20) | 2.04 (1.42, 2.95) | 0.0003 |
| Lacunar stroke | ||||||
| n of strokes | 21 | 19 | 16 | 16 | 15 | |
| Model 2† | 1 (reference) | 1.18 (0.62, 2.27) | 1.03 (0.51, 2.09) | 1.23 (0.58, 2.61) | 1.62 (0.72, 3.67) | 0.32 |
| Model 3‡ | 1 (reference) | 1.17 (0.61, 2.24) | 1.02 (0.50, 2.05) | 1.20, 0.57, 2.55) | 1.49 (0.65, 3.39) | 0.43 |
| Nonlacunar stroke | ||||||
| n of strokes | 48 | 44 | 47 | 49 | 44 | |
| Model 2† | 1 (reference) | 1.10 (0.71, 1.69) | 1.19 (0.77, 1.85) | 1.64 (1.04, 2.58) | 2.02 (1.22, 3.34) | 0.003 |
| Model 3‡ | 1 (reference) | 1.09 (0.71, 1.68) | 1.19 (0.77, 1.86) | 1.63 (1.03, 2.58) | 2.02 (1.22, 3.35) | 0.003 |
| Cardioembolic stroke | ||||||
| n of strokes | 19 | 30 | 17 | 27 | 32 | |
| Model 2† | 1 (reference) | 2.15 (1.16, 3.99) | 1.19 (0.59, 2.43) | 2.12 (1.06, 4.24) | 3.76 (1.85, 7.64) | 0.002 |
| Model 3‡ | 1 (reference) | 2.03 (1.09, 3.76) | 1.03 (0.50, 2.09) | 1.68 (0.83, 3.39) | 2.63 (1.28, 5.37) | 0.04 |
| Hemorrhagic stroke | ||||||
| n of strokes | 21 | 13 | 15 | 11 | 3 | |
| Model 2† | 1 (reference) | 0.80 (0.38, 1.69) | 1.05 (0.49, 2.23) | 1.30 (0.57, 2.97) | 0.50 (0.13, 1.97) | 0.94 |
| Model 3‡ | 1 (reference) | 0.80 (0.38, 1.68) | 1.04 (0.49, 2.21) | 1.28 (0.56, 2.94) | 0.51 (0.13, 2.01) | 0.94 |
Model 1 - Adjusted for age, sex, and race.
Model 2 - Adjusted for model 1 + initial values for body mass index, smoking status and amount, diabetes, systolic blood pressure, antihypertensive medication use, HDL cholesterol, total cholesterol, lipid medication use, C-reactive protein, lipoprotein-associated phospholipase A2 (Lp-PLA2), prevalent atrial fibrillation, coronary heart disease, and heart failure.
Model 3 - Same as Model 2, except incident atrial fibrillation, coronary heart disease, and heart failure were treated as time-dependent covariates.
