ABSTRACT
BACKGROUND
Many older adults become dependent in one or more activities of daily living (ADLs: dressing, bathing, transferring, eating, toileting) when hospitalized, and their prognosis after discharge is unclear.
OBJECTIVE
To develop a prognostic index to estimate one-year probabilities of recovery, dependence or death in older hospitalized patients who are discharged with incident ADL dependence.
DESIGN
Retrospective cohort study.
PARTICIPANTS
449 adults aged ≥ 70 years hospitalized for acute illness and discharged with incident ADL dependence.
MAIN MEASURES
Potential predictors included demographics (age, sex, race, education, marital status), functional measures (ADL dependencies, instrumental activities of daily living [IADL] dependencies, walking ability), chronic conditions (e.g., congestive heart failure, dementia, cancer), reason for admission (e.g., neurologic, cardiovascular), and laboratory values (creatinine, albumin, hematocrit). Multinomial logistic regression was used to develop a prognostic index for estimating the probabilities of recovery, disability or death over 1 year. Discrimination of the index was assessed for each outcome based on the c statistic.
KEY RESULTS
During the year following hospitalization, 36 % of patients recovered, 27 % remained dependent and 37 % died. Key predictors of recovery, dependence or death were age, sex, number of IADL dependencies 2 weeks prior to admission, number of ADL dependencies at discharge, dementia, cancer, number of other chronic conditions, reason for admission, and creatinine levels. The final prognostic index had good to excellent discrimination for all three outcomes based on the c statistic (recovery: 0.81, dependence: 0.72, death: 0.78).
CONCLUSIONS
This index accurately estimated the probabilities of recovery, dependence or death in adults aged 70 years or older who were discharged with incident disability following hospitalization. This tool may be useful in clinical settings to guide care discussions and inform decision-making related to post-hospitalization care.
KEY WORDS: functional status, disability, mortality, hospitalization, prognosis
INTRODUCTION
Hospitalization often marks a critical transitional event in older adults.1–4 In many cases, an elderly patient who is fully independent prior to hospitalization may require help with one or more basic activities of daily living (ADLs: dressing, bathing, transferring, eating, or toileting) at the time of discharge. This change in functional status may result in the need for family members to take on new roles or hire a paid caregiver following hospitalization. In some cases, it reflects a brief period of dependence that is followed by full recovery of functional status. In other cases, it reflects the beginning of an extended period of functional dependence or is a harbinger of death. For both patients and clinicians, it is critically important to be able to accurately predict functional outcomes in elders who are discharged with incident disability following hospitalization, so that patients and family members can be informed of the most likely prognosis and be targeted toward the most appropriate post-hospitalization care and resources.
Systematic reviews have identified a variety of tools to predict the risk of functional decline, nursing home placement and mortality in older adults following hospitalization.5–9 Fewer studies have explored predictors of functional recovery in hospitalized elders.10,11 No tools are available to predict the likelihood of recovery versus continued dependence or death in the important setting of an elderly patient who is discharged with incident disability following hospitalization.
The objective of our study was to develop a prognostic index that can be used to simultaneously predict the risk of three outcomes (recovery, dependence or death) over 1 year, in older adults who are discharged with incident ADL dependence following hospitalization.
METHODS
Participants
Study participants were drawn from two randomized, controlled trials (RCTs) that examined the effects of receiving care in a specialized Acute Care for Elders (ACE) unit versus usual care in hospitalized elders. The trials had similar designs and collected similar data.12,13 Both studies found no significant differences between the intervention and control groups for the outcomes of ADL decline or mortality. Therefore, subjects from the two studies were combined for the current study to increase the sample size. Subjects for the original studies were considered potentially eligible if they were ≥ 70 years of age and admitted to the general medical service of one of two study hospitals (University Hospitals of Cleveland or Akron City Hospital) from 1993 to 1998. Exclusion criteria included admissions that were elective, were to intensive care or subspecialty units, or that resulted in a length of stay less than 2 days. A total of 3,163 participants were enrolled in the original RCTs. Our study focuses on the subset of subjects who were fully independent in all five basic ADLS 2 weeks prior to admission (referred to here as baseline) and had one or more ADLs dependencies at discharge (N = 473). Twenty-four participants were missing 1 year outcome data, leaving a total of 449 hospitalized elders who were discharged with incident ADL dependence for inclusion in our study.
All study procedures were approved by the Institutional Review Boards at the participating sites, and informed consent was obtained orally from patients or their proxies. Analyses described here were approved by the Committee on Human Research at the University of California, San Francisco, and the San Francisco Veterans Affairs Medical Center Research & Development Committee.
Predictor Variables
A standardized questionnaire was administered to study participants at admission by trained research staff. Demographic variables included age, sex, marital status, self-identified race (white or black), years of education, and income. Depressive symptoms were assessed using the 10-item version of the Center for Epidemiologic Studies—Depressive symptom scale (CES-D), in which scores may range from 0 to 10 with scores ≥ 4 suggestive of depression.14 Cognitive function was assessed with the 10-point Short Portable Mental Status Questionnaire (SPMSQ).15 In participants who had evidence of cognitive impairment (defined as more than five errors on the SPMSQ) or were unable to answer questions for themselves, data were collected from surrogates and depressive symptoms were not assessed.
