ABSTRACT
BACKGROUND
Paced respiration has been internationally recommended for vasomotor symptom management, despite limited empirical evidence.
OBJECTIVE
To evaluate efficacy of a paced respiration intervention against breathing control and usual care control for vasomotor and other menopausal symptoms.
DESIGN
A 16-week, 3-group, partially blinded, controlled trial with 2:2:1 randomization and stratification by group (breast cancer, no cancer), in a Midwestern city and surrounding area.
PARTICIPANTS
Two hundred and eighteen randomized women (96 breast cancer survivors, 122 menopausal women without cancer), recruited through community mailings and registries (29 % minority).
INTERVENTIONS
Training, home practice support, and instructions to use the breathing at the time of each hot flash were delivered via compact disc with printed booklet (paced respiration intervention) or digital videodisc with printed booklet (fast shallow breathing control). Usual care control received a letter regarding group assignment.
MAIN MEASURES
Hot flash frequency, severity, and bother (primary); hot flash interference in daily life, perceived control over hot flashes, and mood and sleep disturbances (secondary). Intervention performance, adherence, and adverse events were assessed.
KEY RESULTS
There were no significant group differences for primary outcomes at 8-weeks or 16-weeks post-randomization. Most intervention participants did not achieve 50 % reduction in vasomotor symptoms, despite demonstrated ability to correctly do paced respiration and daily practice. Statistically significant differences in secondary outcomes at 8 and 16 weeks were small, not likely to be clinically relevant, and as likely to favor intervention as breathing control.
CONCLUSIONS
Paced respiration is unlikely to provide clinical benefit for vasomotor or other menopausal symptoms in breast cancer survivors or menopausal women without cancer.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2202-6) contains supplementary material, which is available to authorized users.
KEY WORDS: menopause, vasomotor symptoms, paced respiration, menopausal symptoms
INTRODUCTION
Vasomotor symptoms (hot flashes, night sweats) are the cardinal symptoms of menopause and can be associated with disrupted mood and sleep. Although pharmacological therapies are often the mainstay of treatment,1 women’s preferences or comorbid conditions may preclude their use. Breast cancer survivors, in particular, are unable to take certain pharmacological therapies, due to potential interactions with anti-estrogen therapies.2
Paced respiration has been internationally recommended as a first line therapy for vasomotor symptoms.3 Recommendations are to take slow deep abdominal breaths at the start of a hot flash, breathing in through the nose and out through the mouth.4 However, this recommendation does not mirror tested research protocols. Small studies (n = 14 to 33) tested the following protocol:5–7 During eight, biweekly, one-hour laboratory sessions, participants were connected to monitoring equipment that measured and graphed chest and abdominal expansion. A research assistant instructed participants to breathe 6–8 times per minute while increasing chest expansions on the polygraph. In addition, participants were instructed to practice this at home for 15 min, twice daily. These protocols were significantly more efficacious than attention control5–7 and reduced vasomotor symptoms by ≥ 50 % among healthy menopausal women,5,6 a clinically meaningful change desired by symptomatic women.8,9 However, it is not easily implemented in clinical practice. Larger scale studies have combined verbal or written instructions on paced respiration (with or without daily practice) with other cognitive, behavioral, or pharmacological therapies, but have not tested paced respiration alone for vasomotor symptoms.10–12 There are no large scale studies testing the intensive laboratory-based protocol, none testing a portable and more easily disseminated version of paced respiration training for vasomotor symptoms, and none testing current recommendations for application without daily practice. Thus, international recommendations appear to be based on insufficient empirical evidence.
The purpose of this study was to evaluate efficacy of a paced respiration intervention (daily practice, application at each hot flash) against attention control (fast, shallow breathing) and usual care. Primary outcomes were vasomotor symptoms (hot flash frequency, severity, and bother). Secondary outcomes were hot flash related interference in daily life, perceived control over hot flashes, and mood and sleep disturbances.
METHODS
Design Overview
This was a 16-week, 3-group, partially-blinded, randomized controlled trial with assessments at baseline and at 8-weeks and 16-weeks post-randomization. The intervention group took part in a 2-week post-randomization visit to confirm learning and performance of paced respiration. The institutional review board and cancer center scientific review committee approved the study. Participants provided written, informed consent and authorization to use health information. Consents described a comparison of two breathing methods against usual care to maintain blinding of participants and data collectors. Recruitment occurred April 1, 2009 to February 1, 2011. Study visits occurred May 7, 2009 to June 30, 2011.
