ABSTRACT
OBJECTIVE
To examine the differential effect of medication non-adherence over time on all-cause mortality by race/ethnicity.
RESEARCH DESIGN AND METHODS
Data on a longitudinal cohort of veterans with type 2 diabetes was examined. The main outcome was time to death. Primary independent variables were race/ethnicity and mean medication possession ratio (MPR) categorized into quintiles over the study period. Cox regression was used to model the association between time to death and MPR quintiles and race/ethnicity, adjusting for relevant covariates.
RESULTS
The cohort of 629,563 veterans was followed for 5 years. After adjusting for all covariates, the hazard ratios (HR) for subjects in the lowest versus highest MPR quintile was 12.21 (95 % CI 11.89, 12.55) for non-Hispanic white (NHW), 10.01 (95 % CI 9.18, 10.91) for non-Hispanic black (NHB), 12.65 (95 % CI 11.10, 14.43) for Hispanic and 10.41 (95 % CI 9.06, 11.96) for Other race veterans. Furthermore, type of diabetes therapy (oral versus insulin) maintained a significant relationship with mortality that varied by racial/ethnic group.
CONCLUSIONS
This study demonstrates the differential impact of medication non-adherence on mortality by race. It also demonstrates that type of diabetes therapy (insulin with or without oral agents) is associated with mortality and varies by racial/ethnic group.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2200-8) contains supplementary material, which is available to authorized users.
KEY WORDS: medication non-adherence, veterans, diabetes, mortality, race/ethnicity
INTRODUCTION
Type 2 diabetes is a growing problem that places a severe burden on health care resources in the United States. Diabetes is the leading cause of stroke, blindness, kidney disease and non-traumatic lower limb amputations.1 Diabetes is associated with a two-fold increase in mortality.2 Diabetes affects all age, gender, ethnic and racial groups, but disproportionately affects minority populations, with African Americans and Hispanics having a two-fold to three-fold increased risk of developing diabetes relative to whites.3,4
Medication non-adherence is linked to poor glycemic control,5–7 increased subsequent hospitalization,8–10 higher all-cause mortality,9,11 and greater healthcare costs.12 Although medication adherence has been linked to increased mortality9,11 and prior studies have documented lower medication adherence in ethnic minority populations,5–7,12 there is no data on the differential impact of medication non-adherence over time on mortality across racial/ethnic groups. In addition, there is little data on whether the type of medication used for treating diabetes has an impact on mortality, and whether it differs across racial/ethnic groups. To address this gap in the literature, we examined the differential effect of medication non-adherence over time on all-cause mortality by race/ethnicity over 5 years of follow-up. In addition, we examined whether the type of medication used to treat diabetes (oral agents vs. insulin) differed across racial/ethnic groups and contributed to differences in mortality.
METHODS
Study Population
A national cohort of veterans with type 2 diabetes was created by linking multiple patient and administrative files from the Veterans Health Administration (VHA) National Patient Care and Pharmacy Benefits Management databases. The National Patient Care Database (NPCD) is the source data for the VHA Medical Statistical Analysis System (SAS) Datasets, which are used to analyze veteran clinical data such as diagnosis and procedure codes for outpatient visits and inpatient admissions. The Pharmacy Benefits Management (PBM) database includes utilization information for every prescription filled through Veterans Affairs (VA). The Outpatient Pharmacy Package includes prescriptions dispensed at the site’s pharmacy, either as a new fill or a refill, within that month. In addition, all prescriptions filled by a Consolidated Mail Outpatient Pharmacy (CMOP) are included. The PBM database is at the level of individual prescriptions, thus a veteran can have multiple records on a given day. Our algorithm for identifying veterans with diabetes has been previously validated,13 and is provided as an online appendix document (online Figure 1). Veterans were included in the cohort if they had: 1) type 2 diabetes defined by two or more International Classification of Diseases, Ninth Revision (ICD-9) codes for diabetes (250, 357.2, 362.0, and 366.41) in the previous 24 months (2000 and 2001) and during 2002 from inpatient stays and/or outpatient visits on separate days (excluding codes from lab tests and other non-clinician visits); or 2) prescriptions for insulin or oral hypoglycemic agents (VA classes HS501 or HS502, respectively) in 2002. PBM data were available during the entire period of analysis. When the data were merged based on the criteria above, the total sample included 832,000 veterans. We excluded those not taking prescription diabetic medications (n = 201,255) and added those who had one ICD-9 code for diabetes and prescriptions filled in 2002 (n = 60,493). The subset with complete adherence data resulted in a final cohort of 629,563 veterans, of which 72.0 % were non-Hispanic white (NHW), 13.3 % were non-Hispanic black (NHB) and 5.3 % were Hispanic veterans with type 2 diabetes. There were also 9.4 % of veterans with race/ethnicity classified as ‘Other’. This category includes American Indian or Alaskan Native, Asian, Native Hawaiian or other Pacific Islander, as well as those with mixed race and missing race. The study was approved by our Institutional Review Board (IRB) and local VA Research and Development committee.