Functional status at baseline and admission were assessed with questions related to mobility (ability to walk a block, walk uphill or up stairs and run a short distance), dependence in instrumental activities of daily living (IADLs: shopping, cooking, performing household chores, using transportation, managing money, managing medications and using the telephone), and dependence in ADLs. ADL dependence was also reassessed at discharge and considered as a potential predictor of one-year outcomes. Subjects were considered dependent if they required the assistance of another person to perform the activity. Those who used assistive devices or aids but did not require personal assistance were considered independent.
Additional data were collected from the medical record by trained chart abstractors. Medical diagnoses included those in the Charlson comorbidity index (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, diabetes, hemiplegia, moderate or severe renal disease, diabetes with end organ damage, any tumor, leukemia, lymphoma, moderate or severe liver disease, metastatic cancer, AIDS).16 Laboratory values included those in the Acute Physiology component of the Acute Physiology and Chronic Health Evaluation (APACHE) Score (creatinine, serum albumin, hematocrit).17 The primary reason for admission was classified using a detailed checklist and was categorized as neurologic (e.g., stroke, head trauma, seizure), cardiovascular (e.g., myocardial infarction, congestive heart failure, hyper/hypotension), infectious (e.g., sepsis, meningitis, fever, urinary tract infection), pulmonary (e.g., pneumonia, bronchitis, asthma), hematologic (e.g., anemia), gastrointestinal (e.g., bleeding, liver disease, pancreatitis), metabolic (e.g., hyper/hypoglycemia, renal failure, substance abuse) or other.18
Outcome Variables
Our primary outcome variable was functional status (recovery, dependence or death) at 1 year. Recovery was defined as being fully independent in all ADLs. Dependence was defined as continued dependence in at least one ADL. Functional status was determined based on interviews with study participants or informed surrogates. Death was confirmed by searching the National Death Index.
Analyses
Our analyses were guided by the key principles for development and validation of prognostic indices,19–24 with extension to the setting of a tri-level outcome.25 The association between each potential predictor and our tri-level outcome was first examined in bivariate analyses using Chi-square for categorical variables and one-way analysis of variance for continuous variables. All continuous variables were also examined as categorical variables using logical cut-points when available (e.g., education <12 years), or based on distributional characteristics (e.g., tertiles). For the Charlson comorbidity index and acute physiology score, we examined the individual components of the scores as well as the total scores.
Variables that were associated with the outcome in bivariate analyses (p < 0.10) were then considered in multinomial logistic regression analyses, which are an extension of logistic regression to the setting of multiple outcomes.25 We used an iterative process and best subsets regression to build a model that maximized prognostic accuracy while minimizing over-fitting. Initial models included age, sex, and baseline IADLs. Subsequent models added Charlson comorbidities, ADLs at discharge, reason for admission and laboratory values. Generalized additive models (GAM) and loess smooths were used to assess for nonlinearity in continuous variables. Likelihood ratio tests were performed to determine whether addition of new variables significantly improved model fit. An overall p value for the association between each predictor and the tri-level outcome was calculated, and variables that were no longer significant (p ≥ 0.10) were removed. The final model was used to calculate the predicted probabilities of the three outcomes for each subject, such that the total predicted probability added to 100 %.
The prognostic accuracy of the final model was assessed by calculating the c statistic for each outcome.19,24,25 Because c statistics can only be calculated for dichotomous comparisons, we used the predicted probabilities from the multinomial model to calculate c statistics for recovery (vs. dependence or death), dependence (vs. recovery or death) and death (vs. recovery or dependence). Model calibration for each of the three outcomes was assessed graphically by plotting actual outcomes as a function of decile of predicted risk, and statistically with the Hosmer–Lemeshow statistic. Overfitting was assessed using bootstrapping to estimate model optimism.21,22,26
Finally, exploratory analyses were performed to examine the effects of hospital readmission on 12-month outcomes and the accuracy of the final index.
RESULTS
Table 1 shows baseline characteristics of the total study population, as well as stratified by status of recovered independence, continued dependence or death at 1 year. Overall, 44 % of study participants were 70–79 years old, 43 % were 80–89 and 13 % were ≥ 90. Approximately two-thirds were female, one-third were married, 21 % were black, and 41 % had fewer than 12 years of education. At baseline (2 weeks prior to admission), all subjects were fully independent in all five ADLs; however, 44 % were unable to walk a block and 58 % required assistance with one or more IADLs. By the time of admission, 64 % had developed one or more ADL dependency and nearly one-fourth were dependent in four to five ADLs. In addition, 90 % required assistance with one or more IADLs. At discharge, all subjects had become dependent in at least one ADL, and nearly 20 % were fully dependent.
Table 1.