Setting and Participants
Recruitment transpired primarily through mass mailings to purchased lists of community-dwelling women and registry participants (tumor registries, Dr. Susan Love Research Foundation’s Love/Avon Army of Women registry) and via breast cancer and high-risk clinics in the Midwest. Eligible participants were adult women: reporting two or more hot flashes per 24-hour day of moderate or greater severity (≥ 4 using 0 to 10 point numeric rating scale); desiring treatment; with peri-menopausal or post-menopausal status; in good general physical and mental health; without self-reported breathing difficulties; being metropolitan statistical area residents (60 mile radius) or willing to commute to the study site; and who were English literate. Hot flash frequency and severity were assessed via self-report at initial telephone screening, and confirmed via prospective electronic diaries at baseline. Breast cancer survivors were > 4 weeks post-completion of surgery, radiation, and/or chemotherapy for non-metastatic breast cancer, and had no other cancer history. The non-cancer group had no history of any cancer. Women taking hot flash treatments were not excluded, but had to agree to continue these treatments for the study duration and use (yes/no) was evaluated as a covariate.
Data Collection
Mailings directed interested women to phone a research office. Trained staff determined eligibility, reviewed study requirements, and addressed questions before mailing study materials. Interested women signed a consent and authorization form and returned them by mail. Women who did not return items were telephoned to query interest and address questions.
Trained and blinded data collectors arranged and conducted the 30-minute, private visits. At baseline, they taught women to use electronic hot flash diaries for ≥ 24 h to ≤ 7 days (time determined by participant choice), verified completion of paper questionnaires, and measured height and weight. Once electronic diaries were returned in person or via prepaid mailers, baseline hot flashes were reviewed to confirm eligibility. Visits at 8 and 16 weeks occurred similarly. Compensation ($25) occurred at each visit.
Participants and blinded data collectors were told that a random subset of participants would participate in an extra visit, 2 weeks after randomization, for quality assurance. Such visits were conducted by other, non-blinded staff and only with intervention participants. The goal was to obtain physiological recordings of breath rate to verify correct performance of paced respiration. Staff referred participants with questions or problems back to their study materials and thus, did not act as in-person interventionists. This quality assurance monitoring continued as part of the 8-week and 16-week visits.
Randomization and Intervention
Randomization occurred in a 2:2:1 fashion in blocks of ten, with stratification by group (cancer, non-cancer). Biostatisticians furnished a password protected, electronic randomization list to the project manager responsible for randomization.
The paced respiration intervention group received a compact disc with paper booklet. The booklet reinforced instructions on the first audio track for how to accomplish a target breath rate of 6–8 breaths per minute, practice twice per day for 15 min, and apply the breathing at the onset of each hot flash. Women were instructed to do slow, deep, abdominal breathing in through the nose and out through the mouth as per international recommendations.4 They were also instructed to practice twice per day for 15 min as per the small, laboratory-based studies.5,6 The second and third tracks contained specially composed, digitally recorded music to help entrain the breath rate and structure the length of practice. Details of this intervention are contained in another manuscript (submitted).
The breathing control group received a digital videodisc with paper booklet. The booklet reinforced voice-over and video demonstration to practice twice per day and to apply the fast shallow breathing at the onset of each flash. A previously published report provides additional details and data indicating this program was a suitable attention control.13
The usual care group received an investigator-signed letter explaining they were not selected to receive any study materials during the 16-week follow-up. These participants received paced respiration materials by mail after study completion.
Outcomes and Follow-up
Primary outcomes were hot flash frequency, severity, and bother (vasomotor symptoms), prospectively recorded in real time via an electronic diary at baseline and at 8 and 16weeks. Participants pressed buttons on a small device (Biolog™ ESR, UFI, Morro Bay, CA) to record each hot flash, and rate severity and bother, from 0 (not at all) to 10 (extremely). After diary data were downloaded, frequency, severity, and bother were calculated as 24-hour averages at each time point. Higher scores indicated worse outcomes (greater frequency, severity, bother).
