Abstract
Objective
To isolate and characterize the bioactive secondary metabolites from Aspergillus ochraceus (A. ochraceus) MP2 fungi.
Methods
The anti bacterial activity of marine sponge derived fungi A. ochraceus MP2 was thoroughly investigated against antagonistic human pathogens. The optimum inhibitory concentration of the fungi in the elite solvent was also determined. The promising extracts that showed good antimicrobial activity were subjected to further analytical separation to get individual distinct metabolites and the eluants were further identified by GC MS instrumental analysis. The molecular characterization of the elite fungal strains were done by isolating their genomic DNA and amplify the internal transcribed spacer (ITS) region of 5.8s rRNA using specific ITS primer. The novelty of the strain was proved by homology search tools and elite sequences was submitted to GENBANK.
Results
Three bioactive compounds were characterized to reveal their identity, chemical formula and structure. The first elutant was identified asα- Campholene aldehyde with chemical formula C10 H16 O and molecular weight 152 Da. The second elutant was identified as Lucenin-2 and chemical formula C27 H30 O16 and molecular weight 610 Da. The third elutant was identified as 6-Ethyloct- 3-yl- 2- ethylhexyl ester with Chemical formula C26 H42 O4 with molecular weight 418 Da.
Conclusions
The isolated compounds showed significant antimicrobial activity against potential human pathogens. Microbial secondary metabolites represent a large source of compounds endowed with ingenious structures and potent biological activities.
Keywords: Marine sponge derived fungi, Aspergillus ochraceus, Bioactive metabolites, Antibacterial activity, Analytical characterization, Fungi, Apergillus, Bioactive secondary metabolite, Antagonistic human pathogen, Antimicrobial activity, Bioactive compounds
1. Introduction
Many marine invertebrates produce natural compounds that affect the growth, metabolism, reproduction, and survival of other types of organisms. Hence, they are considered to be bioactive. Those include potentially effective therapeutic agents with antiviral, antibacterial, and antitumor properties produced by invertebrates from the classes Porifera, Cnidaria, Mollusca, Echinodermata, Bryozoa, and Urochordata. Close relations between marine invertebrate species and microorganisms, including symbiotic associations and interactions during larval settlement, have been characterized and this provides insights to the regulation of host-symbiont-microbial community interactions. Many of the compounds isolated from marine organisms, such as sponges, may be produced by associated microbes. Marine sponges are benthic animals found in the widerange of marine environments. The diversity of sponges species is superior in the tropical coral reef environments. The sponges are also very important resources for searching the biologically active substances, which are useful to develop pharmaceuticals, agrochemicals and biochemical reagents and their lead compounds[1]. The origins of these biologically active substances are recently thought to be the metabolites produced by the microorganisms associated with the sponges. And studies have also suggested that some bioactive compounds isolated from marine organisms have been shown to exhibit anti-cancer, anti-microbial, anti-fungal or anti-inflammatory and other pharmacological activities[2]–[9]. These marine invertebrates have evolved chemical defense mechanicsms against other invading organisms, which involve the production of secondary metabolites[10]. Sponges are good homes not only for macro organisms, such as worms, brittlestars, shrimp, crabs, etc., but also for a variety of microorganisms such as bacteria, fungi, and microalgae, which live in the canals, between cells, and even inside the cell[11]. A variety of antimicrobial substances have been isolated from various species of marine sponges[12]. Up to 800 antibiotic compounds have been isolated from marine sponges, a number of which corroborates assumptions that sponges appear to defend themselves against infections by producing and/or accumulating secondary metabolites. Aspergillus ochraceus (A. ochraceus) can produce other secondary metabolites, whose biological activity has not been characterized until now. These molecules may be beneficial (antibiotics) or harmful (mycotoxins) to human health[13]–[15].