Characteristics of 449 Elders Who Became Dependent During Hospitalization
| Characteristic | Total Population (n = 449) | Status at 1 Year | |||
|---|---|---|---|---|---|
| Recovered Independence (n = 164) | Continued Dependence (n = 120) | Death (n = 165) | P-Value | ||
| Demographics | |||||
| Age, y | |||||
| 70–79 | 198 | 89 (45) | 41 (21) | 68 (34) | <0.01 |
| 80–89 | 191 | 64 (34) | 58 (30) | 69 (36) | |
| ≥ 90 | 60 | 11 (18) | 21 (35) | 28 (47) | |
| Sex | |||||
| Female | 300 | 118 (39) | 86 (29) | 96 (32) | 0.01 |
| Male | 149 | 46 (31) | 34 (23) | 69 (46) | |
| Race | |||||
| White | 353 | 132 (37) | 89 (25) | 132 (37) | 0.38 |
| Black | 96 | 32 (33) | 31 (32) | 33 (34) | |
| Marital Status | |||||
| Married | 145 | 54 (37) | 32 (22) | 59 (41) | 0.26 |
| Not Married | 303 | 109 (36) | 88 (29) | 106 (35) | |
| Education | |||||
| < 12 years | 163 | 48 (29) | 49 (30) | 66 (40) | 0.06 |
| ≥ 12 years | 239 | 98 (41) | 58 (24) | 83 (35) | |
| Baseline | |||||
| Able to walk a block | |||||
| Yes | 240 | 101 (42) | 60 (25) | 79 (33) | 0.03 |
| No | 188 | 57 (30) | 51 (27) | 80 (43) | |
| No. IADL dependencies | |||||
| 0 | 188 | 98 (52) | 37 (20) | 53 (28) | <0.001 |
| 1 | 80 | 26 (33) | 21 (26) | 33 (41) | |
| 2 | 58 | 17 (29) | 15 (26) | 26 (45) | |
| ≥ 3 | 123 | 23 (19) | 47 (38) | 53 (43) | |
| Admission | |||||
| No. ADL dependencies | |||||
| 0 | 161 | 59 (37) | 38 (24) | 64 (40) | 0.55 |
| 1 | 67 | 30 (45) | 15 (22) | 22 (33) | |
| 2 | 66 | 18 (27) | 23 (35) | 25 (38) | |
| 3 | 43 | 15 (35) | 12 (28) | 16 (37) | |
| ≥ 4 | 107 | 39 (36) | 32 (30) | 36 (34) | |
| No. IADL dependencies | |||||
| 0 | 44 | 22 (50) | 6 (14) | 16 (36) | 0.08 |
| 1 | 36 | 10 (28) | 8 (22) | 18 (50) | |
| 2 | 42 | 20 (48) | 8 (19) | 14 (33) | |
| 3 | 59 | 22 (37) | 15 (25) | 22 (37) | |
| ≥ 4 | 260 | 84 (32) | 83 (32) | 93 (36) | |
| Chief reason for admission | <0.01 | ||||
| Neurologic | 95 | 28 (29) | 37 (39) | 30 (32) | |
| Cardiovascular | 52 | 12 (23) | 13 (25) | 27 (52) | |
| Infectious | 43 | 23 (53) | 12 (28) | 8 (19) | |
| Pulmonary | 73 | 32 (44) | 10 (14) | 31 (42) | |
| Hematologic | 8 | 2 (25) | 1 (13) | 5 (63) | |
| Gastrointestinal | 82 | 29 (35) | 20 (24) | 33 (40) | |
| Metabolic | 93 | 38 (41) | 26 (28) | 29 (31) | |
| Comorbid conditions | |||||
| Myocardial infarction | 66 | 17 (26) | 18 (27) | 31 (47) | 0.06 |
| Congestive heart failure | 119 | 38 (32) | 27 (23) | 54 (45) | 0.07 |
| Peripheral vasc.disease | 80 | 25 (31) | 26 (33) | 29 (36) | 0.36 |
| Cerebrovascular disease | 74 | 23 (31) | 23 (31) | 28 (38) | 0.48 |
| Dementia | 60 | 7 (12) | 23 (38) | 30 (50) | <0.0001 |
| Chronic lung disease | 81 | 31 (38) | 13 (16) | 37 (46) | 0.04 |
| Peptic ulcer disease | 55 | 14 (25) | 20 (36) | 21 (38) | 0.11 |
| Diabetes | 76 | 36 (47) | 13 (17) | 27 (36) | 0.05 |
| Cancer, solitary tumor | 31 | 5 (16) | 8 (26) | 18 (58) | 0.02 |
| Cancer, metastatic | 27 | 3 (11) | 1 (4) | 23 (85) | <0.0001 |
| Laboratory values | |||||
| Creatinine, mg/dL | |||||
| < 1.5 | 313 | 122 (39) | 95 (30) | 96 (31) | <0.001 |
| 1.5–3.0 | 99 | 28 (28) | 19 (19) | 52 (53) | |
| > 3.0 | 29 | 11 (38) | 4 (14) | 14 (48) | |
| Albumin, g/dL | |||||
| < 3.0 | 79 | 25 (32) | 17 (22) | 37 (47) | 0.17 |
| 3.0–3.4 | 93 | 31 (33) | 24 (26) | 38 (41) | |
| ≥ 3.5 | 277 | 108 (39) | 79 (29) | 90 (32) | |
| Hematocrit | |||||
| ≤ 29 | 80 | 22 (28) | 20 (25) | 38 (48) | 0.18 |
| 30–35 | 112 | 40 (36) | 34 (30) | 38 (34) | |