Secondary outcomes were assessed with well-validated, standardized questionnaires at baseline and at 8 and 16 weeks: the Hot Flash Related Daily Interference Scale (HFRDIS),14 Perceived Control over Hot Flashes Index (PCI),15,16 Positive and Negative Affect Scale (PANAS),17,18 Profile of Mood States-short form (POMS-SF),19,20 and Pittsburgh Sleep Quality Index (PSQI).21 Lower scores on the PCI, PANAS positive affect subscale, and POMS-SF vigor subscale indicated worse outcomes (less control, less positive affect, less vigor). For all others, higher scores indicated worse outcomes (greater interference, greater negative affect, greater mood disturbance, greater sleep problems).
Demographics, menopausal status, prior breath training experience, and breast cancer disease and treatment variables were assessed to describe the sample. Menopausal status was assessed through self-reported menstrual and gynecological history. Adverse events were monitored through participant self-reports.
To assess adherence, intervention participants demonstrated 15 min of paced respiration at 2, 8, and 16 weeks, while wearing an electronic monitoring strap placed around their diaphragm by non-blinded assessors (Model 1132 Pneumotrace II™, UFI, Morro Bay, CA). The strap’s piezo electric sensors recorded expansion and contraction with inhalation and exhalation. After 15 min, the strap was removed. Downloaded data generated minute-by-minute breath rates for each time point. Home practice was recorded on a calendar.22
Statistical Analysis
The required sample size was based on data collected from 60 participants (minimum of two hot flashes per day and average severity ratings ≥ 4): 24-hour hot flash frequency (M = 6.42, SD = 4.54); 24-hour hot flash severity (M = 5.15, SD = 1.68); 24-hour hot flash bother (M = 4.56, SD = 1.77). Power analysis for 16-week pairwise comparisons between the intervention and two control groups on the primary outcomes was done using an estimated 50 % reduction in hot flashes with intervention versus 25 % reduction with control. A ≥ 50 % reduction in hot flashes was seen in prior laboratory-based studies of paced respiration,5 and appears to be a minimally clinically important difference.8,9 A 25 % reduction is a typical placebo response. Under these assumptions with two strata and 2:2:1 randomization, 193 participants were needed to achieve 80 % power to detect a difference between groups with alpha of 0.05 for the primary outcomes, using a type I error rate control similar to the well-known Bonferroni procedure. Total enrollment was estimated at 213 to allow for attrition.
All analyses are based on all randomized subjects, regardless of adherence. Hot flash frequency was the total number of hot flashes and night sweats divided by the total diary recording time to produce an average over 24 hours. Severity and bother were also averaged over 24 hours. If no hot flashes were recorded, frequency, severity, and bother were set to zero.
We used frequency tables, including p-values from analysis of variance (ANOVA), for continuous baseline covariates and from Fisher’s exact tests for categorical baseline covariates to compare randomized groups. Analyses for primary and secondary outcomes at 8 and 16 weeks were based on analysis of covariance (ANCOVA) to compare group differences adjusting for corresponding baseline outcomes. Pairwise comparisons between intervention and breathing control groups at 8 and 16 weeks were also based on ANCOVA. We did not adjust for multiple comparisons since all outcomes were hypothesis driven.
There were three different methods to handle missing data: all completed cases data analysis, last observation carry forward, and multiple imputations. The completed cases analysis (presented below) used all cases with baseline and 8-week data for the 8-week analysis and all cases with baseline and 16-week data for the 16-week analysis. The multiple imputations procedure was based on SAS 9.3, which imputes missing data five times using regression on variables significantly associated with all three primary outcomes of hot flash frequency, severity and bother (i.e. body mass index, income, smoking, hot flash therapies). All methods led to the same conclusions with only slight differences in p-values. Results did not vary by strata (cancer, no cancer) or by use of hot flash therapies (no, yes). We did not adjust for use of aromatase inhibitors (AI) or selective estrogen receptor modulators (SERM), type of hot flash treatment, or any other covariates in Table 1, because no imbalance was noted due to randomization.
Table 1.