Fungi isolated from marine sponge have a high creativity index, i.e., ability to synthesize new and interesting secondary metabolites. Although the natual function is not known, it is assumed that they play an important role in chemical defense and communication of the organism[16]. Many of them have been suggested to act as pheromones, antifeedants or repellents and regulators in the development of organisms. The secondary metabolite does not occur randomly but is correlated with ecological factors[17]. Nevertheless, a growing number of metabolites from sponge-derived fungal strains has been reported in the last years[18]. It provides an overview of sponge species investigated, taxonomy of isolated fungi, and reported metabolites. These structures suggest most of the metabolites to be derived from metabolic pathways is also common to terrestrial fungi. Such a similarity is, for example, obvious for sesquiterpenes of the hirsutane-type[16]. Studies show that secondary metabolites in sponges play a crucial role in their survival in the marine ecosystem[26]. These natural products have interesting biomedical potential, pharmaceutical relevance and diverse biotechnological applications[18]. The biomedical and pharmaceutical importances of these compounds are attributed to their antiviral, antitumor, antimicrobial and general cytotoxic properties[12]. Interestingly, out of the 13 marine natural products that are currently under clinical trials as new drug candidates, 12 are derived from invertebrates. Among them, Porifera remains the most important phylum, as it provides a greater number of natural products, especially novel pharmacologically active compounds[19]. Biochemical characteristics seem to be useful taxonomic markers and good indicators of sponge phylogeny[20].The diversity of biochemical properties of sponges has been demonstrated by the continued discovery of novel compounds that have pharmacological properties [21].
2. Materials and methods
2.1. Collection of sponge
Specimens were collected by SCUBA diving using hammer and chisel from Gulf of Mannar, located at 215 kms from Kanyakumari District, in the narrow strip of peninsular land along the south east coast of Tamilnadu state.
2.2. Isolation of fungi
The sponge sample was washed with sterile water (distilled water: sea water; 1:1) and ground in a mortar and pestle under aseptic conditions. Serial dilution was performed and from each dilution, plating was done in Sabourauds agar by spread plate technique. The plates were then incubated at 27 °C for 5 days. After 5 days, the plates were examined and the pure culture was isolated on pure agar plate.
2.3. Molecular characterization and identification of elite fungi by ITS sequencing
The fungi were grown in culture in potato dextrose broth at room temperature in the dark for 48 to 72 hours. The genomic DNA was isolated and the internal transcribed spacer (ITS) region of 5.8sRNA was amplified using primer ITS1 TO 5′ TCCGTAGGTGAACCTGCGG 3′ and primer ITS5 5′ TCCTCCGCTTATTGATATGC 3′ 7 and sequenced using automated sequencer.
2.4. Mass cultivation of A. ochraceous
A. ochraceus Wilhelm NRRL 3174 was grown on synthetic agar medium (SAM) of the following composition: 3 g/L NH4NO3, 26 g/L K2HPO4, 1 g/L KCl,1 g/L MgSO4·7H2O, 10 mL of mineral solution (containing distilled water per litre, 70 mg Na2B4O7·10H2O, 50 mg (NH4)6·Mo7O24·4H2O, 1 000 mg FeSO4·7H2O, 30 mg CuSO4·5H2O, 11 mg MnSO4·H2O, and 1 760 mg ZnSO4·7H2O; the pH was adjusted to 2 with 2 mol/L HCl), 15 g agar, and 50 g/L glucose. The pH of the medium was adjusted to 6.5 by 2 mol/L HCl and autoclaved at 120 °C for 20 minutes.
2.5. Extraction process
The fungal mycelia were homogenized using sea water. Then the biomass was subjected to an extraction of biologically active components which were carried out with different solvents in the order of increase polarity: Choloroform, butanol and ethyl acetate by soaking at ambient temperature. The crude extracts obtained were dried under rotary vacuum evaporator and screened for anti-bacterial activity.
2.6. Antimicrobial assay
Agar diffusion assay is used widely to determine the antibacterial activity of crude extract. The technique works well with defined inhibitors. Nutrient agar was prepared and was poured in the petri dish and allowed for solidification, 24 hours growing bacterial culture were swabbed on it.The wells (8 mm diameter) were made by using cork borer.The difference concentration of the crude extract were loaded in the well. The plate was then inculated at 37 °C for 24 hours.
