Abstract
Eukaryotic elongation factor-2 kinase (eEF-2K) is a Ca2+/calmodulin-dependent enzyme that negatively regulates protein synthesis. eEF-2K has been shown to be up-regulated in cancer, and to play an important role in cell survival through inhibition of protein synthesis. Post-translational modification of protein synthesis machinery is important for its regulation and could be critical for survival of cancer cells encountering stress. The purpose of our study was to examine the regulation of eEF-2K during stress with a focus on the roles of phosphorylation in determining the stability of eEF-2K. We found that stress conditions (nutrient deprivation and hypoxia) increase eEF-2K protein. mRNA levels are only transiently increased and shortly return to normal, while eEF-2K protein levels continue to increase after further exposure to stress. A seemingly paradoxical decrease in eEF-2K stability was found when glioma cells were subjected to stress despite increased protein expression. We further demonstrated that phosphorylation of eEF-2K differentially affects the enzyme’s turnover under both normal and stress conditions, as evidenced by the different half-lives of phosphorylation-defective mutants of eEF-2K. We further found that the eEF-2K site (Ser398) phosphorylated by AMPK is pivotal to the protein’s stability, as the half-life of S398A mutant increases to greater than 24 h under both normal and stress conditions. These data indicate that eEF-2K is regulated at multiple levels with phosphorylation playing a critical role in the enzyme’s turnover under stressful conditions. The complexity of eEF-2K phosphorylation highlights the intricacies of protein synthesis control during cellular stress.
Keywords: eEF-2K, Phosphorylation, Enzyme stability, Protein synthesis, Glioblastoma, AMPK
1. Introduction
Elongation factor-2 kinase (eEF-2K) is a member of the calcium/calmodulin-dependent kinase (CaMK) family of serine/threonine protein kinases. eEF-2K is responsible for the phosphorylation of eukaryotic elongation factor 2 (eEF-2) [1]. eEF-2 promotes peptide elongation, and its phosphorylation at Thr56 decreases its affinity for the ribosome resulting in termination of peptide elongation [2,3]. As an important regulator of protein synthesis, eEF-2K is normally expressed at varying levels in human and vertebrate tissues [4]. Increased eEF-2K activity has been observed in a variety of circumstances including cellular differentiation [5], cell cycle progression [6], cell growth [7], and neuronal functions [8,9].
Previous studies have shown that eEF-2K plays a critical role in cell survival through its inhibition of protein synthesis and that the enzyme’s protein levels are increased in cancer cell lines and tumors, including human glioblastoma [10–12]. eEF-2K can act as an energy sensor, inducing a cytoprotective effects [13]. Association with cell survival was first seen in hibernating squirrels, where pEF-2 and eEF-2K were increased in their brain and liver samples during stressful environmental conditions that reduced oxygen profusion and nutrient availability [14]. eEF-2K has also been shown to play a role in energy homeostasis through its regulation of autophagy during ER-stress [15] and amino acid deprivation [16]. Inhibition of eEF-2K in cancer cells during stress can inhibit autophagy and lead to cell death during various stresses including metabolic stress [16,17] and growth factor inhibition [18].
eEF-2K activity is regulated by multiple upstream signaling cascades through its phosphorylation. The mTOR/S6 kinase pathway phosphorylates eEF-2K on Ser78 and Ser366 to inhibit the enzyme’s activity [19,20], while the AMPK pathway phosphorylates it at Ser398 activating eEF-2K [21]. These pathways tie into cellular stress and energy homeostasis, as the mTOR/S6 kinase signals during nutrient-rich conditions while AMP kinase signals during times of low ATP and stress [22].