| > 35 | 247 | 98 (40) | 63 (26) | 86 (35) | |
| Discharge | |||||
| No. ADL dependencies | |||||
| 1 | 168 | 88 (52) | 32 (19) | 48 (29) | <0.001 |
| 2 | 78 | 28 (36) | 21 (27) | 29 (37) | |
| 3 | 73 | 25 (34) | 23 (32) | 25 (34) | |
| 4 | 43 | 12 (28) | 17 (40) | 14 (33) | |
| 5 | 87 | 11 (13) | 27 (31) | 49 (56) | |
Values reflect number for the total population and row number (percent) for status at 1 year. P values are based on Chi-square over the three outcomes. Baseline =2 weeks before admission. ADL, activities of daily living; IADL, instrumental activities of daily living. Data missing as follows: marital status (n = 1); education (n = 47); able to walk a block at baseline (n = 21); IADL at admission (n = 8); ADL at admission (n = 5); chief reason for admission (n = 3); comorbid conditions (n = 5); creatinine (n = 8); hematocrit (n = 34); albumin (n = 47).
At 1 year, 36 % of subjects had fully recovered, 27 % remained dependent and 37 % had died. Factors that were significantly associated with status at 1 year (recovery, dependence or death) in bivariate analyses included age, sex, baseline functional status (ability to walk a block, number of IADL dependencies), chief reason for admission, some comorbid medical conditions (dementia, cancer, chronic pulmonary disease), creatinine levels, and functional status at discharge (number of ADL dependencies) (Table 1).
The final prognostic index included age (categorized as 70–79, 80–89, ≥ 90); sex; function at baseline (number of IADL dependencies, categorized as 0, 1–2, ≥ 3); reason for admission (categorized as neurologic, cardiovascular, pulmonary, gastrointestinal, other); dementia; any cancer (solitary tumor, leukemia, lymphoma or metastatic); number of other chronic conditions (categorized as 0, 1, ≥ 2); creatinine ≥ 1.5 mg/dL and function at discharge (number of ADL dependencies, categorized as 1, 2–4, 5). Odds ratios from the three separate logistic regression models that underlie the multinomial logistic model are presented in Table 2. Discrimination of these models was good to excellent, based on the c statistic (recovery: 0.81; dependence: 0.72; death: 0.78). When bootstrapping was used to estimate model optimism, the c statistics were reduced but still good (recovery: 0.76; dependence: 0.66; death: 0.73).
Table 2.
Odds Ratios of Recovery, Dependence and Death in Elders Who Became Dependent During Hospitalization
| Predictor | Recovery | Dependence | Death |
|---|---|---|---|
| Age, y | |||
| 70–79 | 1.0 | 1.0 | 1.0 |
| 80–89 | 0.60 (0.37–0.99) | 1.52 (0.92–2.51) | 1.11 (0.68–1.80) |
| ≥ 90 | 0.37 (0.16–0.85) | 1.65 (0.81–3.38) | 1.30 (0.63–2.68) |
| Gender | |||
| Female | 1.97 (1.17–3.32) | 1.04 (0.62–1.72) | 0.55 (0.34–0.90) |
| Male | 1.0 | 1.0 | 1.0 |
| No. IADL dependencies 2 weeks prior to admission | |||
| 0 | 1.0 | 1.0 | 1.0 |
| 1–2 | 0.36 (0.21–0.63) | 1.69 (0.95–3.00) | 1.85 (1.07–3.20) |
| ≥ 3 | 0.30 (0.16–0.57) | 2.60 (1.43–4.76) | 1.30 (0.71–2.38) |
| Chief reason for admission | |||
| Neurologic | 0.42 (0.21–0.83) | 1.32 (0.71–2.42) | 1.67 (0.85–3.28) |
| Cardiovascular | 0.29 (0.12–0.67) | 0.81 (0.36–1.78) | 3.82 (1.78–8.19) |
| Pulmonary | 0.94 (0.47–1.89) | 0.29 (0.13–0.66) | 2.86 (1.43–5.73) |
| Gastrointestinal | 0.51 (0.26–1.02) | 0.81 (0.41–1.58) | 2.29 (1.17–4.46) |
| Other | 1.0 | 1.0 | 1.0 |
| Chronic conditions | |||
| Dementia | 0.21 (0.08–0.55) | 1.15 (0.60–2.20) | 2.04 (1.03–4.04) |