Dilution assay is a standard method used to compare the inhibition efficiency of the antimicrobial agents. Nutrient broth was inoculated with 24 hours growing bacterial culture and different concentrations of the extract were inoculated. Bacterial culture inoculated in nutrient broth were used as control. The tubes were incubated at 37°C for 24 hours. The optimal densities were measured spectrometrically at 600 nm. The percentage of viable cell was calculated using the following formula:
% Viable cells= Control OD-Test OD×100/ Control
2.7. Thin layer chromatography
TLC is used to separate the compound present in the crude extract. The separation of the compound also depends on the usage of the solvent. The drug with the concentration of 1 mg/mL was plotted on the TLC plate and dried. It was then run with different solvent ratio the spots were identified both in the uv light and in the iodine chamber. The Rf value was calculated using the formula:
Rf value=Distance travelled by the solute / Distance travelled by the solvent
2.8. Gas chromatography-mass spectrometry (GCMS)analysis
The crude extract was quantified using gas chromatograph (GCMS-Shimadzu) equipped with a DB-5 ms column (mm inner diameter 0.25 mm, length 30.0 m, film thickness 0.25 µm) mass spectrometer (ion source 200 °C, RI 70 eV) programmed at (40-650) °C with a rate of 4 °C/min. Injector temperature was 280 °C; carrier gas was He (20 psi), column flow rate was 1.4mL/min, injection mode -split.
3. Results
3.1. Isolation of fungi
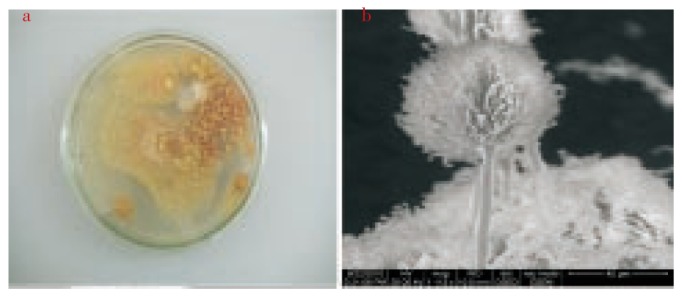
In the present study, the 10−5 dilution of the sponge sample yielded three different isolates. The characterization and analysis was performed for Isolate 1. Pure culture of Isolate 1 (Figure 1a) was obtained and SEM micrograph (Figure 1b) was taken to visualize the morphological features of the fungi.
Figure 1. a:Pure culture of Isolate1; b: SEM micrograph of Isolate 1.
3.2. Molecular characterization and identification of elite fungi
The ITS region is now perhaps the most widely sequenced DNA region in fungi, It is most useful for molecular systematics at the species level, and even within species. In the present study, the DNA was isolated from the Isolate 1 and the ITS region of 5.8s rRNA was amplified using specific primers ITS1 and ITS4 and the sequence was determined using automated sequencers. Blast search sequence similarity was found against the existing non redundant nucleotide sequence database thus, identifying the fungi as Aspergillus ochraceus. The percentage of similarity between the fungi and database suggests it as novel strain. Thus, the novel strain was named as A. ochraceus strain MP2 and made publically available in GenBank with an assigned accession number.
3.3. Anti-microbial assay
The fungi A. ochraceus MP2 was extracted in three solvents of varying polarity (Butanol, chloroform, ethyl aceteate). Three human pathogens namely Klebsiella pneumonia ATCC 15380, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853 were used to test the anti-microbial activity.Their zone of inhibition in varying concentrations of the sample is given in Table 1.
Table 1. Zone of inhibition of fungal filtrate in different solvents (mm).
| Pathogen | Butanol (µL) |
Chloroform (µL) |
Ethyl acetate (µL) |
|||||||||
| 25 | 50 | 75 | 100 | 25 | 50 | 75 | 100 | 25 | 50 | 75 | 100 | |
| Pseudomonas | - | - | - | - | 12 | 12.5 | 14 | 15 | 14 | 16.5 | 18 | 19 |
| Klebsiella | - | - | - | - | 11 | 13 | 15 | 15 | 17 | 19 | 20 | 23 |
| S. aureus | - | - | - | - | 11 | 14 | 18 | 21 | 15 | 17 | 23 | 25 |
Ethyl acetate provided promising results compared to the other solvents. Therefore, the optimum concentration of ethyl acetate producing maximum inhibition of the pathogen was analyzed using the same well diffusion assay for both low concentration [(25-100) µL] and high concentration [(250-1 000) µL] of the solvent. Their zone of inhibition is shown in Table 2.
