ABSTRACT
BACKGROUND
Use of proton pump inhibitors (PPIs) is associated with community-acquired pneumonia (CAP), an association which may be confounded by unobserved patient and prescriber characteristics.
OBJECTIVE
We assessed for confounding in the association between PPI use and CAP by using a ‘falsification approach,’ which estimated whether PPI use is also implausibly associated with other common medical conditions for which no known pathophysiologic link exists.
DESIGN
Retrospective claims-based cohort study.
SETTING
Six private U.S. health plans.
SUBJECTS
Individuals who filled at least one prescription for a PPI (N = 26,436) and those who never did (N = 28,054) over 11 years.
INTERVENTIONS
Multivariate linear regression of the association between a filled prescription for a PPI and a diagnosis of CAP in each 3-month quarter. In falsification analyses, we tested for implausible associations between PPI use in each quarter and rates of osteoarthritis, chest pain, urinary tract infection (UTI), deep venous thrombosis (DVT), skin infection, and rheumatoid arthritis. Independent variables included an indicator for whether a prescription for a PPI was filled in a given quarter, and quarterly indicators for various co-morbidities, age, income, geographic location, and marital status.
KEY RESULTS
Compared to nonusers, those ever using a PPI had higher adjusted rates of CAP in quarters in which no prescription was filled (68 vs. 61 cases per 10,000 persons, p < 0.001). Similar associations were noted for all conditions (e.g. chest pain, 336 vs. 282 cases, p < 0.001; UTI, 151 vs. 139 cases, p < 0.001). Among those ever using a PPI, quarters in which a prescription was filled were associated with higher adjusted rates of CAP (111 vs. 68 cases per 10,000, p < 0.001) and all other conditions (e.g. chest pain, 597 vs. 336 cases, p < 0.001; UTI, 186 vs. 151 cases, p < 0.001), compared to quarters in which no prescription was filled.
CONCLUSION
PPI use is associated with CAP, but also implausibly associated with common medical conditions. Observed associations between PPI use and CAP may be confounded.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2211-5) contains supplementary material, which is available to authorized users.
KEY WORDS: proton pump inhibitors, pneumonia, falsification testing
INTRODUCTION
Observational studies demonstrate a positive association between proton-pump inhibitor (PPI) use and the risk of community-acquired pneumonia (CAP).1–3 Use of PPIs is associated with short-term, but not long-term, increases in the rate of CAP,2 and users of PPIs have higher rates of CAP when compared to similar individuals who recently stopped taking a PPI.1While a plausible mechanism exists—namely, higher rates of CAP due to increased bacterial colonization arising from a reduction in gastric acid suppression 4–6—the association may be confounded. In the absence of randomized controlled trial data, validating whether use of PPIs raises the risk of CAP is important, given widespread use of these medications 7 and recommendations to curtail use based on these associations.8–11
Several biases may explain observed associations between use of PPIs and CAP. Confounding by indication and disease severity are likely, as individuals prescribed PPIs more frequently have other comorbid conditions and more severe gastroesophageal reflux disease than those not using these medications.1–3 Even after adjustment for potential confounders, confounding by indication and disease severity may still be important, as individuals prescribed PPIs are likely to have unobserved health characteristics that predispose to CAP when compared to nonusers.1 The association between PPI use and CAP may also be biased by the omission of relevant physician practice patterns—physicians more likely to prescribe PPIs may also be more likely to diagnose CAP. Although not interpreted as such, the association between antipsychotic medication use and CAP 11—a pathophysiologic mechanism that is potentially weaker than the proposed mechanism explaining the association between PPI use and CAP—suggests that observed associations between PPI use and CAP may be confounded by unobserved severity of patient comorbidities.
We used a ‘falsification approach’ to examine whether the association between PPI use and CAP is confounded. Falsification methods have been suggested elsewhere as a way to test the causal validity of estimated associations in observational studies, particularly when quasi-experimental study designs are unavailable.12–14 Specifically, we asked whether use of PPIs is implausibly associated with higher adjusted rates of common conditions—osteoarthritis, chest pain, urinary tract infection (UTI), deep venous thrombosis (DVT), skin infection, and rheumatoid arthritis—for which known pathophysiologic mechanisms to base an association are few or absent. The theoretical basis of the falsification approach is that a positive association between PPI use and CAP is more likely to be causal, rather than reflect selection bias, if use of PPIs is not also positively associated with other implausible diseases. The presence of one or several implausible associations would suggest that the observed association between PPI use and CAP is confounded. Patient or physician characteristics that are unable to be accounted for in empirical analyses would be more likely responsible for the observed association between PPI use and CAP than a direct effect of PPIs.
