Abstract
Objective
To analyze the total phenolic content, DNA protecting and radical scavenging activity of ethanolic leaf extracts of three Lamiaceae plants, i.e. Anisomelos malabarica (A. malabarica), Leucas aspera (L. aspera) and Ocimum basilicum (O. basilicum).
Methods
The total polyphenols and flavonoids were analyzed in the ethanolic leaf extracts of the lamiaceae plants. To determine the DNA protecting activity, various concentrations of the plant extracts were prepared and treated on cultured HepG2 human lung cancer cells. The pretreated cells were exposed to H2O2 to induce DNA damage through oxidative stress. Comet assay was done and the tail length of individual comets was measured. Nitric oxide and superoxide anion scavenging activities of lamiaceae plants were analyzed.
Results
Among the three plant extracts, the highest amount of total phenolic content was found in O. basilicum (189.33 mg/g), whereas A. malabarica showed high levels of flavonoids (10.66 mg/g). O. basilicum also showed high levels of DNA protecting (85%) and radical scavenging activity.
Conclusions
The results of this study shows that bioactive phenols present in lamiaceae plants may prevent carcinogenesis through scavenging free radicals and inhibiting DNA damage.
Keywords: Cadmium chloride, DNA damage, Lamiaceae plants, Phenols, Comet assay, Antioxidant activity
1. Introduction
Reactive oxygen species are cytotoxic and disrupt the normal metabolism through oxidative damage to lipids, proteins and nucleic acids[1]. Heavy metals present in the environment are of increasing concern due to their carcinogenic effect. Cadmium, a heavy metal, widely used in industries, is considered as a human carcinogen[2]. The human body has limited capacity to respond to cadmium exposure, hence the metal cannot undergo metabolic degradation. This increases the half-time of cadmium in human body to 15 and 20 years[3]. Cadmium produces DNA single strand breaks, DNA-protein cross-links, chromosomal aberrations and changes the gene expression of proto-oncogenes[4]. The mechanism of cadmium induced toxicity was found to be the inhibition of GSH and production of radicals including nitric oxide and superoxide anion[5]. A recent study suggests that low levels of cadmium inhibit the activities of phosphatase and kinases that are involved in DNA repair[6]. Experiments on animal models and cell lines showed that cadmium is involved in the pathogenesis of cancers in organs like breast, lung, prostate and kidney[7]. Cadmium is found to be a potent toxic to sperm cells whose number and motility are reduced[8].
Plants contain a wide variety of secondary metabolites like phenols and flavonoids with antioxidant activities[9],[10]. These perform functions like scavenging free radicals, donating hydrogen atoms or electrons, or chelating metal cations[11]. Lamiaceae members are found to be rich source of polyphenols, flavonoids and vitamins[12],[13]. A wide variety of biological activities like anticholinesterase, antimicrobial, anti-inflammatory and antiviral activities were reported in lamiaceae members[14],[15]. In the present study, lamiaceae members were tested for its anticarcinogenic effect on cadmium induced DNA damage. The plants were also tested for the scavenging of nitric oxide and superoxide anion free radicals.
2. Materials and methods
2. 1. Preparation of leaf extract
Anisomelos malabarica (A. malabarica), Leucas aspera (L. aspera) and Ocimum basilicum (O. basilicum) were collected from the Bharathiar University campus, Coimbatore, India. The leaves were shade dried, ground into fine powder and extracted with ethanol (95%) in a Soxhlet apparatus for 24 h. The extract was concentrated under reduced pressure and controlled temperature (40-50 °C) using a rotary evaporator.
2. 2. Estimation of total phenols and flavonoids
Total phenols were expressed in gallic acid equivalents (GAE)[16]. The dry extract was diluted in water and 100 mL of this solution were transferred to a 10 mL volumetric flask, to which 0.5 mL undiluted Folin-Ciocalteu reagent was added. After 1 min, 1.5 mL 20% (w/v) Na2CO3 were added and the volume was made up to 10 mL with H2O. After 1 h incubation at 25 °C, the absorbance was measured at 760 nm and compared with a pre-prepared gallic acid calibration curve. Flavonoids in the plant extracts were measured as described by Chang et al[17]. An aliquot of 500 µL of plant extract was dissolved in 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminum chloride hexahydrate, 0.1 mL potassium acetate and 2.8 mL of water. The above contents were incubated at room temperature for 40 min. The absorbance was read at 415 nm using a UV-Vis spectrophotometer. Quercetin was used as a standard and the results were expressed as milligram quercetin equivalents.