While phosphorylation is known to regulate eEF-2K activity, the precise role of the modification is largely unknown. Protein turnover is necessary to conserve the integrity of the cellular proteins. Continual turnover of cellular proteins also creates a homeostasis in protein amounts and activity, and a delicate balance exists between breakdown of proteins and their synthesis. eEF-2K has been shown to be degraded by the ubiquitin–proteasome pathway, with a half-life of approximately 6–8 h in human glioma cells [23]. A recent study by Kruiswijk et al. revealed that autophosphorylation of eEF-2K at Ser445 can affect the enzyme’s degradation to promote translation after genotoxic stress [24]. Since nutrient deprivation has been shown to increase eEF-2K protein levels in addition to altering the phosphorylation status of the kinase, here we intended to determine the effects of phosphorylation on eEF-2K protein stability. Rapid changes in eEF-2K protein expression have been implicated in regulating crucial processes within the cell [25–27]; thus, examining the effects of phosphorylation on the enzyme’s stability will be critical for a better understanding how eEF-2K works within the cell and promotes cancer cell survival.
2. Materials and methods
2.1. Cell lines and culture
The human glioma cell lines T98G and LN299were purchased from American Type Culture Collection. T98G(shEF2K) and LN299(shEF2K) cells were stably transfected with a shRNA against eEF-2K in a pcDNA 3.1 vector. T98G cells were cultured in Ham’s F-10/DMEM (10:1); LN229 cells were cultured in DMEM. The cell culture media were supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2/95% air.
2.2. Reagents and antibodies
All cell culture media and reagents were purchased from Hyclone. Cycloheximide was purchased from Sigma. Antibodies to eEF-2K, phospho-eEF-2K (Ser366), S6K, pS6K (Thr389), AMPK, pAMPK (Thr172), and β-actin were purchased from Cell Signaling Technologies while p-eEF-2K (Ser78) was supplied by Santa Cruz. eEF-2K mutants in pcDNA 3.1 vectors were generated by Genewiz.
2.3. Stress conditions
Serum conditions contained 10% FBS. For transient transfections with expression vectors containing phosphorylation mutants eEF-2K at Ser78/Ser366 and Ser398, T98G(shEF2K) cells were transfected with 1 μg of Qiagen-purified DNA and 5 μl of Roche Fugene 6 as previously described [28]. Stress experiments were conducted 24 h after plating or 24 h after transfection. Media was changed to serum-free, glutamine-free, or oxygen-depleted media for serum, amino acid, and oxygen deprivation experiments, respectively. Oxygen deprivation was conducted in a hypoxia chamber at 1% oxygen.
2.4. Real time RT-PCR
Total RNA from T98G cells were extracted using Trizol system according to manufacturer instructions. cDNA was made from harvested total RNA of T98G and LN229 cells (Roche). RT-PCR was performed using TaqMan Gene Expression Assays. GAPDH was used to normalize samples for comparison.
2.5. eEF-2K phosphorylation-defective mutants
Mutation variants of the three eEF-2K phosphorylation sites were generated by site-directed mutagenesis. Phosphorylation-defective mutants were created by exchanging serine coding sequences with alanine coding sequences: eEF-2K Ser78 (TCC) was converted to Ala78 (GCC); Ser366 (TCT) was converted to Ala366 (GCT); and Ser398 (TCT) was converted to Ala398 (GCT). eEF-2K mutant sequences were cloned into Invitrogen pcDNA3.1(+) plasmid vectors.
2.6. Preparation of cellular extracts and Western Blot analysis
Cells were lysed with M-PER mammalian protein extraction reagent (Pierce Biotechnology) which was supplemented with protease inhibitor cocktail (Roche) and a phosphatase inhibitor cocktails 1 and 2 (Sigma) followed by centrifugation. Protein (30 μg) was resolved by SDS–PAGE, and protein signals were detected by ECL method (Perkin Elmer).
2.7. Mining of mRNA functional elements
Web-based tool, RegRNA – A Regulatory RNA Motifs and Elements Finder, was used to find predicted regulatory RNA motifs and elements in eEF-2K [29]. eEF-2K gene accession number NM_013302 was used for database query. 5′- and 3′-UTR regulatory elements of eEF-2K were examined.