For a large sample of privately insured beneficiaries retrospectively followed over 11 years, we compared rates of CAP between users and nonusers of PPIs, as well as within users over time (comparing rates of CAP during months in which a prescription for a PPI was filled to months in which one was not). We compared these results to separate “falsification tests” for osteoarthritis, chest pain, UTI, DVT, skin infection, and rheumatoid arthritis, in which we calculated rates of each condition between users and nonusers of PPIs and within users over time.
MATERIALS AND METHODS
Data
We assembled a data set of prescription drug and medical claims for 54,490 privately insured beneficiaries above the age of 30, continuously enrolled in six employer-based insurance plans from 1997 to 2007. The data were obtained through a benefits consulting firm and have been used elsewhere to examine the impact of benefit design on pharmacy spending,15 medication use by the chronically-ill,16–18 and specialty drug use.16 The six plans were chosen since data was available for these plans over a long period of time (11 years) and because the plans were geographically diverse (although not nationally representative). Since the data were based on insurance claims, no data were missing in any of the health plans. We excluded beneficiaries younger than 30 years, because prior work studying the association between PPI use and CAP has demonstrated rates of CAP in patients younger than 30 to be exceedingly low.1 Data on claims utilization were quarterly, resulting in 2,397,560 person-quarters. The data were de-identified and exempt from review by the Institutional Review Board of the Massachusetts General Hospital.
Drug information included drug name, national drug code, dosage, and days supplied. Outpatient and emergency room medical claims included the date of service, diagnoses, and procedure codes associated with each visit. These data have been used to examine the impact of benefit design on pharmacy spending,15 medication use by the chronically-ill,16–18 and specialty drug use.16
Our level of observation was an individual in a given quarter. In each quarter, we classified an individual as using a PPI if they filled one or more prescriptions in that quarter for a PPI. Prescriptions were identified by generic and brand names and national drug codes. We flagged individuals by quarter according to whether they had at least one outpatient or emergency department claim for CAP, osteoarthritis, chest pain, UTI, DVT, skin infection, or rheumatoid arthritis. UTI and skin infection were chosen since, like CAP, they are common infections treated in the outpatient setting.19, 20 The remaining diseases were chosen as they are common reasons for seeking ambulatory care.21 Disease indicators were identified in the medical claims according to International Classification of Disease, Ninth Revision (ICD-9) diagnoses.
Based on prior work, we also adjusted for common chronic conditions,16–18 which may be associated with higher rates of CAP: asthma or chronic obstructive pulmonary disease, cancer, chronic renal insufficiency, congestive heart failure, coronary artery disease, diabetes, hyperlipidemia, hypertension, and stroke. A beneficiary was determined to have one of these conditions if their medical claims included two or more office visits within a year with the corresponding ICD-9 code.
The final cohorts of users and nonusers of PPIs included patients older than 30 years old who were continuously enrolled in one of six health plans from 1997 to 2007 (inclusion criteria). We did not exclude patients who had a diagnosis of CAP in the first quarter or who filled a prescription for a PPI in the first quarter, since our inclusion of individual fixed effects in the estimation model identified the ‘effect’ of PPI use on CAP using changes in PPI utilization within individuals over time. Intuitively, because individuals were followed longitudinally, we were able to ascertain additional information on the association of PPI use with CAP by also studying individuals who entered the sample on a PPI, but who ceased use within the study period.
Descriptive Analysis
We computed unadjusted quarterly rates of CAP, osteoarthritis, chest pain, UTI, DVT, skin infection, and rheumatoid arthritis among those beneficiaries who never filled a prescription for a PPI during the 11-year period (n = 54,490 beneficiaries; 2,397,560 person-quarters). We compared these rates to disease rates among beneficiaries who filled at least one prescription for a PPI in 11 years, termed “users” (n = 26,436 beneficiaries; 1,163,184 person-quarters). Disease rates among users were calculated separately for quarters in which a PPI prescription was filled (n = 320,981 quarters) and quarters in which no prescription was filled (n = 842,203 quarters). Univariate comparisons between nonusers and users of PPIs (unpaired groups) were made using χ2 analysis at p < 0.05 level of significance. Among users of PPIs, univariate comparisons between quarters in which a prescription for a PPI was filled versus not filled (two paired groups) were made using McNemar’s paired statistical test at p < 0.05 level of significance.