Baseline Characteristics by Group
| Paced respiration intervention (n = 88) | Breathing control (n = 86) | Usual care control (n = 44) | p | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Age | 53.44 (6.84) | 51.93 (6.81) | 53.52 (5.68) | 0.25 |
| Body mass index | 29.06 (7.50) | 29.97 (6.94) | 27.62 (4.95) | 0.18 |
| Years of education | 15.01 (2.34) | 15.05 (2.36) | 15.05 (2.16) | 0.99 |
| Concurrent medications | 2.32 (2.09) | 2.30 (1.98) | 2.41 (1.98) | 0.96 |
| Comorbid conditions | 1.83 (1.24) | 1.73 (1.26) | 1.61 (1.26) | 0.64 |
| Past hot flash therapies tried | 2.08 (1.74) | 2.20 (1.65) | 1.84 (1.63) | 0.52 |
| Current hot flash therapies | 0.45 (0.69) | 0.60 (0.76) | 0.61 (0.75) | 0.29 |
| Years since last menses | 6.87 (7.55) | 7.17 (6.96) | 7.00 (7.88) | 0.97 |
| Electronic hot flash diary days | 3.73 (2.56) | 4.02 (2.56) | 3.95 (2.49) | 0.74 |
| 24-hour hot flash frequency | 7.02 (4.32) | 6.43 (3.96) | 7.31 (4.04) | 0.46 |
| 24-hour hot flash severity | 4.50 (1.60) | 5.23 (1.58) | 5.40 (1.26) | 0.41 |
| n (%) | n (%) | n (%) | ||
| Ethnicity | 0.11 | |||
| Latina | 3 (3 %) | 0 (0 %) | 0 (0 %) | |
| Non-Latina | 85 (97 %) | 86 (100 %) | 44 (100 %) | |
| Race | 0.89 | |||
| White/Caucasian | 61 (69 %) | 62 (72 %) | 32 (73 %) | |
| Other | 27 (31 %) | 24 (28 %) | 12 (27 %) | |
| Marital | 0.99 | |||
| Married/living with partner | 56 (64 %) | 55 (64 %) | 29 (66 %) | |
| Single, widowed | 28 (32 %) | 28 (33 %) | 13 (30 %) | |
| Other | 4 (5 %) | 3 (3 %) | 2 (5 %) | |
| Employment | 0.51 | |||
| Full time | 58 (66 %) | 53 (62 %) | 28 (64 %) | |
| Part time | 9 (10 %) | 13 (15 %) | 9 (20 %) | |
| Not employed | 21 (24 %) | 20 (23 %) | 7 (16 %) | |
| Difficulty paying for basics | 0.28 | |||
| None | 68 (77 %) | 56 (65 %) | 35 (80 %) | |
| Some | 16 (18 %) | 23 (27 %) | 8 (18 %) | |
| A lot | 4 (5 %) | 7 (8 %) | 1 (2 %) | |
| Smoker | 0.74 | |||
| Never | 59 (67 %) | 53 (62 %) | 29 (66 %) | |
| Ever (former, current) | 29 (33 %) | 33 (38 %) | 15 (34 %) | |
| Menopausal status | 0.29 | |||