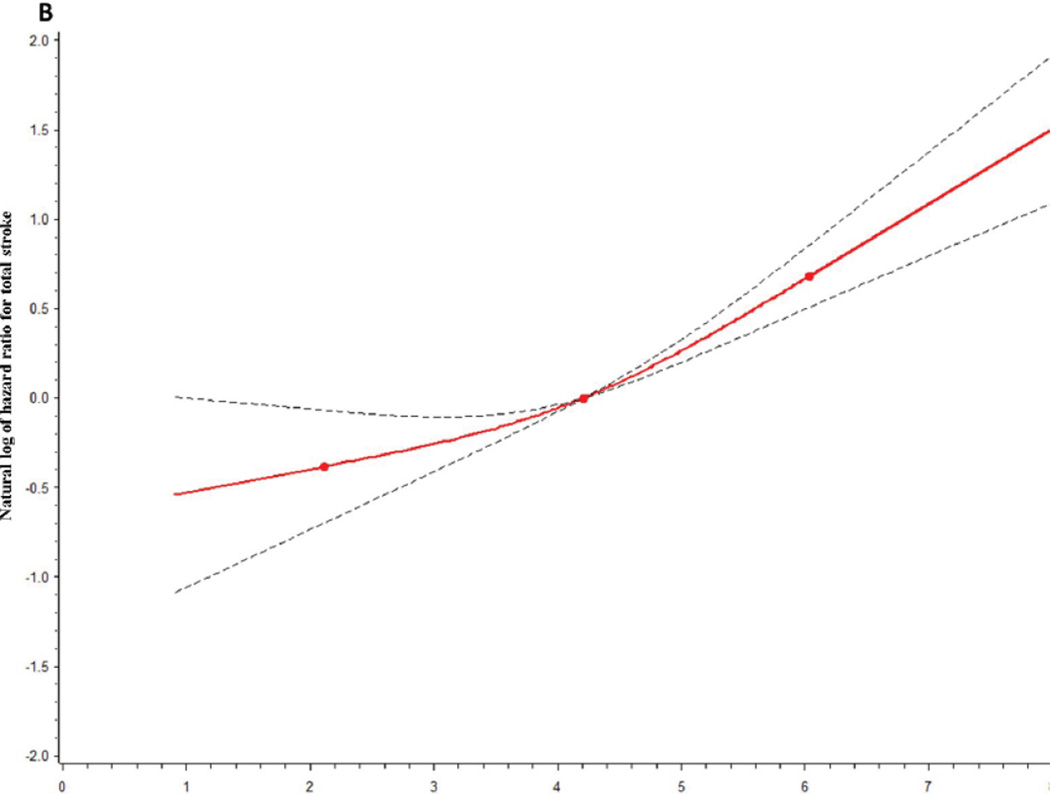
Figure. A and 1 B. Adjusteda restricted cubic splinesb for the association between total stroke and (A) troponin T and (B) N-terminal pro-B type natriuretic peptide.
aAdjusted for Model 2 risk factors as in Table 1.
bThe reference values for the natural log of hazard ratios were 4.21 for Ln NT-pro BNP and −5.30 for Ln troponin T. The dotted lines indicate 95% confidence bands. 3 knots were used, located at the 5th, 50th, and 95th percentiles of the natural logs of TnT (−5.81, −5.30, −4.02) and NT-pro BNP (2.12, 4.21, 6.04).
NT-proBNP also was a risk marker for total stroke (Table 2): Model 1 HRs for total stroke were 1.61 and 3.52 for the highest two quintiles of NT-proBNP, compared with the first. Adjustment using Model 2 moderately attenuated the HRs. Model 3 adjustments attenuated the HRs only slightly more. The patterns of association between stroke subtypes (Table 2) and NT-proBNP were similar to those for TnT. Cardioembolic stroke showed a particularly strong association with NT-proBNP; the Model 2 HR was 12.6 for the highest versus lowest NT-proBNP quintile. Strikingly, approximately 58% (i.e., 72/125) of cardioembolic strokes occurred in the highest quintile of visit 4 NT-proBNP, compared with 3% (4/125) in the lowest quintile (Table 2). Moreover, 32% of cardioembolic strokes occurred in participants who had both NT-proBNP in the highest quintile and were known by ARIC to have atrial fibrillation sometime before their cardioembolic stroke hospitalization, compared with only 6% of the total cohort being so classified. Figure 1B further shows the NT-proBNP relation with total stroke.
Table 2.