This descriptive analysis had two purposes. The first was to explore how contemporaneous rates of CAP and each of the studied conditions varied between those individuals ever filling a prescription for a PPI and those not. The second was to explore how rates of each disease varied within users during quarters in which a prescription for a PPI was filled versus not. Comparing disease rates within the same individuals over time is an arguably better approach to dealing with unobserved patient characteristics that may be associated with both the probability of illness and PPI use.
Multivariate Analysis
For each condition, we estimated a multivariate linear probability model with an indicator for whether a prescription for a PPI was filled in a given quarter; quarterly indicators for each of the common chronic conditions listed above; individual fixed effects; and demographic characteristics including age (continuous variable), income (continuous variable), geographic location (indicator variable for North, South, East, West Census region), and marital status (binary variable). We a priori adjusted for the same chronic conditions in each outcome regression to proxy for overall health status of the patient. Age, geographic location, income, and marital status were included as they are commonly associated with the incidence and/or diagnosis of disease and the likelihood of receiving medical care.15, 17, 18
The fixed effect specification identifies the association between PPI use and each disease by comparing adjusted rates of each disease within individuals over time, and accounting for unobserved, fixed individual-specific health risks that are correlated with both PPI use and the disease outcomes being studied. The assumption behind the fixed effect model is that there exists an unobserved characteristic of a patient that is unchanging over time and that is correlated with both the exposure variable of interest (in this case PPI use) and the outcome being studied (pneumonia). For instance, if certain patients are always more likely to seek medical care, they will be more likely to use a PPI and will be more likely to be diagnosed with CAP, both by virtue of being more likely to see a physician. If that underlying propensity to seek medical care does not change over time, the fixed effect estimation strategy can account for this confounder by comparing rates of CAP within the same individual during periods of PPI use and no PPI use. The fixed effect model is estimated by including an indicator variable for each patient into the multivariate regression. The specification differs from a random effects specification in that the latter is identified through comparisons both within and between individuals.22 We estimated a linear probability model rather than a logistic model since we included individual fixed effects. Individual fixed effects have a more natural interpretation in linear models and allow for fixed unobserved characteristics that are correlated with PPI exposure. We also estimated logistic models with individual random effects and found quantitatively similar results. For each condition, we reported adjusted quarterly rates of disease associated with filling a prescription for a PPI in a given quarter.
We also tested for a “dose response” between length of use of PPIs and risk of CAP, osteoarthritis, chest pain, UTI, DVT, skin infection, and rheumatoid arthritis. The presence of a dose response between PPI use and CAP—i.e. higher rates of CAP associated with greater PPI use—has been argued to support a causal association, 1 even though those with greater exposure (either through higher dosages or more prolonged use) may still have underlying comorbidities that are associated with higher risk of CAP. We divided users of PPIs into terciles of use, based on the number of quarters in which a PPI prescription was filled, and estimated a linear probability model with indicators for tercile of PPI use and adjustments for comorbidities. We reported predicted quarterly rates of each condition by tercile of PPI use.
STATA version 11 (STATA Corp, College Station, Texas) was used for statistical analyses and the 95 % CI reflects 0.025 in each tail or P ≤ 0.05.
RESULTS
Table 1 presents initial demographic and health characteristics of users and nonusers of proton pump inhibitors in the first quarter. Users were defined as those individuals filling at least one prescription for a PPI in the 11 year period. Among users, the mean number of quarters in which a prescription for a PPI was filled was 12.1 (95 % CI 12.0–12.3). Compared to those never filling a prescription for a PPI, users of PPIs were of similar age (66.5 y vs. 66.0 y in first quarter, p < 0.001) and gender (39.9 % male vs. 44.0 %, p < 0.001), but were generally less healthy. For example, users of PPIs were more likely to have cancer (6.2 % v 4.9 %, p < 0.001), chronic renal insufficiency (0.8 % vs. 0.4 %, p < 0.001), hypertension (14.9 % vs. 12.5 %, p < 0.001), diabetes (6.4 % vs. 4.5 %, p < 0.001), hyperlipidemia (3.7 % vs. 3.1 %, p < 0.001), and stroke (1.1 % vs. 0.7 %, p < 0.001).
Table 1.