Outcome Measure
The main outcome measure was time to death. Veterans were followed from time of entry into the study until death, loss to follow-up, or through December 2006. A subject was considered censored if alive by May 2006.
Primary Covariates
The first primary covariate was mean medication possession ratio (MPR) over the study period for each veteran. MPR was calculated for individuals using 1) insulin combined with oral hypoglycemic agents, 2) insulin only, and 3) oral hypoglycemic agents only. MPR was defined as the ratio of days for which a medication (insulin or oral hypoglycemic agents, i.e. VA classes HS501 or HS502, respectively) is supplied to the total days in a specified time interval. In this study, MPR was initially calculated in quarterly (90 days) intervals for both insulin and oral hypoglycemic agents for each patient. Subsequently, an annual average was calculated by taking the mean of the four quarterly MPR values in each year the patient was in the study from 2002 to 2006. Finally, mean MPR was calculated across all study years and utilized in the analysis. If the MPR exceeded 1, the MPR was set to 1. An MPR value of 1 implies perfect medication adherence, whereas an MPR value of zero would imply that no medication had been taken. A mean MPR of 80 %, which is an average of 90-day interval MPRs over the entire study period, means that a patient has, on average, 80 % of their medication supply over the entire study period. The greater the MPR percentage within each 90-day interval, the more medication veterans had in their possession and likely took as prescribed (i.e., greater adherence). MPR was categorized into quintiles to capture the distribution of MPR. In general, MPR values of 0.8 and above are considered to imply good adherence. For example, Lau and colleagues found that patients with type 2 diabetes who did not obtain at least 80% of their oral hypoglycemic medications across 1 year were at higher risk of hospitalization in the following year.
The second primary covariate of interest was race/ethnicity, with NHW serving as the reference group. Race/ethnicity was retrieved from the 2002 outpatient and inpatient Medical SAS data sets. When missing or unknown, the variable was supplemented using the inpatient race1–race6 fields from the 2003 Medical SAS data sets, the outpatient race1–race7 fields from the 2004 Medical SAS data sets, and the VA Vital Status Centers for Medicare and Medicaid Services (CMS) field for race.
Demographic Variables
We controlled for six demographic variables in addition to the primary covariates. Age was treated as continuous and centered at a mean of 66 years. Gender was treated as nominal with males as the reference group. Marital status was classified as single or married (reference group). Percentage service-connectedness, representing the degree of disability due to illness or injury that was aggravated by or incurred in military service, was treated as continuous. For location of residence (urban, rural), urban served as the reference group and highly rural was categorized as rural14. Region accounted for the five geographic regions of the country: Northeast (VISNs 1, 2, & 3), Mid-Atlantic (VISNs 4, 5, 6, 9, & 10), South (VISNs 7, 8, 16, & 17), Midwest (VISNs 11, 12, 15, 19, & 23), and West (VISNs 18, 20, 21, & 22), with the South serving as the reference group.15
Disease Severity Measure
We used VA medication class as a measure of disease severity (1 = Insulin only, 2 = both insulin and oral medication, 3 = oral only). Oral medication was used as the reference group.
Medical Comorbidity Measures
Medical comorbidity variables included anemia, cancer, cardiovascular disease (CVD), cerebrovascular disease, congestive heart failure (CHF), depression, fluid and electrolyte disorders, hypertension, hypothyroidism, liver disease, lung conditions (chronic pulmonary disease, pulmonary circulation disease), obesity, peripheral vascular disease, psychoses, substance abuse (alcohol abuse, drug abuse), and other (acquired immunodeficiency syndrome-AIDS, rheumatoid arthritis, renal failure, peptic ulcer disease and bleeding, weight loss), and were defined based on ICD-9 codes at entry into the cohort. All medical comorbidities were dichotomized as present or absent.