| Cancer | 0.16 (0.07–0.35) | 0.47 (0.21–1.03) | 7.06 (3.62–13.77) |
| Number of other chronic conditions | |||
| 0 | 1.0 | 1.0 | 1.0 |
| 1 | 0.67 (0.37–1.21) | 1.03 (0.56–1.88) | 1.32 (0.72–2.41) |
| ≥ 2 | 0.38 (0.21–0.70) | 1.19 (0.67–2.11) | 1.84 (1.04–3.25) |
| Creatinine, mg/dL | |||
| < 1.5 | 1.0 | 1.0 | 1.0 |
| ≥ 1.5 | 0.83 (0.48–1.44) | 0.41 (0.23–0.73) | 2.38 (1.44–3.93) |
| No. ADL dependencies at discharge | |||
| 1 | 1.0 | 1.0 | 1.0 |
| 2–4 | 0.39 (0.23–0.65) | 1.83 (1.06–3.14) | 1.46 (0.87–2.44) |
| 5 | 0.14 (0.06–0.31) | 1.44 (0.73–2.85) | 3.60 (1.87–6.93) |
| C statistic | 0.81 | 0.72 | 0.78 |
Predicted Versus Actual Outcomes
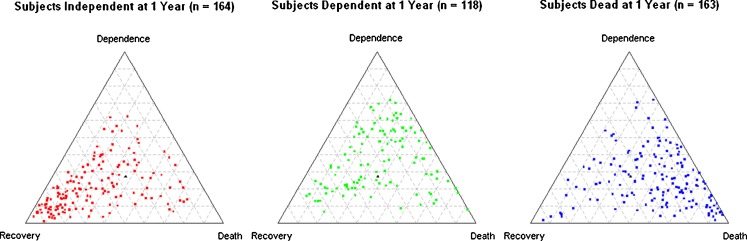
The final multinomial logistic regression model was used to predict the probability of recovery, dependence or death for each subject, such that the sum added to 100 %. Figure 1 shows the predicted probabilities of the three outcomes as a function of actual outcomes. In subjects who recovered (Fig. 1a), the predicted probabilities cluster in the left corner of the triangle, suggesting that the predicted probability of recovery tends to be high in those who actually recover. Similarly, in subjects who died (Fig. 1c), there is a clustering in the right corner, suggesting that the predicted probability of death is high in most people who actually died. In subjects who remained dependent (Fig. 1b), there is a more diffuse pattern, suggesting that dependence is more difficult to predict reliably. This is also reflected in the lower c statistic for dependence.
Figure 1.
a–c. Predicted probabilities of recovery, dependence and death as a function of actual outcomes. The three-way predicted probabilities of recovery, dependence and death are shown for subjects who actually recovered (Fig. 1a), remained dependent (Fig. 1b) or died (Fig. 1c), such that the total predicted probability for each subject adds to 100 %. The predicted probability for each outcome may range from 0 % (base of triangle opposite the outcome of interest) to 100 % (corner of triangle for outcome of interest); the lines parallel to the base reflect predicted probabilities progressing from 10 % to 90 %. To determine a subject’s predicted probability of a given outcome, start at the base opposite the outcome (0 %) and count the number of parallel lines until the subject of interest is reached. For example, in Figure 1a, the predicted probabilities for the subject with the highest predicted probability of recovery (left corner) are 93 % recovery, 5 % dependent and 2 % death. In Figure 1b, the predicted probabilities for the subject with the highest predicted probability of dependence (top) are 72 % dependent, 21 % death and 7 % recovery. In Figure 1c, the predicted probabilities for the subject with the highest predicted probability of death (right corner) are 95 % death, 2 % recovery and 4 % dependence (numbers do not add to 100 % due to rounding).
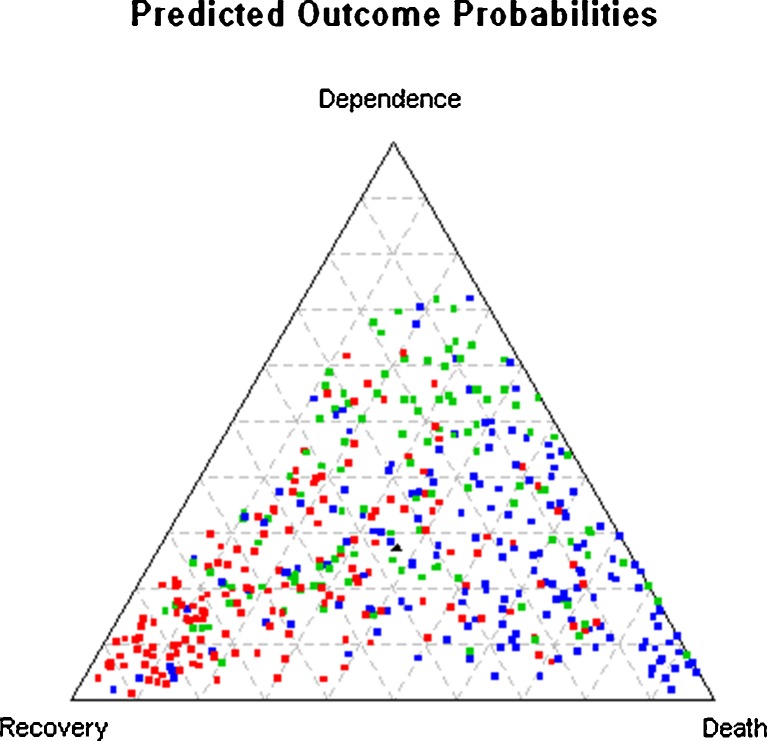
Figure 2 shows Figure 1a–c, with each section laid on top of each other, with colored dots to represent the actual outcomes (red = recovery, green = dependence, blue = death). This provides a visual sense of the true positive to false positive ratio for each outcome. For example, it shows that, in individuals with a high predicted probability of recovery (left corner), some subjects remained dependent (green) or died (blue), but most recovered (red).