Baseline Characteristics of Users and Nonusers of Proton Pump Inhibitors Among Individuals With Employer-Provided Health Insurance
| No. | Nonusers of PPIs | Users of PPIs | p-value |
|---|---|---|---|
| 28,054 | 26,436 | ||
| Mean age (SD), y | 66.0 (0.1) | 66.5 (0.1) | <0.01 |
| Male, No. (%) | 12,344 (44.0) | 10,548 (39.9) | <0.001 |
| Married, No. (%) | 14,672 (52.3) | 14,381 (54.4) | <0.001 |
| Mean zip code income in 1999 (standard deviation of mean), $ | 40,600 (36.9) | 40,500 (38.3) | 0.22 |
| Asthma or COPD, No. (%) | 56 (0.2) | 132 (0.5) | <0.001 |
| Cancer, No. (%) | 1,375 (4.9) | 1,639 (6.2) | <0.001 |
| Chest Pain, No. (%) | 572 (2.0) | 1,131 (4.3) | <0.001 |
| Chronic Renal Insufficiency, No. (%) | 112 (0.4) | 212 (0.8) | <0.001 |
| Congestive heart failure, No. (%) | 140 (0.5) | 238 ( 0.9) | <0.001 |
| Coronary artery disease, No. (%) | 1,347 (4.8) | 2,194 (8.3) | <0.001 |
| Deep Venous Thrombosis, No. (%) | 14 (0.05) | 30 (0.1) | <0.001 |
| Diabetes, No. (%) | 1,262 (4.5) | 1,692 ( 6.4) | <0.001 |
| Hyperlipidemia, No. (%) | 870 (3.1) | 978 (3.7) | <0.001 |
| Hypertension, No. (%) | 3,507 (12.5) | 3,939 (14.9) | <0.001 |
| Osteoarthritis, No. (%) | 620 (2.2) | 1,116 (4.2) | <0.001 |
| Rheumatoid Arthritis, No. (%) | 117 (0.4) | 215 (0.8) | <0.001 |
| Skin Infection (%) | 0.673 (0.05) | 1.02 (0.06) | <0.001 |
| Stroke, No. (%) | 196 (0.7) | 291 (1.1) | <0.001 |
| Urinary Tract Infection, No. (%) | 303 (1.1) | 441 (1.7) | <0.001 |
Notes: Table presents characteristics of individuals older than 30 years with employer-provided health insurance who were continuously enrolled over 11 years. Users of PPIs were defined by whether a prescription for a PPI was filled at least once in 11 years. Baseline gender, marital status, and income characteristics are from the first quarter. Baseline disease prevalence was based on whether an individual’s medical claims included two or more office visits with the corresponding ICD-9 code in the first four quarters
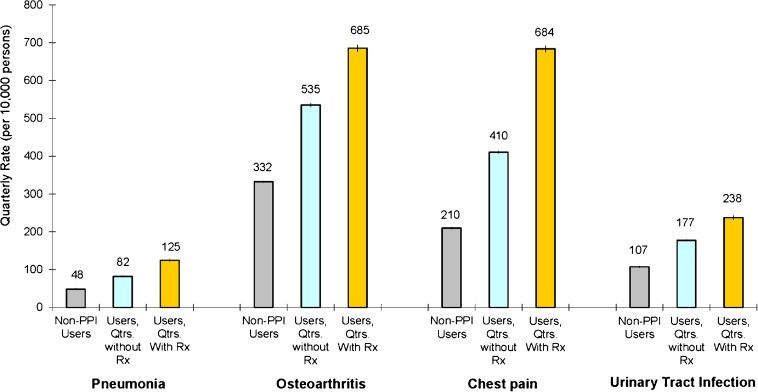
Figure 1 displays unadjusted rates of CAP among nonusers of PPIs, users during quarters in which they did not fill a prescription for a PPI, and users during quarters in which they did fill a prescription. Compared to nonusers, those who ever filled a prescription for a PPI had higher quarterly rates of CAP even during quarters in which a prescription was not filled (89 vs. 51 cases per 10,000 persons, p < 0.001). This suggests that unobserved health characteristics correlated with CAP may be important in explaining the association between PPI use and CAP.
Figure 1.
Unadjusted quarterly rates of community-acquired pneumonia, osteoarthritis, chest pain, and urinary tract infection medical claims among users and nonusers of proton pump inhibitors. Unadjusted quarterly rates of each condition were calculated for those never filling a prescription for a PPI (termed nonusers of PPIs), users (defined as persons who filled at least one prescription for a PPI) during quarters in which a prescription was not filled, and users during quarters in which a prescription was filled. Rates were determined from ICD-9 codes in administrative data and are displayed at the quarterly level per 10,000 persons. 95 % confidence intervals reported in graph.