Statistical Analysis
In preliminary analyses, crude associations were examined between mortality and all measured covariates using chi-square tests for categorical variables and t-tests for continuous variables. Cox regression methods were used to model the association between time to death and MPR (categorized into quintiles) and race. Time to death was defined as the number of months from time of entry into the cohort to time of death or censoring (i.e., day last seen or May 2006). For the Cox model, appropriateness of the assumption of proportionality was determined by examining log{-log(time)} plots and by testing the coefficients of the interactions of time with the respective covariate in multivariate analyses. Initially, unadjusted hazard ratios (HR) for mortality risk were computed for race and MPR quintiles. Then, after fitting the final Cox model adjusted for all covariates (race, socio-demographics, and comorbidities), an interaction between race and MPR was tested. HR estimates stratified by race are reported, since there was significant race and MPR interaction. The Kaplan–Meier method was used to plot the survival functions for MPR quintiles stratified by race. Residual analysis was used to assess goodness-of-fit of each of the models. All data analyses were conducted using SAS v9.2.16
RESULTS
Table 1 shows the demographic and clinical characteristics of the entire study cohort by MPR quintiles. The mean age was 65 years, with most of the sample being men (2.2 % were women). Overall, 22 % of the cohort died, with more than an 8-fold higher proportion dead among those in the lowest MPR quintile (0–49.3 %) compared to those in the highest quintile (94.2–100.0 %). A majority of the sample was NHW (72 %) and the proportion of NHW increased with each increase in MPR quintile. The largest proportion of NHB veterans (17.2 %) was in the lowest MPR quintile and dropped by increasing MPR quintile, while the proportion of Hispanic only slightly declined across increasing MPR quintiles. For medication use, the majority were on oral medication, only (57 %) across all quintiles, followed by insulin then both insulin and oral medication. Nearly 37 % of veterans lived in rural areas and the proportion increased with each increase in MPR quintile (medication adherence). More veterans were from the South (31 %) followed by the Mid-Atlantic, Midwestest, West and Northeast. At baseline, the most prevalent comorbidity was hypertension (78 %). Lung disease, obesity and depression were also common, while the prevalence of other comorbid conditions was below 20%.
Table 1.
Sample Characteristics of a National Cohort of 629,563 Veterans with Type 2 Diabetes by MPR Quintile
| Medication Possession Ratio | ||||||
|---|---|---|---|---|---|---|
| Variable | All | 0 %–49.2 % | 49.3 %–68.6 % | 68.7 %–83.7 % | 83.8 %–94.1 % | 94.2 %–100.0 % |
| Race | ||||||
| Non-Hispanic White | 72.0 | 67.7 | 69.2 | 71.0 | 74.2 | 77.8 |
| Non-Hispanic Black | 13.3 | 17.2 | 16.1 | 13.7 | 11.0 | 8.6 |
| Hispanic | 5.3 | 5.6 | 5.8 | 5.7 | 5.0 | 4.6 |
| Other Race | 9.4 | 9.4 | 8.9 | 9.6 | 9.8 | 9.0 |
| Age (in years), mean (sd) | 65 (11.1) | 66 (12.1) | 66 (11.8) | 65 (11.1) | 65 (10.4) | 65 (9.8) |
| Sex | ||||||