Table 2. Well diffusion assay-standardization (ethyl acetate-low concentration and high concentration).
| Concentration (µL) | Klebsiella (mm) | Pseudomonas (mm) | Staphylococcus (mm) | Micrococcus (mm) | |
| Low concentration | 25 | - | - | - | - |
| 50 | - | 11 | 11 | - | |
| 75 | 11 | 12 | 12 | - | |
| 100 | 12 | 13 | 13 | 11 | |
| High concentration | 250 | 12 | 11 | 21 | 17 |
| 500 | 14 | 16 | 23 | 19 | |
| 750 | 16 | 18 | 23 | 20 | |
| 1 000 | 19 | 20 | 24 | 24 |
Higher concentration of ethyl acetate provided a better inhibition activity compared to their low concentration counterpart. The minimum inhibition concentration (MIC) of the elite solvent was standardised for two pathogens Staphylococcus and Micrococcus using broth dilution assay and the result is in Table 3.
Table 3. Percentage of viable cells of Staphylococcus and Micrococcus in varying concentrations of ethyl acetate.
| Concentration (µL) | % of viable cells of Staphylococcus | % of viable cells of Micrococcus |
| 100 | 7.532 | 7.399 |
| 200 | 12.727 | 13.452 |
| 300 | 13.766 | 16.591 |
| 400 | 26.753 | 18.161 |
| 500 | 27.532 | 28.475 |
| 600 | 30.389 | 34.977 |
| 700 | 46.233 | 40.132 |
| 800* | 52.207 | 46.188 |
| 900** | 71.428 | 54.484 |
| 1 000 | 75.064 | 83.408 |
*MIC for Staphyloccoccus; **MIC for Microccoccus.
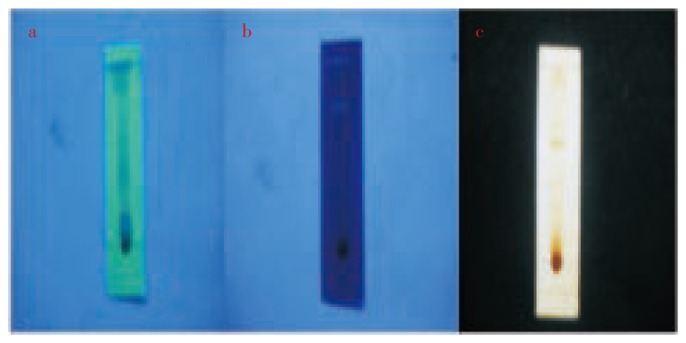
The fungal extract subjected to TLC separation revealed the presence of three bioactive metabolites which was visualised in UV short range spectrum of 254 nm (Figure 2a),UV long range of 365nm (Figure 2b) and iodine chamber ( Figure 2c).
Figure 2. TLC strip visulaised in a. UV short range b. UV long range c. Iodine chamber.
The TLC band was eluted and the bioactive metabolites in the eluant responsible for the anti bacterial activity were characterized using GC-MS. The chromatogram (Figure 3) revealed the presence of different functional groups in the eluant and the solvent. Three dominant compounds were individually characterized to reveal their identity, chemical formula and structure. The first elutant was identified as α- Campholene aldehyde with Chemical formula C10 H16 O and molecular weight 152 Da. The second elutant was identified as Lucenin-2 with Chemical formula C27 H30 O16 and molecular weight 610 Da. The third elutant is identified as 6-Ethyloct - 3-yl- 2- ethylhexyl ester with Chemical formula C26 H42 O4 and molecular weight 418 Da.
4. Discussion
World's oceans cover more than 70% of the earth's surface and marine biota are an enormous yet underutilized resource for the discovery of neutraceuticals, pharmaceuticals and other high value, low volume bioactives[22]. Marine-derived fungi have been recognized as a potential source of structurally novel and biologically potent metabolites, and a growing number of marine fungi have been reported to produce novel bioactive.