Among those ever using a PPI, rates of CAP were higher during quarters in which a prescription was filled for a PPI (137 vs. 89 cases per 10,000 persons, p < 0.001). By estimating the impact of PPI use within individuals during periods of use and non-use, this result could be interpreted as supporting a causal association between PPI use and the risk of CAP. To explore this further, Figure 1 also displays unadjusted rates of osteoarthritis, chest pain, and UTI (rates of DVT, skin infection, and rheumatoid arthritis are displayed in Supplementary Figure 1, available online). When compared to nonusers, those who ever filled a prescription for a PPI had higher rates of osteoarthritis (535 vs. 332 cases per 10,000 persons, p < 0.001), chest pain (410 vs. 210 cases per 10,000 persons, p < 0.001), and UTI (178 vs. 107 cases per 10,000 persons, p < 0.001) during quarters in which a prescription was not filled, again suggesting greater unobserved risks among individuals ever using a PPI. Among those ever using a PPI, quarterly rates of osteoarthritis, chest pain, and UTI were also higher during quarters in which a prescription was filled for a PPI (685 vs. 535 cases per 10,000 persons for osteoarthritis, p < 0.001; 683 vs. 410 cases per 10,000 persons for chest pain, p < 0.001; 238 vs. 178 cases per 10,000 persons for UTI, p < 0.001). When estimating the impact of PPI use within individuals during periods of use and non-use (paired analysis), use of PPIs was associated not only with higher rates of CAP but also with pathophysiologically unrelated conditions.
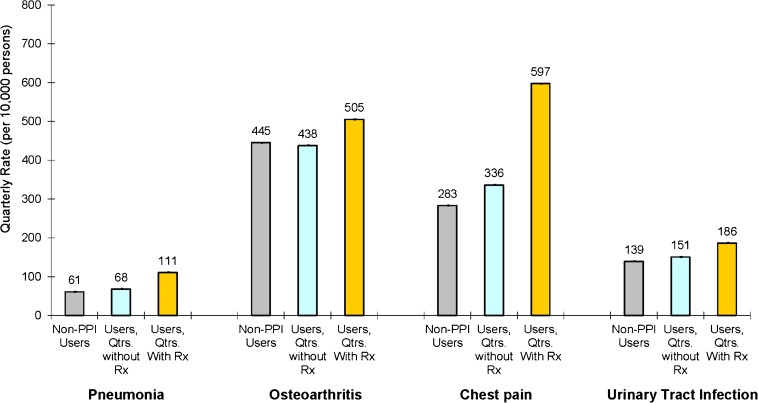
Figure 2 presents adjusted quarterly rates of CAP, osteoarthritis, chest pain, and UTI. Compared to those never filling a prescription for a PPI, users of PPIs continued to have higher adjusted quarterly rates of pneumonia (68 vs. 61 cases per 10,000 persons, p < 0.001), chest pain (336 vs. 282 cases per 10,000 persons, p < 0.001), and UTI (151 vs. 139 cases per 10,000 persons, p < 0.001) during quarters in which they did not fill a prescription. Adjusted differences were smaller than unadjusted differences, primarily due to statistically significantly higher rates of CAP associated with the presence of asthma or chronic obstructive pulmonary disease (COPD), cancer, chronic renal insufficiency, congestive heart failure, coronary artery disease, end stage renal disease, and stroke (Supplementary Table 1, available online). Among those ever using a PPI, adjusted rates of CAP were higher during quarters in which a prescription was filled for a PPI, compared to quarters in which there was no use (111 vs. 68 cases per 10,000 persons per year, p < 0.001). Similar patterns were noted, however, for osteoarthritis (505 vs. 438 cases per 10,000 persons per year, p < 0.001), chest pain (597 vs. 336 cases per 10,000 persons per year, p < 0.001), and UTI (186 vs. 151 cases per 10,000 persons per year, p < 0.001), and as shown in Supplementary Figure 2 (available online) for DVT (25 vs. 16 cases per 10,000 persons per year, p < 0.001), skin infection (143 vs. 124 cases per 10,000 persons per year, p < 0.001), and rheumatoid arthritis (85 vs. 68 cases per 10,000 persons per year, p < 0.001). Quantitatively similar results were obtained with logistic models with individual random effects. An additional sensitivity analysis was conducted to quantify the prevalence and strength of an unmeasured confounder that would explain the observed association between PPI use and the incidence of CAP. A categorical, unmeasured variable of 60 % prevalence among PPI users and 10 % prevalence among nonusers and an odds ratio of CAP of greater than 2.5 would explain the observed association between PPI use and CAP.
Figure 2.
Adjusted quarterly rates of community-acquired pneumonia, osteoarthritis, chest pain, and urinary tract infection medical claims among users and nonusers of proton pump inhibitors. Adjusted estimates are from a multivariate linear probability model with individual fixed effects, quarterly indicators for various comorbidities, and demographic characteristics such as age, marital status, income, and geographic location. Rates are displayed at the quarterly level and are per 10,000 persons. 95 % confidence intervals reported in graph.