3. Results
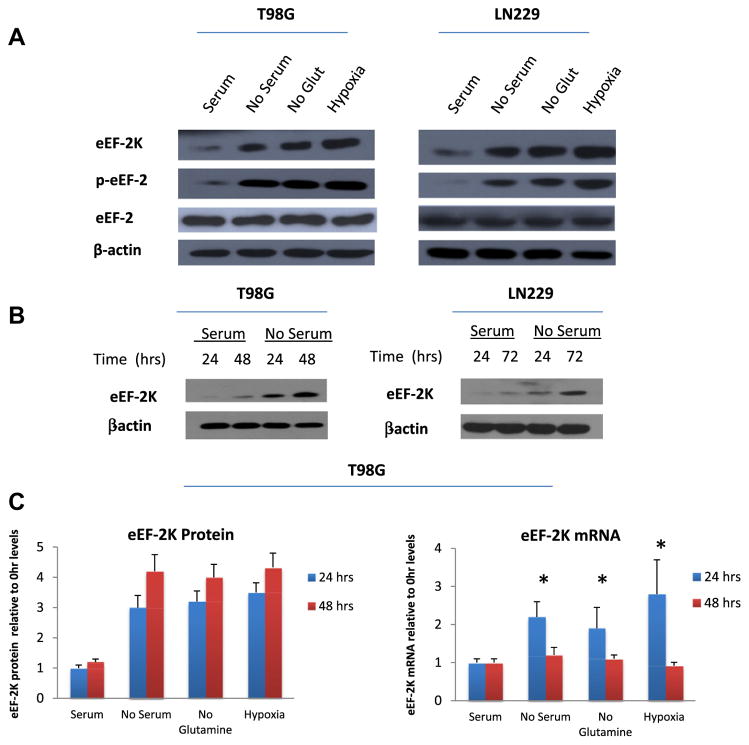
To determine how cellular stresses affect eEF-2K activity and expression, we first determined the effects of nutrient or oxygen deprivation on the expression of eEF-2K mRNA and protein. Fig. 1A shows that incubation of human glioma cells lines, T98G and LN229, with nutrient-deprived media for 48 h increased eEF-2K protein expression. These stressful conditions also increased eEF-2K activity, as indicated by an increased phosphorylation of its substrate, eEF-2 (Fig. 1A).
Fig. 1.
Cellular stresses increase eEF-2K expression while decreasing eEF-2K protein stability. (A) T98G and LN229 cells were cultured in media deficient in serum, glutamine, or oxygen for 24 h. (B) T98G and LN229 cells were cultured in serum-containing or serum-free media for 24, 48, or 72 h. (C) T98G cells were subjected to various stresses for 24 or 48 h. At the end of treatment, eEF-2K protein and mRNA levels were measured. LN229 results (not shown) were similar. The bars represent the mean value ± SD from three independent experiments.
A time course of serum deprivation demonstrates a time-dependent increase of eEF-2K protein levels from 24 to 48 h (Fig. 1B). Analysis of eEF-2K mRNA expression revealed that while mRNA levels transiently increased at 24 h, mRNA levels reverted to baseline by 48 h (Fig. 1C). However, eEF-2K protein levels continued to increase at 48 h despite having a previously reported half-life of ~8 h. These results suggest that enhanced stability of eEF-2K protein could be responsible for the increased eEF-2K expression during stress. Therefore, we next examined the effects of various stresses on eEF-2K protein turnover in human glioma cells. Surprisingly, eEF-2K turnover was increased when glioma cells were subjected to various stresses, with half-lives averaging from 2 to 4 h as opposed to ~8–12 h under normal culture conditions (Fig. 2A).
Fig. 2.
Phosphorylation sites on eEF-2K differentially affect its turnover. (A) T98GshVector and LN229shVector cells were subjected to various stresses for 24 h, followed by treatment with 20 μM of cycloheximide (CHX). Cell samples were collected at time points from 0 to 24 h. LN299 results (not shown) are similar. (B) T98G shVector and LN229shVector cells were treated with various stresses for 24 h. The levels of the respective proteins were examined by Western Blot. (C) LN229shEF2K and T98GshEF2K cells were transfected with the S78A/S366A eEF-2K phosphorylation-defective mutants for 24 h, followed by 24 h of various stress treatments. Cells were then treated with 20 μM of cycloheximide (CHX) and collected at time points from 0 to 24 h. (D) LN229shEF2K and T98GshEF2K cells were transfected with the S398A eEF-2K phosphorylation mutants for 24 h, followed by 24 h of various stress treatments. Cells were then treated with 20 μM of CHX and collected at time points from 0 to 24 h. *p < 0.05.