| Early peri/late peri | 3 (4 %) | 1 (1 %) | 2 (5 %) | |
| Early post | 6 (7 %) | 14 (17 %) | 6 (15 %) | |
| Late post | 73 (89 %) | 68 (82 %) | 33 (80 %) | |
| Current hot flash therapies | 0.77 | |||
| None | 58 (66 %) | 47 (55 %) | 24 (55 %) | |
| Prescription medication | 21 (24 %) | 25 (29 %) | 13 (30 %) | |
| Over-the-counter product | 6 (7 %) | 9 (10 %) | 5 (11 %) | |
| Both | 3 (3 %) | 5 (6 %) | 2 (5 %) | |
| Use of SERM or AI | 0.55 | |||
| No, not currently | 65 (74 %) | 64 (74 %) | 29 (66 %) | |
| Yes, currently | 23 (26 %) | 22 (26 %) | 15 (34 %) | |
| Strata | 0.97 | |||
| Breast cancer survivor | 38 (43 %) | 38 (44 %) | 20 (45 %) | |
| Menopausal woman | 50 (57 %) | 48 (56 %) | 24 (55 %) |
Current hot flash therapies included the following: prescription medications [selective serotonin reuptake inhibitors (n = 44), hormone therapy (n = 4), gabapentin (n = 3), clonidine (n = 2), or a combination of these medications (n = 6)]; over-the counter products [combination product (e.g., soy with multivitamins and black cohosh, n = 8), flax seed oil (n = 6), women’s menopause multivitamin (n = 4), or black cohosh (n = 2)]; or both [a combination of the listed prescription medications and over-the-counter products (n = 10)]
SERM selective estrogen receptor modulator, AI aromatase inhibitor
RESULTS
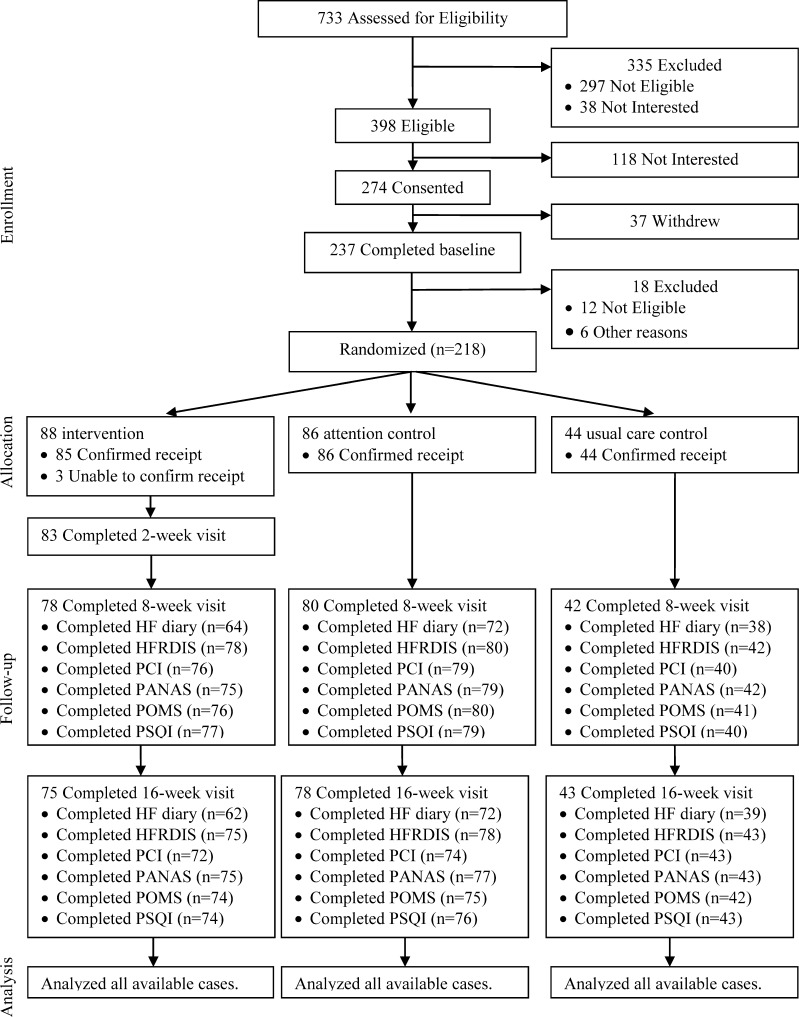
There were 218 women (96 breast cancer survivors and 122 menopausal women) randomized (Fig. 1) with no significant differences at baseline in demographic, clinical, or breast cancer variables (Table 1). Breast cancer participants were stage II or less (90 %), a mean of 5 years post-diagnosis (SD = 5), and had received chemotherapy (53 %) and/or radiation therapy (67 %).
Figure 1.
Participant flow diagram for primary and secondary outcomes. Legend: Accrual diagram showing attrition. HF hot flash, HFRDIS Hot Flash Related Daily Interference, PCI Perceived Control Index, PANAS Positive and Negative Affect Scale, POMS Profile of Mood Disturbance Scale, PSQI Pittsburgh Sleep Quality Index.