Dose Response
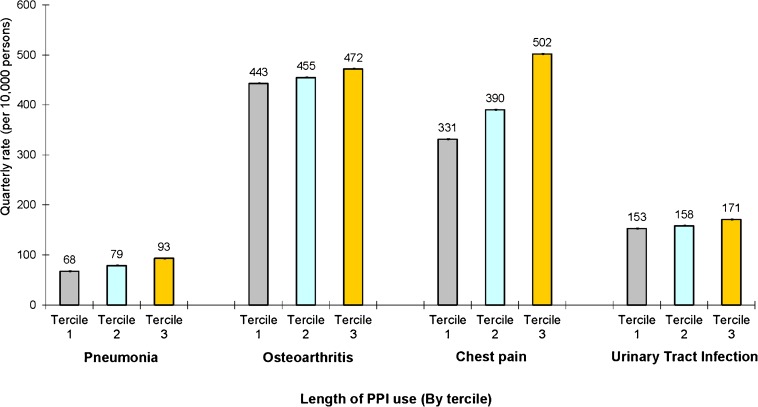
Figure 3 presents quarterly rates of CAP, osteoarthritis, chest pain, and UTI among those ever using a PPI, according to the amount of PPI use over the study period. Length of use was divided into terciles based on the number of quarters a prescription for a PPI was filled. The mean number of quarters in which a prescription for a PPI was filled was 1.64 (SD 0.77) for the bottom tercile, 8.6 (SD 3.5) for the middle, and 25.9 (SD. 7.10) for the top. The mean quarterly rate of CAP was higher in the top tercile of PPI users (93 cases per 10,000 persons, 95 % CI 112–122) than the bottom tercile (68 cases per 10,000 persons, 95 % CI 82–91), p < 0.001. A similar association was observed for osteoarthritis (472 vs. 443 cases per 10,000 persons, p < 0.001), chest pain (502 vs. 331 cases per 10,000 persons, p < 0.001), and UTI (171 vs. 153 cases per 10,000 persons, p < 0.001).
Figure 3.
Adjusted quarterly rates of community-acquired pneumonia, osteoarthritis, chest pain, and urinary tract infection medical claims, according to length of proton pump inhibitor use. This figure presents adjusted quarterly rates of each condition among users of PPIs according to tercile of use (based on number of quarters over the study period in which a prescription for a PPI was filled). Estimates are from a multivariate linear probability model with indicators for tercile of PPI use, quarterly indicators for various comorbidities, and demographic characteristics such as age, marital status, income, and geographic location. Rates are displayed at the quarterly level and are per 10,000 persons. 95 % confidence intervals reported in graph.
DISCUSSION
Use of PPIs has been positively associated with CAP in several observational studies,1–3 an association which may be confounded. We used a falsification approach to assess for confounding in the association of PPI use with CAP in a large cohort of PPI users and nonusers. We found that current PPI use was not only associated with higher adjusted rates of CAP, but also implausibly associated with higher rates of osteoarthritis, chest pain, UTI, DVT, skin infection, and rheumatoid arthritis. The absence of a known physiologic mechanism by which use of PPIs could lead to higher rates of each these conditions suggests that the observed association between PPI use and CAP may be confounded by unobserved patient and physician characteristics among users of PPIs. Furthermore, higher rates of each of these conditions among users of PPIs during the months in which a PPI is used suggest that these unobserved characteristics may change over time and confound even a longitudinal cohort analysis that uses individuals as their own controls.
In our study as in others, patients using PPIs had greater observed comorbidities than nonusers.1–3 While adjusting for these comorbidities has been shown to reduce the estimated association between PPI use and CAP,2 residual confounding in underlying risk of CAP may still exist, as demonstrated by our study. In addition to confounded health risks, physicians that are more likely to prescribe a PPI may also be more likely to diagnose CAP (or bill insurance for any of the conditions studied) when compared to other physicians. Both patient and physician characteristics that are unable to be accounted for may explain the observed association between PPI use and CAP.