2.3. Cell culture and treatment
Human lung cancer HepG2 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% L-glutamine. After reaching 80% confluence, the cells were incubated with different concentrations of A. malabarica, L. aspera and O. basilicum leaf extract individually for 4 h at 37 °C. The pretreated cells were exposed to 150 µM/mL of CdCl2 for 1 h. The cells were washed with PBS and harvested using trypsin/EDTA. The cell suspension was centrifuged at 1 500 rpm for 5 min. The supernatant was discarded and the cell pellet was resuspended in PBS.
2.4. Analysis of DNA damage using comet assay
Comet assay was performed as descried by Singh et al[18]. The cell suspension was mixed with 75 µL of 0.5% low melting agarose and layered on the slides precoated with 1% normal melting agarose. After solidification of agarose, 75 µL of 0.5% low melting agarose was layered. The slides were immersed in lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris and 1% Sodium laurylasarcosine; 1% Triton X-100 and 10% DMSO) for 1 h at 4 °C. The slides were then placed in an electrophoresis tank filled with 300 mM NaOH and 10 mM Na2EDTA (pH 13) for 40 min. The slides were electrophoresed at 25 V/300mA for 20 min at 4 °C. After electrophoresis, the slides were washed with neutralizing buffer (0.4 M Tris, pH 7.5) for 5 min at 4 °C and treated with ethanol for 5 min. The slides were dried and stained with 50 µL of ethidium bromide(20 µg/mL) and viewed under Nikon fluorescent microscope with a 515-560 nm excitation filter. Comets like structures were seen in cells with DNA damage. The tail length and head diameter of the comet structures were measured using an ocular micrometer. The DNA damage was quantified as comet tail length (µm) = (maximum total length) - (head diameter)[19].
2.5. Analysis of nitric oxide
The HepG2 cells were cultured and treated with plant extracts and CdCl2 as described above. Nitric oxide was measured in the supernatant of culture medium using Griess reagent. 50µL of supernatant of culture medium was mixed with equal amount of Griess reagent and incubated for 10 min. The absorbance was read at 550 nm using a spectrophotometer. Cells treated with cadmium chloride were used as controls.
2.6. Analysis of superoxide anion
The superoixde anion was measured using nitroblue tetrazolium (NBT) as described by Siwik et al[20]. After treatment with the plant extracts and cadmium chloride, the cells were incubated in PBS containing 100 µM/L of NBT for 90 min. The cells were harvested and centrifuged at 10 000 rpm for 10 min. The cell pellet was resuspended in 100 µM/L of pyridine and heated at 80 °C for 90 min. The purple color formed by formazan was measured at 540 nm using UV-Vis spectrophotometer. The amount of NBT reduced to formazan was used to calculate the levels of superoxide anion.
2. Statistical analysis
All the values were expressed as mean ± SD. Students ‘t’ test was performed to analyze significance between groups using SPSS software. A value of P<0.05 was considered as significant level.