| Male | 97.8 | 97.3 | 97.6 | 97.8 | 98.0 | 98.2 |
| Female | 2.2 | 2.7 | 2.4 | 2.2 | 2.0 | 1.8 |
| Marital Status | ||||||
| Married | 65.1 | 59.3 | 61.9 | 65.3 | 68.2 | 70.6 |
| Single | 34.9 | 40.7 | 38.1 | 34.7 | 31.8 | 29.4 |
| Service % Disability, mean (sd) | 13 (26.4) | 12 (25.4) | 12 (26.1) | 13 (26.6) | 13 (26.7) | 13 (27) |
| Rural Status | ||||||
| Live in Rural Area | 36.8 | 32.3 | 34.1 | 36.4 | 39.2 | 41.6 |
| Live in Urban Area | 63.2 | 67.7 | 65.9 | 63.6 | 60.8 | 58.4 |
| Geographic Region | ||||||
| Northeast | 11.0 | 11.5 | 11.3 | 10.8 | 10.9 | 10.6 |
| Mid-Atlantic | 21.6 | 20.8 | 21.3 | 21.3 | 22.0 | 22.9 |
| Mid-West | 21.1 | 18.8 | 19.7 | 20.9 | 22.5 | 23.4 |
| South | 30.8 | 31.6 | 31.9 | 31.7 | 30.0 | 29.1 |
| West | 15.2 | 16.7 | 15.6 | 15.2 | 14.6 | 14.0 |
| Comorbidity | ||||||
| Anemia | 7.5 | 11.0 | 8.6 | 6.8 | 5.7 | 5.2 |
| Cancer | 7.3 | 9.1 | 7.9 | 6.7 | 6.2 | 6.5 |
| Cerebrovascular Disease | 11.4 | 15.2 | 13.0 | 10.7 | 9.3 | 8.9 |
| Congestive Heart Failure | 11.1 | 16.8 | 13.6 | 10.4 | 8.3 | 6.7 |
| Cardiovascular Disease | 3.6 | 4.8 | 4.0 | 3.3 | 3.0 | 2.8 |
| Depression | 12.7 | 13.6 | 13.5 | 13.0 | 12.2 | 11.4 |
| Fluid/Electrolyte Disorders | 4.9 | 7.9 | 5.7 | 4.5 | 3.6 | 3.0 |
| Hypertension | 78.1 | 75.3 | 77.5 | 78.1 | 78.8 | 80.5 |
| Hypothyroidism | 6.2 | 6.4 | 6.3 | 6.1 | 6.1 | 6.0 |
| Liver Disease | 3.2 | 4.7 | 3.9 | 2.9 | 2.4 | 2.0 |
| Lung Disease | 13.8 | 16.9 | 15.4 | 13.0 | 12.1 | 11.6 |
| Obesity | 12.8 | 10.9 | 12.1 | 13.0 | 13.8 | 14.2 |
| Psychoses | 4.4 | 5.7 | 4.8 | 4.3 | 3.9 | 3.4 |
| Peripheral Vascular Disease | 11.9 | 14.6 | 13.6 | 11.6 | 10.3 | 9.5 |
| Substance Abuse | 3.9 | 6.2 | 5.0 | 3.7 | 2.7 | 2.1 |
| Other Disease | 3.6 | 5.7 | 4.3 | 3.3 | 2.7 | 2.3 |
| Medication Class | ||||||
| Insulin | 22.0 | 28.5 | 30.0 | 25.5 | 17.8 | 8.7 |
| Insulin + Oral Agents | 21.0 | 18.5 | 19.3 | 27.4 | 29.9 | 10.6 |
| Oral Agents | 57.0 | 53.0 | 50.9 | 46.9 | 52.3 | 80.7 |
| Vital Status | ||||||
| Dead | 22.0 | 47.3 | 35.1 | 16.7 | 5.9 | 5.4 |
| Months to Death, mean (SD) | 54 (13.1) | 44 (19.7) | 51 (13.9) | 57 (8) | 60 (2.7) | 59 (6.2) |
All values represent percentages unless otherwise indicated
Other Race = includes 0.3 % Asian/Pacific Islander, 0.2 % American Indian/Alaska Native and 8.9 % other)
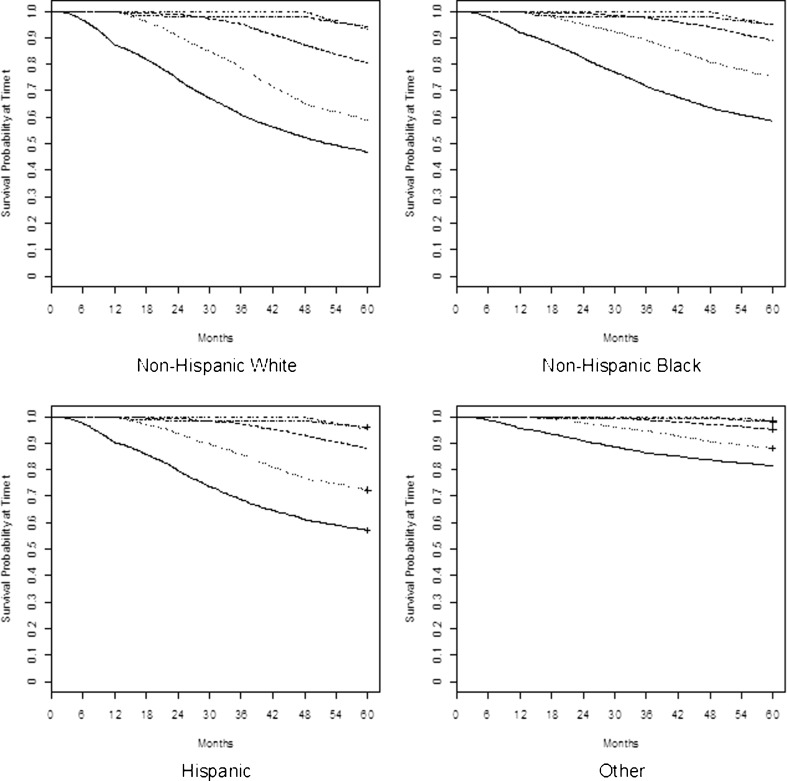
Figure 1 shows the Kaplan–Meier estimates of the survival curve by MPR quintile for each race/ethnicity group. Within each race/ethnicity group, there is a strong association between MPR quintile and mortality, with the largest gap between the lowest and highest quintiles. Comparing across the four racial/ethnic groups, there are differences in survival by MPR quintiles (or levels of medication adherence); the most pronounced differences are shown among NHW.
Figure 1.
Kaplan–Meier Survival Curves by MPR Quintile for each Race/Ethnicity Group. MPR = medication possession ratio.  MPR 0–49.2 % (first quintile),
MPR 0–49.2 % (first quintile),  MPR 49.3–68.6 % (second quintile).
MPR 49.3–68.6 % (second quintile).  MPR 68.7–83.7 % (third quintile).
MPR 68.7–83.7 % (third quintile).  MPR 83.8–94.1 % (fourth quintile).
MPR 83.8–94.1 % (fourth quintile).  MPR 94.2–100.0 % (fifth quintile).