Strengths and Weaknesses
The results of this study have important implications, not only for assessing the risks associated with PPI use, but for validating the causal plausibility of observational studies more generally. Use of PPIs has been linked in observational studies to gastrointestinal infections by Salmonella and Clostridium,23–26 and has been associated with risk of hip fracture,27 particularly at high doses. The proposed mechanism of impacting the risk of hip fracture is an interference with calcium absorption and inhibition of osteoclastic proton pumps.27 While each of these associations may indeed be causal, our results strongly raise the possibility that even sophisticated attempts to account for confounding may not be enough. Falsification methods, such as those employed in this study, offer an intuitive approach to assessing the likelihood of confounding in observational studies. The theoretical basis of this approach is that an association between an exposure variable (in this case PPI use) and an outcome variable (e.g. CAP) is more likely to be causal—rather than reflect selection bias—if the exposure variable is not also associated with other implausible outcomes (e.g. UTI and skin infection) that would be expected to be present if residual confounding persists (e.g. due to health severity). Prior work arguing for a causal effect of social networks on the rise in obesity28 has, for example, been called into question as the same social network analysis predicts height, acne, and severe headaches, all of which would be expected to bear no causal relationship to social networks.29 Recent evidence of an association between antipsychotic medication use and CAP11 may reflect a causal association or just as easily be interpreted as a positive falsification test—use of antipsychotic medications may proxy for unobserved health risks that are correlated with CAP in the same way as use of PPIs.
Although this study demonstrates an arguably implausible association between PPI use and the risk of several common conditions, there are limitations. First, filling a prescription for a PPI is an imperfect proxy for actual PPI use. Moreover, given the availability of over-the-counter PPIs during the time period studied, some individuals may be incorrectly classified as being nonusers. While this misclassification of exposure to PPIs may bias the association between PPI use and CAP, it should have the same effect on the associations between PPI use and the other conditions studied. A second limitation of this study is a reliance on administrative claims data to identify disease conditions. While the absence of corroborating clinical data such as culture specimens or radiographic imaging may result in false positive and negative classifications of CAP, the resulting bias should not vary with the other conditions we studied. A third limitation is that our study population was comprised of privately insured patients, which may limit generalizability.
An additional limitation of our analysis is that it is unable to identify which unmeasured patient or provider characteristics are responsible for the association between PPI use and each of the disease outcomes we studied. The persistence of the PPI association in models which use individuals as their own controls suggests that unobserved characteristics that are not fixed within an individual over time are important. For example, higher rates of each disease outcome during quarters in which PPI users fill a prescription for a PPI suggest that unobserved characteristics in those specific quarters are potentially confounded with the risk of disease. Potential confounders may include an increased likelihood of patients to seek medical care during certain periods (thereby raising rates of both PPI use and disease outcomes) or greater medical care provided by physicians during certain periods (i.e. a concomitant increase in both PPI prescribing and the diagnose of disease by physicians).
An important statistical limitation of the falsification approach we employed is that by chance alone one may identify a statistically significant association between an exposure variable (PPI use) and an implausibly associated disease, creating a falsely positive falsification test. As in our study, it is important to choose multiple, prevalent diseases with which to conduct these tests. Finally, the absence of established mechanisms linking use of PPIs to conditions such as UTI, skin infection, DVT, or chest pain does not preclude the presence of a causal relationship between use of PPIs and each of these diseases.
Despite its limitations, this study raises important questions about whether the observed association between PPI use and CAP is confounded. Applying falsification testing to data from prior studies that first demonstrated the association would be a useful first step to validating a causal association between use of PPIs and the risk of CAP. More generally, falsification testing may be a useful tool to evaluating causal relationships in observational studies.
Electronic supplementary material
(PDF 183 kb)
ACKNOWLEDGEMENTS
Contributors
All authors were involved in the study design and concept, interpretation of results and editing of manuscript. Drs. Jena and Sun analysed the data set and wrote the initial manuscript draft. Dr. Jena obtained ethical approval for the project. Dr. Goldman obtained funding. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
National Institute on Aging. The design, conduct, analysis, interpretation, and presentation of the data are the responsibility of the investigators, with no involvement from the funding sources.
Prior Presentations
None
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Contributor Information
Anupam B. Jena, Phone: +1-617-4328322, Email: jena@hcp.med.harvard.edu.
Eric Sun, Phone: +1-301-3251458, FAX: +1-310-4517007, Email: esun1@stanford.edu.
Dana P. Goldman, Phone: +1-213-8634492, FAX: +1-310-4517007, Email: dana.goldman@usc.edu.