As multiple pathways are known to signal and phosphorylate eEF-2K, we examined the effects of stress on upstream signaling of eEF-2K. The mTOR pathway deactivates eEF-2K by phosphorylating the enzyme at Ser78 and Ser366, while the AMPK pathway activates eEF-2K through phosphorylation at Ser398 during cellular stress. Fig. 2B shows that cellular stress decreased mTOR signaling and thus S6K phosphorylation while it increased AMPK activation. Since both mTOR/S6K and AMPK are regulators of eEF-2K through its phosphorylation, we created the phosphorylation-defective mutants for S78A/S366A and S398A phosphorylation sites on eEF-2K, and then examined the effects of eEF-2K phosphorylation on the enzyme’s stability during stress. Fig. 2C shows that mutation of the mTOR/S6 kinase sites (S78/366A) resulted in an increased stability of eEF-2K (t1/2 > 24 h) under normal culture conditions. Turnover rates for S78/366A mutants measured during stress conditions decreased to the basal level seen for wild-type eEF-2K under normal culture conditions (t1/2 ~ 8 h). AMPK phosphorylation-site mutants (S398A) of eEF-2K also show increased stability under normal conditions (t1/2 > 24 h), and this stability of eEF-2K continued under all the stress conditions (t1/2 > 24 h) (Fig. 2D). These observation indicate that phosphorylation of eEF-2K at these two sets of sites differentially affects the protein’s turnover in glioma cells.
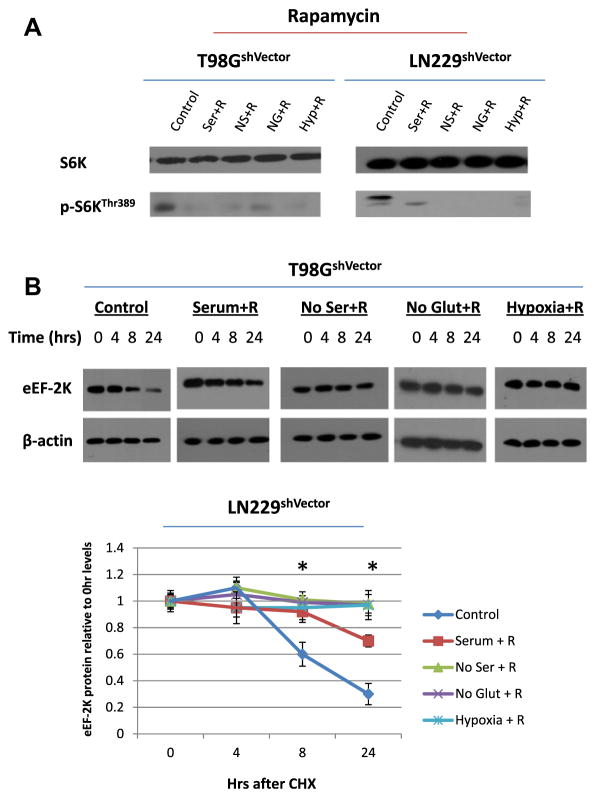
To verify the effects of upstream signaling pathways on eEF-2K stability, we used pharmacological inhibitors of these cascades to compare with the results of mutating their respective phosphorylation sites. Fig. 3A shows that the mTOR inhibitor rapamycin decreased phosphorylation of S6K under all culture conditions including stress. Rapamycin treatment differed from the mTOR/S6 kinase phosphorylation-site mutants of eEF-2K as it decreased the turnover of eEF-2K under both normal and stress conditions (t1/2 > 24 h) (Fig. 3B). Pharmacological inhibition of AMPK activation by Compound C decreased phosphorylation of AMPK (Fig. 4A). eEF-2K stability was decreased under all culture conditions with Compound C treatment (t1/2 < 4 h), which was the opposite result of mutating the AMP kinase phosphorylation-sites of eEF-2K (Fig. 4B). These results indicate that eEF-2K is regulated not only by their known phosphorylation sites but by additional mechanisms.