secondary metabolites[23]–[25].Of the 18 000 marine natural products described, over 30% are from sponges and of the antitumor natural product patent registrations in recent years over 75% are from sponges[26],[27]. Especially, the genus Aspergillus has been known to be a major contributor to the secondary metabolites of marine fungal origin, for example, four sesquiterpenoids with a unique nitrobenzyl ester from Aspergillus versicolor (A. versicolor), two modified cytotoxic tripeptides from Aspergillus sp., novel pentacyclic oxindole alkaloid from Aspergillus tamari[28], four prenylated indole alkaloids from Aspergillus sp.[29] and two cyclopentapeptides from A. versicolor[30]. This paper reports the isolation of three bioactive compounds from A. ochraceus. The antimicrobial activities of the isolated pure compounds in ethyl acetate solvent were reported since ethyl acetate extract showed promising results in Aspergillus flavus[31]. The isolated compounds showed significant antimicrobial activity against potential human pathogens. Microbial secondary metabolites has a large source of compounds endowed with ingenious structures and potent biological activities. Many of the products currently used for human or animal therapy, in animal husbandry and in agriculture are produced by microbial fermentation, or derived from chemical modification of a microbial product[32]. The present study of screening bioactive secondary metabolites revealed A. ochraceus as a source for the production of three effective metabolites. These metabolites can be further exploited for the biotechnological applications in medicine and agriculture.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Pietra Secondary metabolites from marine microorganisms: bacteria, protozoa, algae and fungi. Achievements and prospects. Nat Prod Rep. 1997;14:453–464. doi: 10.1039/np9971400453. [DOI] [PubMed] [Google Scholar]
- 2.Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet. 2003;3:338–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gul W, Hamann MT. Indole alkaloid marine natural products: an established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005;78:442–453. doi: 10.1016/j.lfs.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haefner B. Drugs from the deep: marine natural products as drug candidates. Drug Discov Today. 2003;8:536–544. doi: 10.1016/s1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- 5.Jha RK, Xu Z. Biomedical compounds from marine organisms. Mar Drugs. 2004;2:123–146. [Google Scholar]
- 6.Proksch P, Edrada RA, Ebel R. Drugs from the seas-current status and microbiological implications. Appl Microbiol Biotechnol. 2002;59:125–134. doi: 10.1007/s00253-002-1006-8. [DOI] [PubMed] [Google Scholar]
- 7.Mayer AM, Hamann MT. Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp Biochem Physio. 2005;140:265–286. doi: 10.1016/j.cca.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkateswara Rao J, Desaiah D, Vig PJS, Venkateswarlu Marine biomolecules inhibit rat brain nitric oxide synthase. Toxicol. 1998;129:103–110. doi: 10.1016/s0300-483x(98)00067-5. [DOI] [PubMed] [Google Scholar]
- 9.Venkateswarlu Y, Srinivasa Reddy N, Venkatesham U. Novel bioactive compounds from the soft corals: chemistry and biomedical applications. Bio-organic compounds: Chem Biomed Appl. 2001;6:101–128. [Google Scholar]
- 10.Li Kam Wah H, Jhaumeer-Laulloo S, Choong Kwet Yive R, Bonnard I, Banaigs B. Biological and chemical study of some soft corals and sponges collected in Mauritian waters, Western Indian Ocean. J Mar Sci. 2006;5:115–121. [Google Scholar]
- 11.Kobayashi M, Higuchi K, Murakami N, Tajima H, Aoki S. Callystatin A, a potent cytotoxic polyketide from the marine sponge,Callyspongia truncata. Tetrahedron Lett. 1999;38:2859–2862. [Google Scholar]
- 12.Wang G. Diversity and biotechnological potential of the sponge-associated microbial consortia. J Ind Microbiol Biotechnol. 2006;33:545–551. doi: 10.1007/s10295-006-0123-2. [DOI] [PubMed] [Google Scholar]
- 13.Jayant N, Suresh R. Moderate protective effect of 6- MFA, a microbial metabolite obtained from Aspergillus ochraceus on immunological liver injury in mice. Comp Immunol Microbiol Infect Dis. 1999;22:15–25. doi: 10.1016/s0147-9571(98)00021-6. [DOI] [PubMed] [Google Scholar]