MPR 94.2–100.0 % (fifth quintile).
Table 2 shows the HR and corresponding 95% CI for association between MPR quintiles and mortality stratified by race/ethnicity. After adjusting for all covariates, the HR for subjects in the lowest MPR quintile (<49.3 %) versus highest MPR quintile (>94.1 %) was 12.21 (95% CI 11.89,12.55) for NHW, 10.01 (95 % CI 9.18,10.91) for NHB, 12.65 (95% CI 11.10,14.43) for Hispanic, and 10.41 (95% CI 9.06,11.96) for Other race groups. HRs at the second lowest quintile relative to the highest quintile, decreased to 7.75, 5.11, 7.09 and 5.92 for NHW, NHB, Hispanic and Others, respectively. In contrast at the second highest quintile (83.8 < MPR < 94.2 %), the HRs were almost equal to one showing no association between MPR and mortality.
Table 2.
Adjusted Hazard Rate Ratios (and 95% CI) for All-Cause Mortality From a Multivariate Cox Model for a Given Difference in Risk Factor Level Stratified by Race/Ethnicity
| NHW | NHB | Hispanic | Other | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CL | HR | 95% CL | HR | 95% CL | HR | 95% CL |
| MPR Quintile | ||||||||
| MPR: 0–49.2% | 12.21 | (11.89,12.55) | 10.01 | (9.18,10.91) | 12.65 | (11.10,14.43) | 10.41 | (9.06,11.96) |
| MPR: 49.3–68.6% | 7.75 | (7.54,7.96) | 5.11 | (4.68,5.58) | 7.09 | (6.20,8.11) | 5.92 | (5.13,6.83) |
| MPR: 68.7–83.7% | 3.32 | (3.22,3.42) | 2.14 | (1.95,2.35) | 2.86 | (2.48,3.30) | 2.44 | (2.09,2.85) |
| MPR: 83.8–94.1% | 1.10 | (1.06,1.13) | 0.96 | (0.86,1.08) | 1.07 | (0.90,1.27) | 0.79 | (0.65,0.96) |
| Highest Quintile | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Medication Class | ||||||||
| Oral Agents | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Insulin | 0.94 | (0.93,0.96) | 0.99 | (0.95,1.02) | 1.09 | (1.03,1.16) | 1.15 | (1.07,1.24) |
| Insulin + Oral | 0.90 | (0.89,0.92) | 0.91 | (0.87,0.95) | 0.88 | (0.82,0.95) | 1.05 | (0.97,1.13) |
| Male (vs. female) | 1.73 | (1.64,1.82) | 2.29 | (2.00,2.64) | 2.64 | (1.88,3.70) | 2.01 | (1.67,2.43) |
| Single (vs. married) | 1.07 | (1.06,1.08) | 1.09 | (1.06,1.12) | 1.06 | (1.01,1.12) | 1.27 | (1.19,1.34) |
| Rural (vs. urban) | 1.04 | (1.03,1.06) | 1.04 | (1.00,1.08) | 0.99 | (0.92,1.07) | 1.14 | (1.07,1.22) |
| Geographic Region | ||||||||
| Northeast | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Mid-Atlantic | 1.12 | (1.10,1.14) | 1.09 | (1.03,1.15) | 0.93 | (0.75,1.15) | 0.95 | (0.83,1.08) |
| Mid-West | 1.13 | (1.10,1.15) | 1.18 | (1.11,1.26) | 1.14 | (1.00,1.31) | 1.19 | (1.04,1.35) |
| South | 1.12 | (1.10,1.14) | 1.11 | (1.05,1.18) | 1.19 | (1.07,1.31) | 1.07 | (0.95,1.21) |
| West | 1.04 | (1.02,1.07) | 1.04 | (0.97,1.11) | 1.13 | (1.01,1.26) | 1.30 | (1.15,1.47) |
| Comorbidity (yes versus no) | ||||||||
| Substance Abuse | 0.88 | (0.86,0.92) | 0.87 | (0.83,0.92) | 0.87 | (0.79,0.97) | 1.18 | (1.06,1.33) |
| Anemia | 1.32 | (1.30,1.35) | 1.34 | (1.29,1.39) | 1.40 | (1.30,1.50) | 1.64 | (1.46,1.83) |
| Cancer | 1.64 | (1.61,1.67) | 1.89 | (1.81,1.97) | 1.81 | (1.68,1.96) | 3.49 | (3.12,3.90) |
| Cerebrovascular Disease | 1.44 | (1.42,1.46) | 1.52 | (1.47,1.58) | 1.67 | (1.57,1.78) | 1.66 | (1.50,1.85) |
| CHF | 1.92 | (1.89,1.94) | 1.94 | (1.86,2.01) | 2.17 | (2.03,2.32) | 2.91 | (2.64,3.21) |
| CVD | 1.23 | (1.20,1.26) | 1.20 | (1.13,1.29) | 1.17 | (1.06,1.30) | 1.59 | (1.35,1.87) |
| Depression | 0.92 | (0.90,0.93) | 0.86 | (0.82,0.91) | 0.87 | (0.81,0.94) | 1.00 | (0.91,1.09) |
| Hypertension | 1.06 | (1.04,1.07) | 1.40 | (1.33,1.47) | 1.38 | (1.28,1.48) | 1.31 | (1.23,1.40) |
| Hypothyroidism | 1.08 | (1.05,1.10) | 1.13 | (1.04,1.23) | 1.12 | (1.02,1.22) | 1.13 | (0.98,1.29) |