Fig. 3.
Inhibition of the mTOR pathway with rapamycin increases eEF-2K stability regardless of stress. (A) T98G shVector and LN229shVector cells were treated with 100 ng/ml rapamycin and exposed to various stresses for 48 h. eEF-2K protein expression was then examined. (B) T98G shVector and LN229shVector cells were treated with 100 ng/ml rapamycin and exposed to various stresses for 24 h. Cells were then treated with 20 μM of CHX and collected at time points from 0 to 24 h. Results shown are the representative of three similar experiments.
Fig. 4.
Inhibition of AMPK pathway with compound C decreases eEF-2K stability under both stressful and non-stressful conditions. (A) T98G shVector and LN229shVector cells were treated with 10 μM of compound C and exposed to various stresses for 48 h. eEF-2K protein expression was then examined. (B) T98G shVector and LN229shVector cells were treated with 20 μM compound C and exposed to various stresses for 24 h. Cells were then treated with 20 μM of CHX and collected at time points from 0 to 24 h. Results shown are the representative of three similar experiments.
To explore the mechanism by which cellular stress increases eEF-2K expression, an effect not fully accounted for in eEF-2K mRNA levels, we examined the eEF-2K mRNA transcript sequence for RNA functional elements. Using RNA regulatory motifs and element predictions, we revealed that eEF-2K’s 5-UTR region is predicted to contain multiple internal ribosome entry sites (IRESs) starting at basepairs 181, 387, 551, 1255, and 1470. IRESs are responsible for the initiation of translation by internal ribosome binding of the mRNA when 5′-cap-dependent translation is inhibited [30]. Numerous upstream open reading frames (uORFs) were predicted as well in the 5′UTR of eEF-2K; uORFs can initiate and enhance translation during specific conditions [31]. These two types of RNA functional elements may elucidate the mechanism behind increased eEF-2K protein levels despite a decrease in mRNA levels.
4. Discussion
We report here that post-translational modification of eEF-2K, a critical regulator of protein synthesis, is pivotal in regulating the enzyme’s protein levels during periods of cellular stress, including nutrient and oxygen deprivation. After determining that cellular stresses can increase eEF-2K protein levels without an increase in eEF-2K mRNA levels (Fig. 1), we were surprised to find that cellular stress decreased eEF-2K protein stability in human glioma cells (Fig. 2A). This surprising result led us to investigate the role of phosphorylation-status of eEF-2K in determining its stability.
Phosphorylation control of eEF-2K is a complex process, with a variety of signaling pathways converging on eEF-2K in a seemingly paradoxical way. Sites Ser78 and Ser366 are phosphorylated by mTOR [19,20], but stress proteins like AMPK can also phosphorylate this site [21]. In fact, AMPK phosphorylation of its main site, Ser398, can actually induce phosphorylation of mTOR sites Ser78 and Ser366 on eEF-2K [32]. In some types of cells, autophosphorylation is overshadowed by stronger phosphorylation signals, while autophosphorylation of eEF-2K seems to play a role in others. A recent study reveals that eEF-2K autophosphorylation at Ser445 is involved in the degradation of enzyme after genotoxic stress release [24]. eEF-2K can also be phosphorylated by a variety of other proteins including PKA, stress-activated proteins, and cdc2-cyclin B at other phosphorylation sites [33–35]. Contradictory results on the effects of eEF-2K phosphorylation have been found, perhaps partially due to the use of different cell systems including cardiomyocytes, HEK293 cells, and cancer cells. The discrepancy between studies highlights the importance of cell types, culture conditions, and the complex regulation and balance of eEF-2K phosphorylation sites.