Adjusted hazard ratios and 95% confidence intervals (HR, 95% CI) for incident stroke by N-terminal pro-B type natriuretic peptide quintiles, ARIC, 1996–2009.
| N-terminal pro-B natriuretic peptide quintile (pg/ml) | ||||||
|---|---|---|---|---|---|---|
| ≤27.2 pg/ml | 27.3–51.9 pg/ml | 52.0–87.3 pg/ml | 87.4–155.1 pg/ml | ≥155.2 pg/ml | ||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | P trend | |
| N for Model 1* | 2,181 | 2,185 | 2,180 | 2,177 | 2,179 | |
| N for Models 2† and 3‡ | 2,063 | 2,085 | 2,058 | 2,071 | 2,073 | |
| Total stroke | ||||||
| Rate/1000 person-years* | 2.09 | 3.15 | 2.80 | 3.34 | 6.51 | |
| n of strokes | 61 | 87 | 77 | 89 | 193 | |
| Model 1* | 1 (reference) | 1.51 (1.08, 2.10) | 1.35 (0.96, 1.90) | 1.61 (1.15, 2.26) | 3.52 (2.59, 4.78) | <.0001 |
| Model 2† | 1 (reference) | 1.57 (1.11, 2.22) | 1.35 (0.94, 1.94) | 1.51 (1.05, 2.16) | 2.88 (2.05, 4.05) | <.0001 |
| Model 3‡ | 1 (reference) | 1.56 (1.10, 2.20) | 1.32 (0.92, 1.89) | 1.44 (1.01, 2.07) | 2.63 (1.87, 3.71) | <.0001 |
| Ischemic stroke | ||||||
| n of strokes | 56 | 73 | 65 | 76 | 174 | |
| Model 2† | 1 (reference) | 1.49 (1.03, 2.15) | 1.31 (0.89, 1.92) | 1.45 (0.99, 2.12) | 2.92 (2.04, 4.17) | <.0001 |
| Model 3‡ | 1 (reference) | 1.48 (1.02, 2.13) | 1.27 (0.87, 1.87) | 1.37 (0.93, 2.01) | 2.61 (1.83, 3.74) | <.0001 |
| Lacunar stroke | ||||||
| n of strokes | 16 | 17 | 18 | 16 | 20 | |
| Model 2† | 1 (reference) | 1.35 (0.67, 2.75) | 1.41 (0.69, 2.89) | 1.10 (0.51, 2.39) | 1.26 (0.58, 2.75) | 0.78 |
| Model 3‡ | 1 (reference) | 1.34 (0.66, 2.73) | 1.43 (0.70, 2.92) | 1.10 (0.51, 2.40) | 1.22 (0.56, 2.66) | 0.84 |
| Nonlacunar stroke | ||||||
| n of strokes | 36 | 46 | 34 | 34 | 82 | |
| Model 2† | 1 (reference) | 1.47 (0.92, 2.35) | 1.03 (0.62, 1.72) | 1.06 (0.63, 1.79) | 2.30 (1.43, 3.69) | 0.004 |
| Model 3‡ | 1 (reference) | 1.48 (0.93, 2.35) | 1.03 (0.62, 1.72) | 1.06 (0.63, 1.78) | 2.29 (1.42, 3.67) | 0.004 |
| Cardioembolic stroke | ||||||
| n of strokes | 4 | 10 | 13 | 26 | 72 | |
| Model 2† | 1 (reference) | 2.28 (0.70, 7.44) | 3.23 (1.04, 10.04) | 5.55 (1.87, 16.4) | 12.6 (4.36, 36.1) | <.0001 |
| Model 3‡ | 1 (reference) | 2.21 (0.68, 7.21) | 2.93 (0.94, 9.09) | 4.70 (1.58, 13.9) | 9.01 (3.11, 26.1) | <.0001 |
| Hemorrhagic stroke | ||||||
| n of strokes | 5 | 14 | 12 | 13 | 19 | |
| Model 2† | 1 (reference) | 2.42 (0.77, 7.58) | 1.79 (0.55, 5.86) | 2.09 (0.65, 6.72) | 2.72 (0.84, 8.76) | 0.20 |
| Model 3‡ | 1 (reference) | 2.41 (0.77, 7.57) | 1.77 (0.54, 5.80) | 2.07 (0.64, 6.68) | 2.70 (0.84, 8.69) | 0.21 |
Model 1 - Adjusted for age, sex, and race.