| Liver disease | 1.37 | (1.33,1.41) | 1.35 | (1.27,1.44) | 1.72 | (1.58,1.88) | 2.10 | (1.85,2.37) |
| Lung Disease | 1.45 | (1.43,1.48) | 1.46 | (1.40,1.52) | 1.32 | (1.23,1.42) | 1.70 | (1.55,1.86) |
| Fluid/Electrolyte Disorders | 1.22 | (1.20,1.25) | 1.26 | (1.21,1.32) | 1.42 | (1.31,1.54) | 1.39 | (1.23,1.58) |
| Obesity | 0.79 | (0.78,0.81) | 0.73 | (0.70,0.78) | 0.75 | (0.68,0.82) | 0.90 | (0.82,0.98) |
| Other Disease | 1.23 | (1.20,1.26) | 1.35 | (1.28,1.41) | 1.33 | (1.21,1.46) | 1.53 | (1.32,1.77) |
| Psychoses | 0.95 | (0.93,0.98) | 1.05 | (0.99,1.11) | 1.10 | (1.00,1.21) | 1.29 | (1.12,1.49) |
| PVD | 1.46 | (1.44,1.48) | 1.47 | (1.42,1.53) | 1.57 | (1.47,1.67) | 1.81 | (1.64,2.01) |
NHW non-Hispanic white; NHB non-Hispanic black; HR hazard ratio; 95% CL 95% confidence limits; MPR medication possession ratio; CHF congestive heart failure; CVD cardiovascular disease; PVD peripheral vascular disease
Other Race = includes 0.3 % Asian/Pacific Islander, 0.2 % American Indian/Alaska Native and 8.9 % other)
Racial/ethnic differences in the relationship between MPR quintile and mortality are evident in the lowest two MPR quintiles relative to the highest MPR quintile. The mortality risk associated with being in the lowest versus highest MPR quintile is roughly 10-fold in NHB and Other race veterans, but 12-fold in NHW and almost 13-fold in Hispanic veterans. Similarly, the mortality risk associated with being in the second lowest versus highest MPR quintile is 5-fold in NHB and almost 6-fold in Other race veterans, but close to 8-fold in NHW and over 7-fold in Hispanic veterans. Additionally, race modified the effect of gender on mortality; the mortality hazard in men relative to women was 2.29 (95% CI: 2.00, 2.64) in NHB and 2.64 (95 % CI: 1.88, 3.70) in Hispanics, but only 1.73 (95% CI: 1.64, 1.82) in NHW. There were no observed race/ethnicity-based differences in the hazard of death by geographic region or location of residence.
For most of the comorbid conditions such as anemia, cancer, cerebrovascular disease, CHF, liver disease, lung disease and PVD, the Other race group had a slightly higher hazard of death followed by Hispanic, NHB and NHW in that order. The risk of death in those with cancer as compared to those without was highest in the Other race group, with a hazard ratio of 3.49 compared to hazard ratios of 1.64 in NHW, 1.81 in Hispanics and 1.89 in NHB.
VA medication class showed an association with mortality such that individuals using insulin only or insulin combined with oral hypoglycemic agents generally had lower mortality risk than those using only oral hypoglycemic agents. The two exceptions to this were Hispanic and Other race Veterans using insulin only; respective HRs for Hispanic and Other race veterans were 1.09 (95 % CI 1.03, 1.16) and 1.15 (95 % CI 1.07, 1.24).
DISCUSSION
This study demonstrates clear evidence of increased mortality with lower medication adherence and a differential impact of medication non-adherence on mortality, with NHBs in lower quintiles of MPR having lower mortality compared to NHWs. In multivariate analyses adjusted for demographic, geographic, and clinical/comorbidity variables, the risk of death was 10-fold to 12-fold higher comparing the lowest level to the highest level of adherence, with the risk being highest in Hispanics (HR 12.65) and lowest in NHB (HR 10.01). Furthermore, the type of diabetes therapy (insulin with or without oral agents) was associated with mortality and varied by racial/ethnic group, with Hispanic veterans having a higher risk of death with insulin only use.