Our results revealed that mutations at the mTOR phosphorylation sites, Ser78 and Ser366, increased stability of the eEF-2K protein under normal conditions; however, stress conditions decreased the stability of eEF-2K back to normal levels (Fig. 2C). Thus, it appears that phosphorylation at the mTOR sites decreases eEF-2K stability under normal conditions, but has no effect on eEF-2K under stress conditions. When the AMPK’s phosphorylation sites on eEF-2K were mutated (S398A), stability of the kinase was increased; and this stability is not affected by culture conditions including stress (Fig. 2D). These results indicate that the AMPK site, Ser398, is pivotal to eEF-2K stability, regardless of conditions; phosphorylation at this site appears to decrease stability of eEF-2K. The mTOR mutants might still be reactive to stress, due to the fact that AMPK can still affect the phosphorylation of the kinase at Ser398; this might explain results showing that the mTOR-site mutants reduced the stability of eEF-2K under stress conditions. Because we show that all of our stress conditions activate AMPK, and AMPK is known to phosphorylate Ser78 and Ser366 in addition to Ser398 [32], our results showing that the stability of S398A mutants are not affected during stress indicate that Ser78 and Ser398 phosphorylations might not be as important as phosphorylation at Ser398. This reduced stability of eEF-2K under stress signaling by Ser398 is unexpected; however, quick turnover of a regulatory protein is not unheard of for translational factors [36]. The tight regulation would allow elongation to vary with the fluctuations in global translation rate.
The unmatched levels of mRNA, protein levels, and protein turnover found in this study of eEF-2K are uncommon, but discordant mRNA levels and protein stability have been found previously in cells, such as with c-myc [37] and stabilization of mRNAs involved in reduction of global translation during ER-stress [38]. These findings could be keys to understanding the seemingly paradoxical changes in eEF-2K protein levels and stability. We propose that the unexpected decrease in eEF-2K protein stability during stress may be a compensatory mechanism for an additional level of regulation at the post-transcriptional level that increases eEF-2K translation. Due to the importance of translation regulation during stress, it is reasonable to have increased translation of a regulator of protein synthesis while decreasing the same protein’s stability in order to quickly adapt to changing nutrient levels. Upon mining databases of RNA functional elements, we found that eEF-2K contains multiple predicted IRESs and uORFs in its 5′-UTR, which would regulate the rate and specific translation of eEF-2K protein. Thus, it is possible that eEF-2K mRNA and protein levels are carefully regulated by the cell, which may quickly and efficiently increase eEF-2K translation during stress while destabilizing both the protein and its mRNA in order to allow for fast resumption of global protein synthesis when favorable conditions return. Further studies will examine the post-transcriptional regulation of EF-2K during stress as these data demonstrate its complex and tight regulation.
The differential regulation of eEF-2K stability by phosphorylation in response to upstream signaling and stress should be carefully considered when studying protein synthesis. The results presented here, including the pharmacological and mutation discrepancies (Figs. 3 and 4), indicate that eEF-2K stability and phosphorylation are delicately balanced by numerous upstream pathways, and the field would benefit from further studies of upstream signaling and phosphorylation eEF-2K. Ser398 appears to be a pivotal phosphorylation site regulating eEF-2K turnover, so exploring the different pathways that affect this site could be critical for understanding the complex regulation of translation. The discordant levels eEF-2K mRNA and protein demonstrate the complexity of protein synthesis control and the ability of stress to alter phosphorylation and genetic expression at multiple levels. Our findings on the effect of phosphorylation on eEF-2K stability in response to stress give further insight into how cells balance and regulate protein synthesis machinery under varied cellular conditions.
Acknowledgments
This study was supported by a Grant (R01CA135038) from the US Public Health Service.