Model 2 - Adjusted for model 1 + body mass index, smoking status and amount, diabetes, systolic blood pressure, antihypertensive medication use, HDL cholesterol, total cholesterol, lipid medication use, C-reactive protein, lipoprotein-associated phospholipase A2 (Lp-PLA2), atrial fibrillation, coronary heart disease, and heart failure.
Model 3 - Same as Model 2, except incident atrial fibrillation, coronary heart disease, and heart failure were treated as time-dependent covariates.
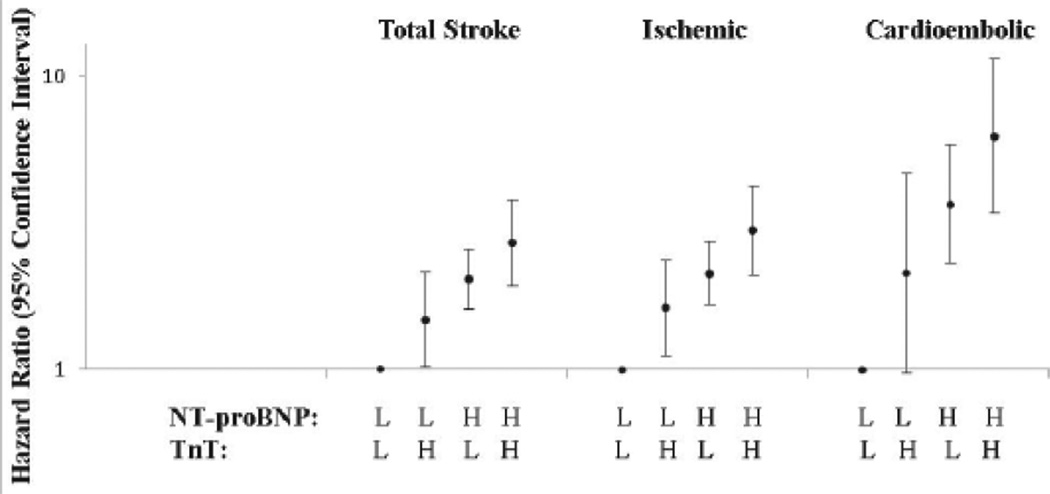
As shown in Figure 2 and Supplemental Table 3, when considered together, elevated TnT and NT-proBNP each were associated with total and ischemic stroke, with no multiplicative interactions (p for interactions > 0.10). NT-proBNP seemingly had a stronger association than did TnT with stroke. For example, for total stroke, the HR for participants with high TnT and low NT-proBNP was 1.48 and the HR for high NT-proBNP and low TnT was 2.03, with the HR for both biomarkers high being 2.70. For cardioembolic stroke, these HRs were 2.13, 3.67, and 6.26, respectively.
Figure 2. Adjusteda hazard ratios for incident stroke by joint NT-proBNP and TnT levelsb, ARIC, 1996–2009.
aAdjusted for Model 2 risk factors as in Table 1.
bLow (L) NT-proBNP group is <155.2 pg/ml, high (H) is ≥155.2. Low (L) TnT group is <0.014 µg/L, high (H) is ≥0.014.
Based on Model 2, 42% of participants had a 10-year stroke risk of <2%, 39% had a risk of 2–4.99%, 14% had a risk of 5–9.99%, and 5% had a ≥10% risk. Measurement of NT-proBNP, but not TnT, modestly improved prediction of 10-year stroke risk beyond Model 2 risk predictions (Supplemental Table 4). The AUC (95% confidence interval) for Model 2 was 0.743 (0.719, 0.768), and it increased to 0.753 (0.729, 0.777) by adding NT-proBNP to the prediction model and to only 0.755 (0.731, 0.779) by further adding TnT. The net reclassification index was 0.06 upon adding NT-proBNP to Model 2 and 0.05 upon adding both markers (Supplemental Table 4).