Our findings are consistent with prior studies. In a large retrospective study of 11,532 patients with diabetes enrolled in a managed care organization, 21.3 % were medication non-adherent (measured as proportion of days covered <80 %) and non-adherent patients had higher HbA1c, blood pressure and lipids. In addition, there was a 37 % higher risk of hospitalization and an 81 % higher risk of death over 1 year of follow-up.9 Our results add to the current body of research on medication non-adherence in diabetes by: 1) extending the length of follow-up to 5 years; 2) examining differential effects of medication adherence on mortality by race/ethnicity; 3) examining the contribution of class of diabetes medication used and how it differs by race/ethnicity; and 4) using quintiles of MPR to better capture differences across the distribution of MPR. Evidence on the use of other diabetes-related medications for CVD risk reduction has been presented in a meta-analysis on adherence to oral hypoglycemic agents, antihypertensive drugs, and drugs for dyslipidemia, which showed an average adherence rate of 59 %—that is, only 59 % of patients took all of their medications for more than 80% of days on a treatment regimen.17
Our study also sheds light on the contribution of medication class on mortality across quintiles of MPR. We found that individuals on insulin only or insulin combined with oral hypoglycemic agents generally had lower mortality risk than those using only oral hypoglycemic agents. This is most likely a function of the increased intensity of treatment usually associated with initiation of insulin, despite strong evidence that medication adherence to insulin is lower than medication adherence to oral agents. An MPR-based analysis of medication adherence among 6,090 insured patients newly diagnosed with diabetes showed poorer medication adherence was associated with initiating insulin therapy compared to initiating an oral agent.18 Initial treatment with insulin carried three-fold higher odds of failing to refill a second prescription and 2.6 greater odds of discontinuing treatment after 12 months.18 While further research is needed to understand whether medication class mediates the relationship between MPR and mortality, it should be noted that insulin-specific medication adherence may be improved with greater patient education and comfort with multiple daily self-injections, greater electronic capability for patients to monitor and graph daily blood glucose, or provisions for continuous insulin delivery devices for those with persistently low adherence and poor glycemic control.
Another salient finding from this study is that despite lower medication adherence among racial/ethnic minority veterans, the mortality risk among NHB was lower across each MPR quintile compared to other racial/ethnic groups. In a previous longitudinal study of racial differences in mortality among veterans with type 2 diabetes, a mortality advantage was shown among older NHB veterans compared to their white counterparts, such that NHB had a 16 % lower risk of death in adjusted analyses.19 Lower mortality among NHB compared to NHW veterans has also been reported in other diseases.20,21 The exact reasons for these differences are unclear and need to be further studied. However, we can offer the following potential brief explanations: that there are more intensive efforts at treatment and monitoring to improve control, use of more social-professional support, and survivor bias among NHB veterans; or greater likelihood of dual use with more fragmented care and tighter glycemic control with poorer outcomes among NHW veterans.
Strengths of our study include: the study population, all veterans with type 2 diabetes receiving care at the VHA who had prescriptions for diabetes medication in 2002; its longitudinal design with 5 years of follow-up data; the extensive data available on comorbidities; our ability to identify racial/ethnic group in over 90 % of the cohort; our longitudinal information on type of medication used to treat diabetes; and our information on medication non-adherence as measured by an MPR quintiles. There are also limitations of our study. First, MPR is really only a proxy for medication adherence, and because of wastage, the reliability of MPR for insulin needs further validation; however, prior studies of insulin MPR have been conducted using VA data.22 Second, the VA medical record does not include information on socioeconomic status, which is likely to be an important factor. Third, we were missing information on racial/ethnic group in 8.9 % of the population and cannot rule out that this introduced bias. However, we do not anticipate this to adversely affect the validity of our results, since the characteristic of those with race and without race information was very similar. Importantly, the distribution of MPR was very similar in those with missing and those with non-missing race information. Fourth, women make up a small proportion and women veterans may not be representative of women in the general population. Fifth, multi-medication regimens examined are limited to diabetes medications (non-diabetes medications were not available), and MPR does not account for issues like stockpiling. Finally, we were not able to further risk-adjust for other important risk factors for CVD (such as smoking and hyperlipidemia), as well as macrovascular and microvascular complications of diabetes.
In summary, this study demonstrates clear evidence of increased mortality with lower medication adherence and a differential impact of medication non-adherence on mortality, with NHBs in lower quintiles of MPR having lower mortality compared to NHWs. It also demonstrates that the type of diabetes therapy (insulin with or without oral agents) was associated with mortality and varied by racial/ethnic group. These findings support that monitoring the extent of adherence needs to become a high priority in the overall clinical management of diabetes, especially among those with poorer glycemic control. Beyond recognition of a simple medication non-adherence, understanding the extent to which patient (knowledge/literacy), provider (therapeutics), and system-level (insurance/cost, access) factors may contribute to non-adherence will serve to further enhance diabetes management.