REFERENCES
- 1.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292(16):1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149(6):391–398. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 3.Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case–control study. Arch Intern Med. 2007;167(9):950–955. doi: 10.1001/archinte.167.9.950. [DOI] [PubMed] [Google Scholar]
- 4.Howden CW, Hunt RH. Relationship between gastric secretion and infection. Gut. 1987;28(1):96–107. doi: 10.1136/gut.28.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannella RA, Broitman SA, Zamcheck N. Influence of gastric acidity on bacterial and parasitic enteric infections. A perspective. Ann Intern Med. 1973;78(2):271–276. doi: 10.7326/0003-4819-78-2-271. [DOI] [PubMed] [Google Scholar]
- 6.Ruddell WS, Axon AT, Findlay JM, Bartholomew BA, Hill MJ. Effect of cimetidine on the gastric bacterial flora. Lancet. 1980;1(8170):672–674. [PubMed] [Google Scholar]
- 7.IMS. Leading Therapy Classes in 2002 Global Pharmaceutical Sales. IMS World Review. Vol. 2010; 2010.
- 8.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139(4):1115–1127. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Logan IC, Sumukadas D, Witham MD. Gastric acid suppressants–too much of a good thing? Age and ageing. 2010;39(4):410–411. doi: 10.1093/ageing/afq057. [DOI] [PubMed] [Google Scholar]
- 10.Lodato F, Azzaroli F, Turco L, et al. Adverse effects of proton pump inhibitors. Best Pract Res Clin Gastroenterol. 2010;24(2):193–201. doi: 10.1016/j.bpg.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Trifiro G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case–control study. Ann Intern Med. 2010;152(7):418–25, W139-40. [DOI] [PubMed]
- 12.Popper KR.The Logic of Scientific Discovery Routledge; 2002.
- 13.Heckman JJ, Hotz VJ. Choosing among alternative nonexperimental methods for estimating the impact of social programs: the case of manpower training. J Am Stat Assoc. 1989;84(408):862–874. doi: 10.1080/01621459.1989.10478848. [DOI] [Google Scholar]
- 14.Bertrand M, Duflo E, Mullainathan S. How much should We trust differences-in-differences estimates? Q J Econ. 2004;119(1):249–275. doi: 10.1162/003355304772839588. [DOI] [Google Scholar]
- 15.Joyce GF, Escarce JJ, Solomon MD, Goldman DP. Employer drug benefit plans and spending on prescription drugs. JAMA. 2002;288(14):1733–1739. doi: 10.1001/jama.288.14.1733. [DOI] [PubMed] [Google Scholar]
- 16.Goldman DP, Joyce GF, Lawless G, Crown WH, Willey V. Benefit design and specialty drug use. Health Aff (Millwood) 2006;25(5):1319–1331. doi: 10.1377/hlthaff.25.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman DP, Joyce GF, Escarce JJ, et al. Pharmacy benefits and the use of drugs by the chronically ill. JAMA. 2004;291(19):2344–2350. doi: 10.1001/jama.291.19.2344. [DOI] [PubMed] [Google Scholar]
- 18.Solomon MD, Goldman DP, Joyce GF, Escarce JJ. Cost sharing and the initiation of drug therapy for the chronically ill. Arch Intern Med. 2009;169(8):740–748. doi: 10.1001/archinternmed.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/S0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 20.Ellis Simonsen SM, van Orman ER, Hatch BE, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134(2):293–299. doi: 10.1017/S095026880500484X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Department of H, Human Services. Centers for Disease C, Prevention. National Center for Health S. National Ambulatory Medical Care Survey, 2009. Inter-university Consortium for Political and Social Research (ICPSR) [distributor]; 2011.
- 22.Ware JH, Dockery DW, Louis TA, Xu XP, Ferris BG, Jr, Speizer FE. Longitudinal and cross-sectional estimates of pulmonary function decline in never-smoking adults. Am J Epidemiol. 1990;132(4):685–700. doi: 10.1093/oxfordjournals.aje.a115710. [DOI] [PubMed] [Google Scholar]
- 23.Garcia Rodriguez LA, Ruigomez A. Gastric acid, acid-suppressing drugs, and bacterial gastroenteritis: how much of a risk? Epidemiology. 1997;8(5):571–574. doi: 10.1097/00001648-199709000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Neal KR, Scott HM, Slack RC, Logan RF. Omeprazole as a risk factor for campylobacter gastroenteritis: case–control study. BMJ. 1996;312(7028):414–415. doi: 10.1136/bmj.312.7028.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case–control studies. CMAJ. 2004;171(1):33–38. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent clostridium difficile infection. Arch Intern Med. 2010;170(9):772–778. doi: 10.1001/archinternmed.2010.73. [DOI] [PubMed] [Google Scholar]
- 27.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 28.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 29.Cohen-Cole E, Fletcher JM. Detecting implausible social network effects in acne, height, and headaches: longitudinal analysis. BMJ. 2008;337:a2533. doi: 10.1136/bmj.a2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 183 kb)