References
- 1.Proud CG. Peptide-chain elongation in eukaryotes. Mol Biol Rep. 1994;19:161–170. doi: 10.1007/BF00986958. [DOI] [PubMed] [Google Scholar]
- 2.Ryazanov AG, Davydova EK. Mechanism of elongation factor 2 (EF-2) inactivation upon phosphorylation. Phosphorylated EF-2 is unable to catalyze translocation. FEBS Lett. 1989;251:187–190. doi: 10.1016/0014-5793(89)81452-8. [DOI] [PubMed] [Google Scholar]
- 3.Nairn AC, Palfrey HC. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987;262:17299–17303. [PubMed] [Google Scholar]
- 4.Pavur KS, Petrov AN, Ryazanov AG. Mapping the functional domains of elongation factor-2 kinase. Biochemistry. 2000;39:12216–12224. doi: 10.1021/bi0007270. [DOI] [PubMed] [Google Scholar]
- 5.End D, Hanson M, Hashimoto S, Guroff G. Inhibition of the phosphorylation of a 1000,000-dalton soluble protein in whole cells and cell-free extracts of PC12 pheochromocytoma cells following treatment with nerve growth factor. J Biol Chem. 1982;257:9223–9225. [PubMed] [Google Scholar]
- 6.Smith EM, Proud CG. Cdc2-cyclin B regulates eEF2 kinase activity in a cell cycle- and amino acid-dependent manner. EMBO J. 2008;27:1005–1016. doi: 10.1038/emboj.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palfrey HC, Nairn AC, Muldoon LL, Villereal ML. Rapid activation of calmodulin-dependent protein kinase III in mitogen-stimulated human fibroblasts. Correlation with intracellular Ca2+ transients. J Biol Chem. 1987;262:9785–9792. [PubMed] [Google Scholar]
- 8.Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- 9.Weatherill DB, McCamphill PK, Pethoukov E, Dunn TW, Fan X, Sossin WS. Compartment-specific, differential regulation of eukaryotic elongation factor 2 and its kinase within Aplysia sensory neurons. J Neurochem. 2011;117:841–855. doi: 10.1111/j.1471-4159.2011.07251.x. [DOI] [PubMed] [Google Scholar]
- 10.Parmer TG, Ward MD, Yurkow EJ, Vyas VH, Kearney TJ, Hait WN. Activity and regulation by growth factors of calmodulin-dependent protein kinase III (elongation factor 2-kinase) in human breast cancer. Br J Cancer. 1999;79:59–64. doi: 10.1038/sj.bjc.6690012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagaglio DM, Cheng EH, Gorelick FS, Mitsui K, Nairn AC, Hait WN. Phosphorylation of elongation factor 2 in normal and malignant rat glial cells. Cancer Res. 1993;53:2260–2264. [PubMed] [Google Scholar]
- 12.Parmer TG, Ward MD, Hait WN. Effects of rottlerin, an inhibitor of calmodulin-dependent protein kinase III, on cellular proliferation, viability, and cell cycle distribution in malignant glioma cells. Cell Growth Differ. 1997;8:327–334. [PubMed] [Google Scholar]
- 13.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Matsushita M, Nairn AC, Damuni Z, Cai D, Frerichs KU, Hallenbeck JM. Mechanisms for increased levels of phosphorylation of elongation factor-2 during hibernation in ground squirrels. Biochemistry. 2001;40:11565–11570. doi: 10.1021/bi010649w. [DOI] [PubMed] [Google Scholar]
- 15.Boyce M, Py BF, Ryazanov AG, Minden JS, Long K, Ma D, Yuan J. A pharmacoproteomic approach implicates eukaryotic elongation factor 2 kinase in ER stress-induced cell death. Cell Death Differ. 2008;15:589–599. doi: 10.1038/sj.cdd.4402296. [DOI] [PubMed] [Google Scholar]
- 16.Wu H, Yang JM, Jin S, Zhang H, Hait WN. Elongation factor-2 kinase regulates autophagy in human glioblastoma cells. Cancer Res. 2006;66:3015–3023. doi: 10.1158/0008-5472.CAN-05-1554. [DOI] [PubMed] [Google Scholar]
- 17.Wu H, Zhu H, Liu DX, Niu TK, Ren X, Patel R, Hait WN, Yang JM. Silencing of elongation factor-2 kinase potentiates the effect of 2-deoxy-D-glucose against human glioma cells through blunting of autophagy. Cancer Res. 2009;69:2453–2460. doi: 10.1158/0008-5472.CAN-08-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Li H, Ren X, Niu T, Hait WN, Yang J. Cytoprotective effect of the elongation factor-2 kinase-mediated autophagy in breast cancer cells subjected to growth factor inhibition. PLoS One. 2010;5:e9715. doi: 10.1371/journal.pone.0009715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol. 2004;24:2986–2997. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;20:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 22.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 23.Arora S, Yang JM, Hait WN. Identification of the ubiquitin-proteasome pathway in the regulation of the stability of eukaryotic elongation factor-2 kinase. Cancer Res. 2005;65:3806–3810. doi: 10.1158/0008-5472.CAN-04-4036. [DOI] [PubMed] [Google Scholar]