Electronic Supplementary Material
(PDF 131 kb)
Acknowledgements
Funders
This study was supported by grant #IIR-06-219 funded by the VHA Health Services Research and Development (HSR&D) program. The funding agency did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The manuscript represents the views of the authors and not those of the VA or HSR&D. All authors had access to the data and contributed to the manuscript
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008.
- 2.Xu JQ, Kochanek KD, Murphy SL, Tejada-Vera B. Deaths: Final data for 2007. National vital statistics reports; vol 58 no 19. Hyattsville, MD: National Center for Health Statistics. 2010. Available online: http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_19.pdf. Accessed August 3, 2012. [PubMed]
- 3.Mokdad, A.. Diabetes trends in the U.S.: 1990–1998. Diabetes Care. 2000;23(9): 1278–83. [PMID: 10977060]. [DOI] [PubMed]
- 4.Cowie C. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 5.Adams AS, Trinacty CM, Zhang F, et al. Medication adherence and racial differences in A1C control. Diabetes Care. 2008;31(5):916–921. doi: 10.2337/dc07-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant R, Adams AS, Trinacty CM, et al. Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care. 2007;30(4):807–812. doi: 10.2337/dc06-2170. [DOI] [PubMed] [Google Scholar]
- 7.Trinacty CM, Adams AS, Soumerai SB, et al. Racial differences in long-term adherence to oral antidiabetic drug therapy: a longitudinal cohort study. BMC Health Serv Res. 2009;9:24. doi: 10.1186/1472-6963-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27(9):2149–2153. doi: 10.2337/diacare.27.9.2149. [DOI] [PubMed] [Google Scholar]
- 9.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 10.Heisler M, Choi H, Rosen AB, et al. Hospitalizations and deaths among adults with cardiovascular disease who underuse medications because of cost: a longitudinal analysis. Med Care. 2010;48(2):87–94. doi: 10.1097/MLR.0b013e3181c12e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo YF, Raji MA, Markides KS, Ray LA, Espino DV, Goodwin JS. Inconsistent use of diabetes medications, diabetes complications, and mortality in older Mexican Americans over a 7-year period: data from the Hispanic established population for the epidemiologic study of the elderly. Diabetes Care. 2003;26(11):3054–3060. doi: 10.2337/diacare.26.11.3054. [DOI] [PubMed] [Google Scholar]
- 12.Balkrishnan R, Rajagopalan R, Camacho FT, Huston SA, Murray FT, Anderson RT. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther. 2003;25(11):2958–2971. doi: 10.1016/S0149-2918(03)80347-8. [DOI] [PubMed] [Google Scholar]
- 13.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–B21. doi: 10.2337/diacare.27.suppl_2.B10. [DOI] [PubMed] [Google Scholar]
- 14.West AN, Lee RE, Shambaugh-Miller MD, et al. Defining “rural” for veterans’ health care planning. J Rural Health. Fall;26(4):301–9. [PMID: 21029164]. [DOI] [PubMed]
- 15.Department of Veterans Affairs Field Research Advisory Committee. (2004). Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Field Research Advisory Committee Operating Procedures. Available online: http://www.research.va.gov/about/frac/FRAC-ops.pdf Accessed August 3, 2012.
- 16.SAS statistical software, version 9.2. SAS Institute Inc, Cary, NC.
- 17.Cramer JA, Benedict A, Muszbek N, et al. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62:76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hertz RP, Unger AN, Lustik MB. Adherence with pharmacotherapy for type 2 diabetes: a retrospective cohort study of adults with employer-sponsored health insurance. Clin Ther. 2005;27(7):1064–1073. doi: 10.1016/j.clinthera.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Lynch CP, Gebregziabher M, Echols C, Gilbert GE, Zhao Y, Egede LE. Racial Disparities in All-Cause Mortality Among Veterans with Type 2 Diabetes. J Gen Intern Med. Jun 8 2010. [PMID: 20532659]. [DOI] [PMC free article] [PubMed]
- 20.Jha AK, Shlipak MG, et al. (2001). “Racial differences in mortality among men hospitalized in the Veterans Affairs health care system.” JAMA. Jan 17 2001;285(3): 297–303. [PMID: 11176839]. [DOI] [PubMed]
- 21.Volpp KG, Stone R, Lave JR, et al. Is thirty-day hospital mortality really lower for black veterans compared with white veterans? Health Serv Res. 2007;42(4):1613–1631. doi: 10.1111/j.1475-6773.2006.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cramer JA, Pugh MJ. The influence of insulin use on glycemic control: how well do adults follow prescriptions for insulin? Diabetes Care. 2005;28:78–83. doi: 10.2337/diacare.28.1.78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 131 kb)