Vasomotor Frequency, Severity, and Bother
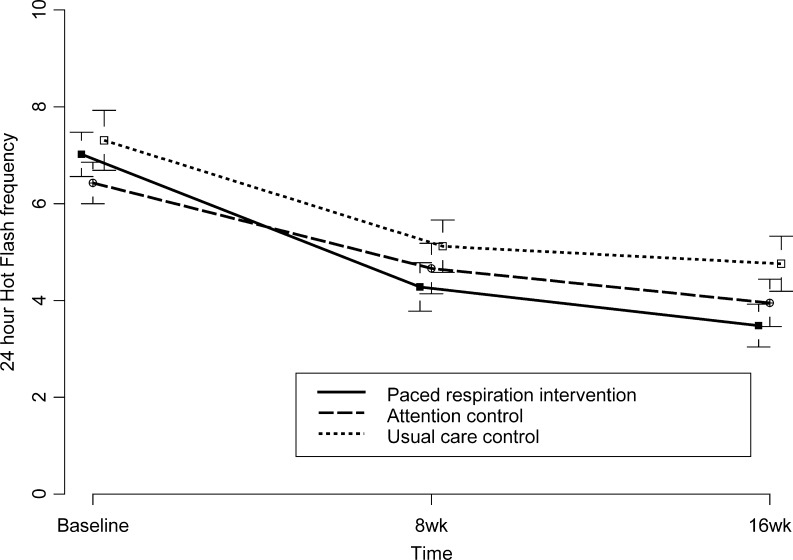
Figure 2 shows unadjusted mean vasomotor symptom frequency across time. Figure 2A and 2B depict severity and bother over time (appendix available online). After adjusting for baseline, there were no statistically significant differences among randomized groups at 8 or 16 weeks (p > 0 .09). Paced respiration was not significantly more efficacious than breathing control or usual care for vasomotor symptoms (p’s > 0.05) or in producing a 50 % reduction in hot flashes. Percentages achieving 50 % reduction in hot flashes from baseline to 8 weeks and sustaining at 16 weeks were: 38 % intervention, 29 % breathing control, and 22 % usual care control. Table 2 shows unadjusted means by group and time for primary outcomes. Table 3 (appendix available online) includes data for secondary outcomes. Figure 3 (appendix available online) depicts effect sizes and 95 % confidence intervals for comparisons between intervention and breathing control.
Figure 2.
24-hour average hot flash frequency by group over time. Legend: Data points are unadjusted means and standard errors with 95 % confidence interval error bars. Frequency was 24-hour average. No significant differences at 8 or 16 weeks.
Table 2.
Primary Outcomes by Group and Time with Comparison Between Intervention and Breathing Control: Unadjusted Means and (Standard Deviation)
| Paced respiration intervention n = 88 | Breathing control n = 86 | Usual care control n = 44 | p | |
|---|---|---|---|---|
| Hot flash frequency | ||||
| Baseline | 7.02 (4.32) | 6.43 (3.96) | 7.31 (4.04) | |
| 8 weeks | 4.28 (4.04) | 4.66 (4.40) | 5.12 (3.35) | 0.18 |
| 16 weeks | 3.48 (3.45) | 3.95 (4.19) | 4.76 (3.54) | 0.09 |
| Hot flash severity | ||||
| Baseline | 4.50 (1.60) | 5.23 (1.58) | 5.40 (1.26) | |
| 8 weeks | 4.50 (2.06) | 4.75 (1.86) | 5.20 (1.41) | 0.82 |
| 16 weeks | 4.33 (2.06) | 4.65 (2.07) | 5.20 (1.89) | 0.88 |
| Hot flash bother | ||||
| Baseline | 4.52 (1.72) | 4.78 (1.72) | 4.69 (1.32) | |
| 8 weeks | 4.02 (1.94) | 4.48 (2.11) | 4.78 (1.41) | 0.39 |
| 16 weeks | 3.89 (1.93) | 4.22 (2.19) | 4.76 (1.73) | 0.94 |
Hot Flash Interference, Perceived Control, and Mood and Sleep Disturbances
There were few statistically significant differences in secondary outcomes at 8 and 16 weeks, but these small group differences are not likely to be clinically significant (appendix available online). At 8 weeks, significant differences were seen between paced respiration and breathing control for PSQI global scores only. From baseline to 8 weeks, PSQI global scores improved 0.61 points in the paced respiration intervention group and worsened by 1.06 points in the breathing control group (p = 0.20). At 16 weeks, significant differences were seen for PANAS negative affect, PSQI sleep duration, and PSQI sleep medications, but affect and sleep duration favored breathing control. From baseline to 16 weeks, negative affect increased (worsened) 0.91 points with intervention and decreased (improved) 1.84 points in breathing control (p = 0.03). Similarly, sleep duration difficulty worsened 0.07 points with intervention and improved 0.20 points in breathing control (p = 0.40). In contrast, from baseline to 16 weeks, use of sleep medications decreased with intervention by 0.14 points and increased with breathing control by 0.14 points (p = 0.03).