Figure 2.
Predicted probabilities of recovery, dependence and death in all subjects combined. Figure 2 is created by stacking Figure 1a–c on top of each other, with colors used to reflect the actual outcomes for each subject (red = recovery, green = dependence, blue = death). The black triangle in the center reflects the marginal predicted probabilities for the three outcomes (36 % recovery, 27 % dependence, 37 % death).
Calibration plots suggested no significance differences between predicted and actual probabilities across deciles of predicted risk for all three outcomes (recovery, p = 0 .93; dependence, p = 0 .12; death, p = 0 .67).
Impact of Hospital Readmission
Cumulative readmission rates were 8 % (n = 38) at 30 days, 16 % (n = 74) at 90 days, 20 % (n = 91) at 6 months and 23 % (n = 102) at 12 months. Hospital readmission at any point was significantly associated with lower odds of recovery and higher odds of death, but not with continued dependence at 12 months. Inclusion of hospital readmission in the index had minimal impact on model discrimination based on c statistics for the different outcomes.
DISCUSSION
Our prognostic index can be used to predict the probability of recovery, dependence or death in the setting of a patient aged 70 years or older who is being discharged from the hospital with incident ADL dependence. The key predictors can all be obtained relatively easily, and include age, sex, number of IADL dependencies two weeks prior to admission, number of ADL dependencies at discharge, medical diagnoses, primary reason for admission, and creatinine level. Together, these predictors enabled accurate stratification of the study population into those with a high likelihood of recovery, dependence or death.
The prognostic accuracy achieved for the three outcomes in the current study based on the c statistic (recovery, 0.81; dependence, 0.72; death, 0.78) is consistent with, or superior to, that achieved with single outcomes in other studies. Numerous tools have been developed to predict the single outcomes of functional decline27–34 or mortality35–42 in hospitalized elders. Prognostic accuracy is generally moderate to good, with c statistics ranging from 0.64 to 0.79.8,9 We are unaware of any tools to predict recovery or to predict multiple outcomes simultaneously. Therefore, our index provides a unique and important contribution to the field.
Clinical Implications
We have created a web-based calculator for our tool (http://campuslifeservices.ucsf.edu/documentsmail/portfolio/client/agingcalculator/) and envision that it could be used by physicians, nurses and/or social workers as part of the discharge planning process, to inform discussions with patients and family members on likely prognosis over 1 year and to develop care plans. In patients with a high predicted probability of recovery, discussions could focus on the importance of rehabilitation and on the need for temporary assistance with daily activities. In patients with a high predicted probability of continued disability, discussions could focus more on planning for provision of more extensive care at home, or transitioning to an assisted living facility. In patients with a high predicted probability of death, discussions could focus more on end-of-life goals and provision of palliative care. In some patients, the predicted probabilities of all three outcomes may be low (< 50 %). This also is important information for clinicians, patients and family members, because it suggests that recovery, dependence and death over the coming year are all likely enough that plans should be made to accommodate all three outcomes.
Limitations
Our study has several limitations that should be considered. First, our index was developed based on secondary data analyses using a retrospective cohort study design. In addition, data were collected in the mid-1990s, potentially raising concern about whether results will apply under current conditions. However, the predictors identified have face validity in today’s medical setting, and are consistent with those that have been included in single-outcome tools. Although data related to functional status are not always routinely collected in hospitalized elders, they are simple to collect from either patients or family members, and there is substantial evidence that functional status is a key predictor of a wide range of outcomes.11,43,44
Finally, our index has not been externally validated. Since we are unaware of another study of hospitalized elders that could be used for external validation, the best approach may be for clinicians to use the index and determine for themselves whether it is helpful for informing clinical decision-making. Additional studies will be needed to assess the impact of the index on clinical practice.
Conclusion
Our prognostic index can be used to predict the probabilities of recovery, dependence and death in adults aged 70 years or older who are discharged with incident ADL dependence. This tool may be useful in clinical settings to guide care planning and inform discussions regarding prognosis with patients and family members.
Acknowledgements
We would like to thank all of the research study participants and investigators who have contributed to this study over the years. Dr. Landefeld was affiliated with the University of California, San Francisco, when this work was performed.