Discussion
This large prospective epidemiological investigation found that, independent of other measured risk factors, higher plasma levels of TnT and NT-proBNP in the general population were moderately strong risk markers for incident ischemic stroke. The stroke associations were somewhat stronger for NT-proBNP than TnT. The associations also were strongest for future cardioembolic stroke (e.g., 9-fold for the highest versus lowest categories of NT-proBNP and 2.6 fold for TnT), followed by nonlacunar atherothrombotic stroke, and not found for lacunar or for hemorrhagic strokes. The combination of both elevated TnT and NT-proBNP tripled the risk of ischemic stroke, compared with low levels of both biomarkers. Our findings, drawn from a general population sample and including stroke subtypes, support growing evidence associating high levels of natriuretic peptides with increased risk of stroke3,5,8–13 and with more limited evidence on troponin and stroke.13–15
Elevated plasma TnT and NT-proBNP are generally considered cardiac biomarkers, reflecting cardiac myonecrosis and heart failure, respectively. These biomarkers are also markers of future major adverse cardiac events and even mortality from non-cardiovascular causes (unpublished ARIC data). They also are correlated with multiple other vascular conditions and risk factors (Supplemental Tables 1 and 2). It therefore was important to establish that TnT and NT-proBNP remained associated with nonlacunar ischemic strokes even after adjustment for risk factors and cardiac conditions. Although elevated TnT and NT-proBNP should not be considered causal risk factors for ischemic stroke, they do seem to be independently associated with stroke occurrence, especially in the highest categories. Clinical use of stroke “risk equations” is not widespread, but our analysis showed that NT-proBNP adds modestly to traditional risk factors for identifying patients at high stroke risk for possible prevention. If our findings received external validation in other populations and measurement proved cost-effective, NT-proBNP might be useful clinically in stroke risk prediction in the general population.
Stronger associations of TnT and NT-proBNP with the cardioembolic subtype of stroke were hypothesized in advance, given the pathophysiology of cardioembolic stroke. NT-proBNP is a strong predictor of atrial fibrillation, which is a common contributor to cardioembolic stroke.26 The association of TnT and NT-proBNP with atherothrombotic stroke is harder to explain but may reflect (1) coexisting atherosclerotic cerebrovascular and cardiac disease or (2) possible misclassification of some cardioembolic strokes as non-lacunar ischemic strokes in the absence of a clear cardioembolic source.
Drawbacks of this study warrant consideration. Firstly, we had only single measures of the plasma biomarkers. In so far as there is random biological variability in the biomarkers, observed associations with stroke incidence would tend to be weakened. We also could not examine, with a single measure, the association of change in biomarkers with incident stroke. Secondly, although we had visit 4 electrocardiograms and adjusted for prevalent atrial fibrillation, CHD, and heart failure, we did not have visit 4 echocardiographic assessments to identify the structural or functional cardiac abnormalities that may have led to visit 4 TnT or NT-proBNP elevations. Thirdly, our assessment of incident atrial fibrillation, CHD, and heart failure was based on hospitalization discharge diagnoses. Incident non-hospitalized and other forms of heart disease therefore could not be accounted for. Yet, at the time of stroke, we did use all available echocardiographic and carotid imaging data in the hospital record to help subclassify strokes. Fourthly, although our validation of stroke events was thorough, it was based, as in most epidemiological studies, on medical reviews and not standardized neurologic examinations of all potential stroke patients. Finally, our non-lacunar ischemic stroke group could not be separated well into large vessel disease versus other ischemic or cryptogenic ischemic strokes. We did have information on whether these strokes had carotid artery imaging and were believed by reviewers to be carotid atheroembolic; however this subgroup was too small for meaningful analysis. It remains possible that the biomarker relations would differ among these smaller non-lacunar subtypes.
Conclusions
In conclusion, we found that in the general population that elevated plasma TnT and NT-proBNP concentrations are associated with increased risk of cardioembolic and other nonlacunar ischemic strokes.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Ms. Bell was supported by an NHLBI training grant, T32 HL07779.