Adherence and Adverse Events
Most intervention participants achieved and sustained paced respiration across time points. At each time point, mean (standard deviation) breath rates were: 2 weeks, 7.35 (2.45); 8 weeks, 6.61 (1.60); and 16 weeks, 6.97 (1.92). Percentages of participants achieving eight or fewer breaths per minute at each time point were: 2 weeks, 75 %; 8 weeks, 84 %; and 16 weeks, 75 %. In addition, during the 16 weeks, the mean number of intervention practice sessions per participant was 109.83 (SD = 67.76), or approximately once per day (16 weeks × 7 days per week = 112). Similarly, during the 16 weeks, the mean number of breathing control practice sessions per participant was 133.93 (SD = 78.27), or approximately once per day. No adverse events were reported.
DISCUSSION
Paced respiration is unlikely to provide clinical benefit for vasomotor or other menopausal symptoms in breast cancer survivors or menopausal women without cancer. It was not significantly more efficacious than attention control or usual care control for primary outcomes and most secondary outcomes. Although some minor benefits for paced respiration were seen for sleep, the observed small differences are not likely to be clinically meaningful, may have been due to nighttime sleep’s natural variability, and statistical significance may have been spurious, since other outcomes were shown to favor breathing control. These negative study findings provide important evidence against international recommendations for paced respiration to alleviate vasomotor symptoms.
Negative study findings cannot be attributed to poor learning or lack of ability. Most intervention participants demonstrated their learned ability to perform and sustain paced respiration at the target breath rate at each time point using the CD-based materials. This suggests that the previously tested, more structured, laboratory-based intervention may not be necessary for teaching women how to do paced respiration.5–7
Negative study findings may be due to daily rather than twice daily practice, but even daily practice is not part of international recommendations. International recommendations are to apply at the onset of each flash only, even though prior laboratory studies involved twice-daily, at-home practice sessions.5–7Twice daily practice may be impractical with women’s busy lives. It is also possible that women did not practice twice daily because they did not perceive the practice as beneficial, in which case, practice rates may simply be another indicator of poor efficacy.
The major problem in any study of this nature is our limited understanding of vasomotor symptom etiology, which severely hampers efforts to create or identify appropriate interventions. Paced respiration was previously hypothesized to be efficacious due to its ability to decrease sympathetic activation, but this hypothesis remains unsupported.6
There were some study limitations. Participants, who were compensated volunteers, could have led to some sample bias. The 16-week intervention duration was relatively short. However, because our intervention was less intense than the previously tested laboratory-based protocol, we extended our study from the 8-week length of prior studies to 16 weeks to allow enough time to identify any benefits. Finally, although prior studies used sternal skin conductance monitoring as a physiological measure of vasomotor symptoms,5–7 components for the Biolog™ hot flash monitoring system were unavailable and/or problematic during the years of this study.23 It would have been interesting to evaluate response via physiologically-recorded hot flashes.
Strengths were inclusion of an attention control and the stratified sample. The control was rationally structured to do the opposite of the active intervention to avoid symptom alleviation. Without the control, the intervention would have looked more promising in relation to usual care. With its inclusion, we were able to show that paced respiration is no more beneficial than fast, shallow breathing. In addition, efficacy findings were similar across two groups of women and, therefore, are more informative to practitioners.
Clinical implications include the following. First, if patients request information or advice concerning paced respiration, clinicians should be prepared to discuss the relative lack of empirical support for this therapy. Second, recommendations for the use of paced respiration as a first line hot flash therapy should be viewed skeptically in light of our study findings. Greater benefit is likely to be seen with other more proven interventions, such as hormones, selective serotonin re-uptake inhibitors, or other emerging non-pharmacological therapies. Third, clinicians who regularly use printed or electronic patient education materials should ensure that information concerning paced respiration is updated to reflect currently available evidence.
In conclusion, paced respiration was not more efficacious than fast, shallow breathing or usual care in providing clinical benefit for vasomotor or other menopausal symptoms in this sample of breast cancer survivors and menopausal women without cancer.
Electronic supplementary material
(PDF 23.6 kb)
(PDF 24.4 kb)
(PDF 310 kb)
Acknowledgments
Contributors
There are no additional contributors who meet criteria for authorship.