- 14.Marquardt RR, Frohlich AA. A review of recent advances in understanding ochratoxicosis. J Anim Sci. 1992;70:3968–3988. doi: 10.2527/1992.70123968x. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz R, Liesch J, Hensens O, Zitano L, Honeycutt S, Garrity G, et al. et al. A novel antifungal agent: fermentation, isolation, structural elucidation and biological properties. J Antibiot. 1988;41:1774–1779. doi: 10.7164/antibiotics.41.1774. [DOI] [PubMed] [Google Scholar]
- 16.Fiedler HP, Bruntner C, Riedlinger J, Bull AT, Knutsen G, Goodfellow M, et al. et al. Proximicin A, B and C, novel aminofuran antibiotic and anticancer compounds isolated from marine strains of the actinomycete Verrucosispora. J Antibiot. 2008;61:158–163. doi: 10.1038/ja.2008.125. [DOI] [PubMed] [Google Scholar]
- 17.Wagner Dobler I, Beil W, Lang S, Meiners M, Laatsch H. Integrated approach to explore the potential of marine microorganisms for the production of bioactive metabolites. Adv Biochem Eng Biotechnol. 2002;74:207–238. doi: 10.1007/3-540-45736-4_10. [DOI] [PubMed] [Google Scholar]
- 18.Jensen PR, Fenical W. Strategies for the discovery of secondary metabolites from marine bacteria: ecological perspectives. Annu Rev Microbiol. 1994;48:559–584. doi: 10.1146/annurev.mi.48.100194.003015. [DOI] [PubMed] [Google Scholar]
- 19.Gunasekera AS, Sfanos KS, Harmody DK, Pomponi SA, McCarthy PJ, Lopez JV. An enhanced database of the microorganisms associated with deeper water marine invertebrates. Appl Microbiol Biotechnol. 2004;66:373–376. doi: 10.1007/s00253-004-1763-7. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi J, Ishibashi M. Bioactive metabolites of symbiotic marine microorganisms. Chem Rev. 1993;93:1753–1769. [Google Scholar]
- 21.Ridley CP, Faulkner DJ, Haygood MG. Investigation of Oscillatoria spongeliae dominated bacterial communities in four dictyoceratid sponges. Appl Environ Microbiol. 2005;71:7366–7375. doi: 10.1128/AEM.71.11.7366-7375.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabdono A, Radjasa OK. Microbial symbionts in marine sponges: marine natural product factory. J Coastal Dev. 2008;11(2):57–61. [Google Scholar]
- 23.Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2006;23:26–78. doi: 10.1039/b502792f. [DOI] [PubMed] [Google Scholar]
- 24.Bugni TS, Ireland CM. Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Nat Prod Rep. 2004;21:143–163. doi: 10.1039/b301926h. [DOI] [PubMed] [Google Scholar]
- 25.Saleem M, Ali MSS, Hussain JA, Ashraf M, Lee YS. Marine natural products of fungal origin. Nat Prod Rep. 2007;24:1142–1152. doi: 10.1039/b607254m. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner DJ. Marine natural products. Nat Prod Rep. 2000;17:7–55. doi: 10.1039/a809395d. [DOI] [PubMed] [Google Scholar]
- 27.Sipkema D, Franssen MCR, Osinga R, Tramper J, Wijffels RH. Marine sponges as pharmacy. Mar Biotechnol. 2005;7:142–146. doi: 10.1007/s10126-004-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suda M, Mugishima T, Komatsu K, Sone T, Tanaka M, Mikami Y, et al. et al. Speradine A, a new pentacyclic oxindole alkaloid from a marine-derived fungus Aspergillus tamari. Tetrahedron. 2003;59:3227–3230. [Google Scholar]
- 29.Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, et al. et al. Notoamides A-D: prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp. Angew Chem Int Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]
- 30.Fremlin LJ, Piggott AM, Lacey E, Capon RJ. Cottoquinazoline A and Cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J Nat Prod. 2009;72:666–670. doi: 10.1021/np800777f. [DOI] [PubMed] [Google Scholar]
- 31.Meenupriya J, Thangaraj M. Isolation and molecular characterization of bioactive secondary metabolites from Callyspongia spp. associated fungi. Asian Pac J Trop Med. 2010;10:738–740. [Google Scholar]
- 32.Lazzarini L, Cavaletti G, Toppo, Marinelli F. Rare genera of actinomycetes as potential producers of new antibiotics. Antonie Leeuwenhoek. 2000;78:399–405. [PubMed] [Google Scholar]