- 24.Kruiswijk F, Yuniati L, Magliozzi R, Low TY, Lim R, Bolder R, Mohammed S, Proud CG, Heck AJ, Pagano M, Guardavaccaro D. Coupled Activation and Degradation of eEF2K Regulates Protein Synthesis in Response to Genotoxic Stress. Sci Signal. 2012;5:ra40. doi: 10.1126/scisignal.2002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Severinov KV, Melnikova EG, Ryazanov AG. Downregulation of the translation elongation factor 2 kinase in Xenopus laevis oocytes at the final stages of oogenesis. New Biol. 1990;2:887–893. [PubMed] [Google Scholar]
- 26.Celis JE, Madsen P, Ryazanov AG. Increased phosphorylation of elongation factor 2 during mitosis in transformed human amnion cells correlates with a decreased rate of protein synthesis. Proc Natl Acad Sci USA. 1990;87:4231–4235. doi: 10.1073/pnas.87.11.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brady MJ, Nairn AC, Wagner JA, Palfrey HC. Nerve growth factor-induced down-regulation of calmodulin-dependent protein kinase III in PC12 cells involves cyclic AMP-dependent protein kinase. J Neurochem. 1990;54:1034–1039. doi: 10.1111/j.1471-4159.1990.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen LB, Calvin SA, Colvin KE, Wright M. FuGENE 6 Transfection Reagent: the gentle power. Methods. 2004;33:104–112. doi: 10.1016/j.ymeth.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Huang HY, Chien CH, Jen KH, Huang HD. RegRNA: an integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res. 2006;34:W429–W434. doi: 10.1093/nar/gkl333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le SY, Maizel JV., Jr A common RNA structural motif involved in the internal initiation of translation of cellular mRNAs. Nucleic Acids Res. 1997;25:362–369. doi: 10.1093/nar/25.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer HA, Thomas AA. Control of eukaryotic protein synthesis by upstream open reading frames in the 5′-untranslated region of an mRNA. Biochem J. 2002;367:1–11. doi: 10.1042/BJ20011706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- 33.Diggle TA, Subkhankulova T, Lilley KS, Shikotra N, Willis AE, Redpath NT. Phosphorylation of elongation factor-2 kinase on serine 499 by cAMP-dependent protein kinase induces Ca2+/calmodulin-independent activity. Biochem J. 2001;353:621–626. doi: 10.1042/0264-6021:3530621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goedert M, Cuenda A, Craxton M, Jakes R, Cohen P. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997;16:3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knebel A, Haydon CE, Morrice N, Cohen P. Stress-induced regulation of eukaryotic elongation factor 2 kinase by SB 203580-sensitive and -insensitive pathways. Biochem J. 2002;367:525–532. doi: 10.1042/BJ20020916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chauvin C, Jean-Jean O. Proteasomal degradation of human release factor eRF3a regulates translation termination complex formation. RNA. 2008;14:240–245. doi: 10.1261/rna.728608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spotts GD, Hann SR. Enhanced translation and increased turnover of c-myc proteins occur during differentiation of murine erythroleukemia cells. Mol Cell Biol. 1990;10:3952–3964. doi: 10.1128/mcb.10.8.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawai T, Fan J, Mazan-Mamczarz K, Gorospe M. Global mRNA stabilization preferentially linked to translational repression during the endoplasmic reticulum stress response. Mol Cell Biol. 2004;24:6773–6787. doi: 10.1128/MCB.24.15.6773-6787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]