Contributors
None.
Funders
Support for this study was provided by the National Institutes of Health (Landefeld: R01 AG029233, AG10418) and the S. D. Bechtel, Jr. Foundation.
Prior Presentations
Findings will be presented at the Gerontological Society of America meeting in San Diego, in November, 2012.
Conflicts of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: "She was probably able to ambulate, but I'm not sure". JAMA. 2011;306(16):1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]
- 2.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219–223. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Sager MA, Franke T, Inouye SK, Landefeld CS, Morgan TM, Rudberg MA, Sebens H, Winograd CH. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156(6):645–652. doi: 10.1001/archinte.1996.00440060067008. [DOI] [PubMed] [Google Scholar]
- 4.Sager MA, Rudberg MA. Functional decline associated with hospitalization for acute illness. Clin Geriatr Med. 1998;14(4):669–679. [PubMed] [Google Scholar]
- 5.De Saint-Hubert M, Schoevaerdts D, Cornette P, D'Hoore W, Boland B, Swine C. Predicting functional adverse outcomes in hospitalized older patients: a systematic review of screening tools. J Nutr Health Aging. 2010;14(5):394–399. doi: 10.1007/s12603-010-0086-x. [DOI] [PubMed] [Google Scholar]
- 6.Hoogerduijn JG, Schuurmans MJ, Duijnstee MS, de Rooij SE, Grypdonck MF. A systematic review of predictors and screening instruments to identify older hospitalized patients at risk for functional decline. J Clin Nurs. 2007;16(1):46–57. doi: 10.1111/j.1365-2702.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 7.McCusker J, Kakuma R, Abrahamowicz M. Predictors of functional decline in hospitalized elderly patients: a systematic review. J Gerontol A Biol Sci Med Sci. 2002;57(9):M569–M577. doi: 10.1093/gerona/57.9.M569. [DOI] [PubMed] [Google Scholar]
- 8.Sutton M, Grimmer-Somers K, Jeffries L. Screening tools to identify hospitalised elderly patients at risk of functional decline: a systematic review. Int J Clin Pract. 2008;62(12):1900–1909. doi: 10.1111/j.1742-1241.2008.01930.x. [DOI] [PubMed] [Google Scholar]
- 9.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd CM, Landefeld CS, Counsell SR, Palmer RM, Fortinsky RH, Kresevic D, Burant C, Covinsky KE. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd CM, Ricks M, Fried LP, Guralnik JM, Xue QL, Xia J, Bandeen-Roche K. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women's Health and Aging Study I. J Am Geriatr Soc. 2009;57(10):1757–1766. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, Quinn LM, Allen KR, Covinsky KE, Landefeld CS. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572–1581. doi: 10.1111/j.1532-5415.2000.tb03866.x. [DOI] [PubMed] [Google Scholar]
- 13.Barnes DE, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J, Chren MM, Landefeld CS. Acute care for elders units produced shorter hospital stays at lower cost while maintaining patients' functional status. Health Aff (Millwood) 2012;31(6):1227–1236. doi: 10.1377/hlthaff.2012.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159(15):1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 15.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9(8):591–597. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG., Jr Resuscitation: how do we decide? A prospective study of physicians' preferences and the clinical course of hospitalized patients. JAMA. 1986;255(10):1316–1322. doi: 10.1001/jama.1986.03370100110027. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 20.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3(2):143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 24.McNeil BJ, Hanley JA. Statistical approaches to the analysis of receiver operating characteristic (ROC) curves. Med Decis Making. 1984;4(2):137–150. doi: 10.1177/0272989X8400400203. [DOI] [PubMed] [Google Scholar]
- 25.Biesheuvel CJ, Vergouwe Y, Steyerberg EW, Grobbee DE, Moons KG. Polytomous logistic regression analysis could be applied more often in diagnostic research. J Clin Epidemiol. 2008;61(2):125–134. doi: 10.1016/j.jclinepi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. Jama. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 27.Sager MA, Rudberg MA, Jalaluddin M, Franke T, Inouye SK, Landefeld CS, Siebens H, Winograd CH. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc. 1996;44(3):251–257. doi: 10.1111/j.1532-5415.1996.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 28.McCusker J, Bellavance F, Cardin S, Trepanier S. Screening for geriatric problems in the emergency department: reliability and validity. Identification of Seniors at Risk (ISAR) Steering Committee. Acad Emerg Med. 1998;5(9):883–893. doi: 10.1111/j.1553-2712.1998.tb02818.x. [DOI] [PubMed] [Google Scholar]