Dr. Ballantyne has received grant support from Roche Diagnostics. Dr. Nambi is on the advisory board for Roche.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The other authors declare no commercial conflicts of interest (but receive National Institutes of Health grant funding).
References
- 1.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 4.Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Govin R, Saleheen D, et al. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 5.Rutten JH, Mattace-Raso FU, Steyerberg EW, Lindemans J, Hofman A, Wieberdink RG, et al. Amino-terminal pro-B-type natriuretic peptide improves cardiovascular and cerebrovascular risk prediction in the population: the Rotterdam study. Hypertension. 2010;55:785–791. doi: 10.1161/HYPERTENSIONAHA.109.143313. [DOI] [PubMed] [Google Scholar]
- 6.Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: A systematic review. Cerebrovasc Dis. 2009;28:220–226. doi: 10.1159/000226773. [DOI] [PubMed] [Google Scholar]
- 7.Rost NS, Biffi A, Cloonan L, Chorba J, Kelly P, Greer D, et al. Brain natriuretic peptide predicts functional outcome in ischemic stroke. Stroke. 2012;43:441–445. doi: 10.1161/STROKEAHA.111.629212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 9.Winkler K, Wanner C, Drechsler C, Lilienthal J, März W, Krane V for the German Diabetes and Dialysis Study Investigators. Change in N-terminal-pro-B-type-natriuretic-peptide and the risk of sudden death, stroke, myocardial infarction, and all-cause mortality in diabetic dialysis patients. Eur Heart J. 2008;29:2092–2099. doi: 10.1093/eurheartj/ehn278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, Nakamura M, Onoda T, Ohsawa M, Tanno K, Itai K, et al. Predictive value of plasma B-type natriuretic peptide for ischemic stroke: A community-based longitudinal study. Atherosclerosis. 2009;207:298–303. doi: 10.1016/j.atherosclerosis.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 11.Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R for the PEACE Investigators. Prognostic value of B-type natriuretic peptides in patients with stable coronary artery disease. The PEACE Trial. J Am Coll Cardiol. 2007;50:205–214. doi: 10.1016/j.jacc.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Doi Y, Ninomiya T, Hata J, Hirakawa Y, Mukai N, Ikeda F, et al. N-terminal pro-brain natriuretic peptide and risk of cardiovascular events in a Japanese community: the Hisayama study. Arterioscler Thromb Vasc Biol. 2011;31:2997–3003. doi: 10.1161/ATVBAHA.111.223669. [DOI] [PubMed] [Google Scholar]
- 13.Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: A Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) Substudy. Circulation. 2012;125:1605–1616. doi: 10.1161/CIRCULATIONAHA.111.038729. [DOI] [PubMed] [Google Scholar]
- 14.Kavsak PA, Xu L, Yusuf S, McQueen MJ. High-sensitivity cardiac troponin I measurement for risk stratification in a stable high-risk population. Clin Chem. 2011;57:1146–1153. doi: 10.1373/clinchem.2011.164574. [DOI] [PubMed] [Google Scholar]
- 15.Everett BM, Cook NR, Magnone MC, Bobadilla M, Kim E, Rifai N, et al. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: The Women’s Health Study. Circulation. 2011;123:2811–2818. doi: 10.1161/CIRCULATIONAHA.110.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baecke JAH, Burema J, Fritters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:932–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 19.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 21.The National Survey of Stroke. National Institute of Neurological and Communicative Disorders and Stroke. Stroke. 1981;12:I1–I91. [PubMed] [Google Scholar]
- 22.Ohira T, Shahar E, Chambless LE, Rosamond W, Mosley TH, Jr, Folsom A. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2006;37:2493–2498. doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 23.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 24.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology. 2010;21:128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambless LE, Cummiskey CP, Cui G. Several methods to assess improvement in risk prediction models: Extension to survival analysis. Stat Med. 2011;30:22–38. doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 26.Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.