Funders
The project described was supported by Award Number 5 R01 CA132927 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The funding agency had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Prior Presentations
None.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Trial registration: http://clinicaltrials.gov, NCT00819182
REFERENCES
- 1.Rada G, Capurro D, Pantoja T, Corbalan J, Moreno G, Letelier LM, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923. [DOI] [PubMed]
- 2.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–64. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 3.Menopause Practice: a Clinician’s Guide. 4. Mayfield Heights: North American Menopause Society; 2010. [Google Scholar]
- 4.The Menopause Guidebook. 7. Mayfield Heights: North American Menopause Society; 2012. [Google Scholar]
- 5.Freedman RR, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol. 1992;167(2):436–9. doi: 10.1016/s0002-9378(11)91425-2. [DOI] [PubMed] [Google Scholar]
- 6.Freedman RR, Woodward S, Brown B, Javiad JI, Pandey GN. Biochemical and thermoregulatory effects of behavioral treatment for menopausal hot flashes. Menopause. 1995;2(4):211–8. doi: 10.1097/00042192-199502040-00005. [DOI] [Google Scholar]
- 7.Germaine LM, Freedman RR. Behavioral treatment of menopausal hot flashes: evaluation by objective methods. J Consult Clin Psychol. 1984;52(6):1072–9. doi: 10.1037/0022-006X.52.6.1072. [DOI] [PubMed] [Google Scholar]
- 8.Butt DA, Deng LYR, Lewis JE, Lock M. Minimal decrease in hot flashes desired by postmenopausal women in family practice. Menopause. 2007;14(2):203–7. doi: 10.1097/01.gme.0000235370.32103.4c. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter JS, Storniolo AM, Johns S, Monahan PO, Azzouz F, Elam JL, et al. Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. Oncologist. 2007;12(1):124–35. doi: 10.1634/theoncologist.12-1-124. [DOI] [PubMed] [Google Scholar]
- 10.Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17(10):1301–9. doi: 10.1007/s00520-009-0587-5. [DOI] [PubMed] [Google Scholar]
- 11.Ganz PA, Greendale GA, Petersen L, Zibecchi L, Kahn B, Belin TR. Managing menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. J Natl Cancer Inst. 2000;92(13):1054–64. doi: 10.1093/jnci/92.13.1054. [DOI] [PubMed] [Google Scholar]
- 12.Mann E, Smith M, Hellier J, Hunter MS. A randomised controlled trial of a cognitive behavioural intervention for women who have menopausal symptoms following breast cancer treatment (MENOS 1): trial protocol. BMC Cancer. 2011;11:44. doi: 10.1186/1471-2407-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter JS, Neal JG, Payne J, Kimmick G, Storniolo AM. Cognitive–behavioral intervention for hot flashes. Oncol Nurs Forum. 2007;34(1):E1–8. doi: 10.1188/07.ONF.E1-E8. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manag. 2001;22(6):979–89. doi: 10.1016/S0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds F. Relationships between catastrophic thoughts, perceived control and distress during menopausal hot flushes: exploring the correlates of a questionnaire measure. Maturitas. 2000;36(2):113–22. doi: 10.1016/S0378-5122(00)00142-0. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds FA. Perceived control over menopausal hot flushes: exploring the correlates of a standardised measure. Maturitas. 1997;27(3):215–21. doi: 10.1016/S0378-5122(97)00048-0. [DOI] [PubMed] [Google Scholar]
- 17.Koller M, Kussman J, Lorenz W, Jenkins M, Voss M, Arens E, et al. Symptom reporting in cancer patients: the role of negative affect and experienced social stigma. Cancer. 1996;77(5):983–95. doi: 10.1002/(SICI)1097-0142(19960301)77:5<983::AID-CNCR27>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–70. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 19.Curran SL, Andrykowski M, Studts JL. Short form of the Profile of Mood States (POMS-SF): psychometric information. Psychol Assess. 1995;7(1):80–3. doi: 10.1037/1040-3590.7.1.80. [DOI] [Google Scholar]
- 20.McNair DM, Lorr M, Droppelman LF. POMS Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Taffe J, Dennerstein L. Menstrual diary data and menopausal transition: methodologic issues. Acta Obstet Gynecol Scand. 2002;81(7):588–94. doi: 10.1034/j.1600-0412.2002.810703.x. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter JS, Newton KM, Sternfeld B, Joffe H, Reed SD, Ensrud KE, et al. Laboratory and ambulatory evaluation of vasomotor symptom monitors from the Menopause Strategies Finding Lasting Answers for Symptoms and Health network. Menopause. 2012;19(6):664–71. doi: 10.1097/gme.0b013e31823dbbe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 23.6 kb)
(PDF 24.4 kb)
(PDF 310 kb)