- 29.Huyse FJ, de Jonge P, Slaets JP, Herzog T, Lobo A, Lyons JS, Opmeer BC, Stein B, Arolt V, Balogh N, Cardoso G, Fink P, Rigatelli M. COMPRI–an instrument to detect patients with complex care needs: results from a European study. Psychosomatics. 2001;42(3):222–228. doi: 10.1176/appi.psy.42.3.222. [DOI] [PubMed] [Google Scholar]
- 30.Hustey FM, Mion LC, Connor JT, Emerman CL, Campbell J, Palmer RM. A brief risk stratification tool to predict functional decline in older adults discharged from emergency departments. J Am Geriatr Soc. 2007;55(8):1269–1274. doi: 10.1111/j.1532-5415.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 31.Inouye SK, Wagner DR, Acampora D, Horwitz RI, Cooney LM, Jr, Hurst LD, Tinetti ME. A predictive index for functional decline in hospitalized elderly medical patients. J Gen Intern Med. 1993;8(12):645–652. doi: 10.1007/BF02598279. [DOI] [PubMed] [Google Scholar]
- 32.Cornette P, Swine C, Malhomme B, Gillet JB, Meert P, D'Hoore W. Early evaluation of the risk of functional decline following hospitalization of older patients: development of a predictive tool. Eur J Public Health. 2006;16(2):203–208. doi: 10.1093/eurpub/cki054. [DOI] [PubMed] [Google Scholar]
- 33.Wu AW, Yasui Y, Alzola C, Galanos AN, Tsevat J, Phillips RS, Connors AF, Jr, Teno JM, Wenger NS, Lynn J. Predicting functional status outcomes in hospitalized patients aged 80 years and older. J Am Geriatr Soc. 2000;48(5 Suppl):S6–15. doi: 10.1111/j.1532-5415.2000.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 34.Mehta KM, Pierluissi E, Boscardin WJ, Kirby KA, Walter LC, Chren MM, Palmer RM, Counsell SR, Landefeld CS. A clinical index to stratify hospitalized older adults according to risk for new-onset disability. J Am Geriatr Soc. 2011;59(7):1206–1216. doi: 10.1111/j.1532-5415.2011.03409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter LC, Brand RJ, Counsell SR, Palmer RM, Landefeld CS, Fortinsky RH, Covinsky KE. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285(23):2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 36.Pilotto A, Ferrucci L, Franceschi M, D'Ambrosio LP, Scarcelli C, Cascavilla L, Paris F, Placentino G, Seripa D, Dallapiccola B, Leandro G. Development and validation of a multidimensional prognostic index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151–161. doi: 10.1089/rej.2007.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inouye SK, Bogardus ST, Jr, Vitagliano G, Desai MM, Williams CS, Grady JN, Scinto JD. Burden of illness score for elderly persons: risk adjustment incorporating the cumulative impact of diseases, physiologic abnormalities, and functional impairments. Med Care. 2003;41(1):70–83. doi: 10.1097/00005650-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Teno JM, Harrell FE, Jr, Knaus W, Phillips RS, Wu AW, Connors A, Jr, Wenger NS, Wagner D, Galanos A, Desbiens NA, Lynn J. Prediction of survival for older hospitalized patients: the HELP survival model. Hospitalized Elderly Longitudinal Project. J Am Geriatr Soc. 2000;48(5 Suppl):S16–24. doi: 10.1111/j.1532-5415.2000.tb03126.x. [DOI] [PubMed] [Google Scholar]
- 39.Fischer SM, Gozansky WS, Sauaia A, Min SJ, Kutner JS, Kramer A. A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31(4):285–292. doi: 10.1016/j.jpainsymman.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Levine SK, Sachs GA, Jin L, Meltzer D. A prognostic model for 1-year mortality in older adults after hospital discharge. Am J Med. 2007;120(5):455–460. doi: 10.1016/j.amjmed.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Drame M, Novella JL, Lang PO, Somme D, Jovenin N, Laniece I, Couturier P, Heitz D, Gauvain JB, Voisin T, De Wazieres B, Gonthier R, Ankri J, Jeandel C, Saint-Jean O, Blanchard F, Jolly D. Derivation and validation of a mortality-risk index from a cohort of frail elderly patients hospitalised in medical wards via emergencies: the SAFES study. Eur J Epidemiol. 2008;23(12):783–791. doi: 10.1007/s10654-008-9290-y. [DOI] [PubMed] [Google Scholar]
- 42.Di Bari M, Balzi D, Roberts AT, Barchielli A, Fumagalli S, Ungar A, Bandinelli S, De Alfieri W, Gabbani L, Marchionni N. Prognostic stratification of older persons based on simple administrative data: development and validation of the "Silver Code," to be used in emergency department triage. J Gerontol A Biol Sci Med Sci. 2010;65(2):159–164. doi: 10.1093/gerona/glp043. [DOI] [PubMed] [Google Scholar]
- 43.Covinsky KE, Justice AC, Rosenthal GE, Palmer RM, Landefeld CS. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med. 1997;12(4):203–208. doi: 10.1046/j.1525-1497.1997.012004203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Covinsky KE, Palmer RM, Counsell SR, Pine ZM, Walter LC, Chren MM. Functional status before hospitalization in acutely ill older adults: validity and clinical importance of retrospective reports. J Am Geriatr Soc. 2000;48(2):164–169. doi: 10.1111/j.1532-5415.2000.tb03907.x. [DOI] [PubMed] [Google Scholar]