3. Results
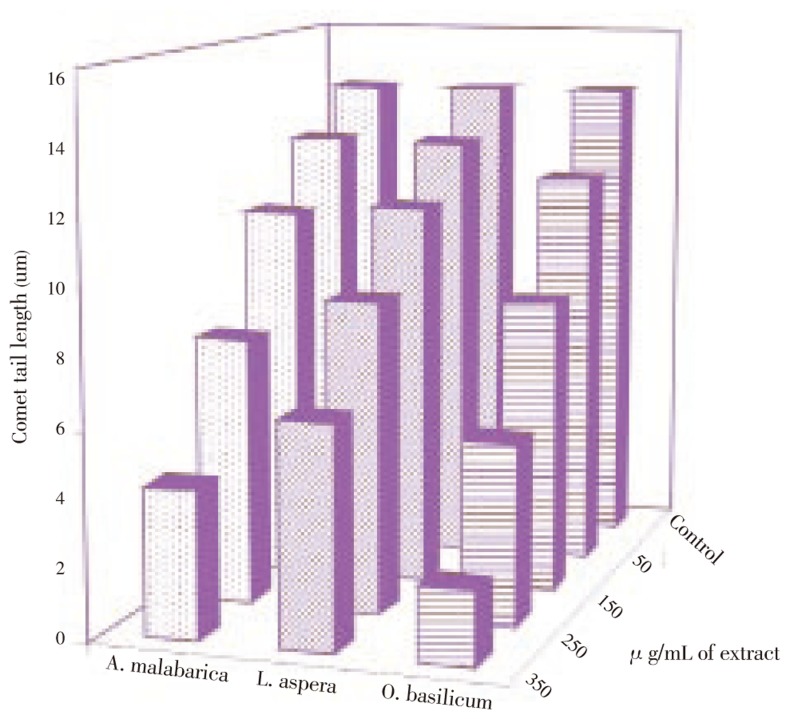
The phenolic content of the three plants, O. basilicum, L. aspera and A. malabarica ranged from 141 mg to 189 mg per gram of dry weight. High levels of phenols were found in O. basilicum leaf extract (Table 1). The anticarcinogenic activity of three plant extracts was tested using comet assay. The cells pretreated with A. malabarica, L. aspera and O. basilicum leaf extracts showed DNA protective effect in a dose dependent manner. Among the three plants, O. basilicum showed a maximum DNA protecting activity. When compared with controls, the O. basilicum extracts treated cells showed a significant (P<0.05) decrease in comet tail length. At 350 µg/mL this plant extract protected 85% of DNA from cadmium toxicity. L. aspera and A. malabarica also protected the cells from cadmium toxicity. At 350 µg/mL, 55% and 70% of DNA was protected by L. aspera and A. malabarica (Figure 1). When compared with other two members, the amount of total phenol content was highest in O. bascillicum leaf extract and further it showed significant DNA protecting ability.
Table 1. Total phenols and flavonoids of three lamiaceae plants (Mean ± SD).
| Plants | Total phenols (mg/g gallic acid equivalent) | Flavonoids (mg/g quercetin equivalent) |
| A. malabarica | 176.33 ± 4.16 | 10.66 ± 1.52 |
| L. aspera | 141.33 ± 2.51 | 7.66 ± 1.15 |
| O. basilicum | 189.33 ± 1.52 | 8.33 ± 0.57 |
Figure 1. DNA protective effect of lamiaceae plants on cadmium chloride induced genotoxicity.
* denotes that data significantly (P<0.05) different from the control.
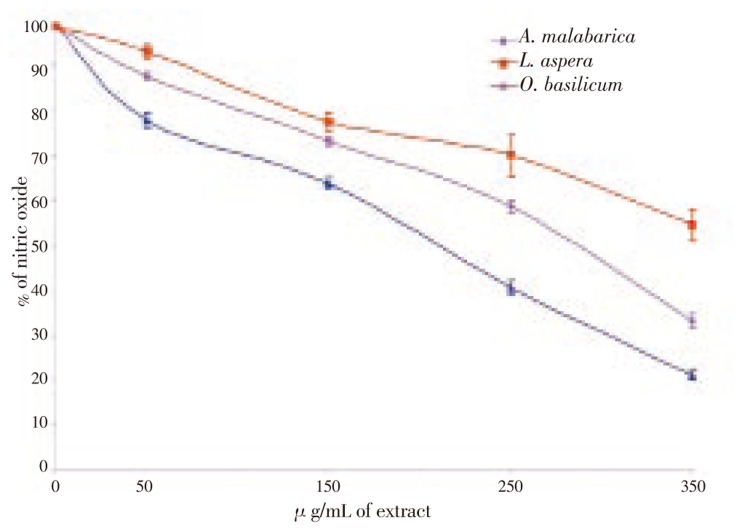
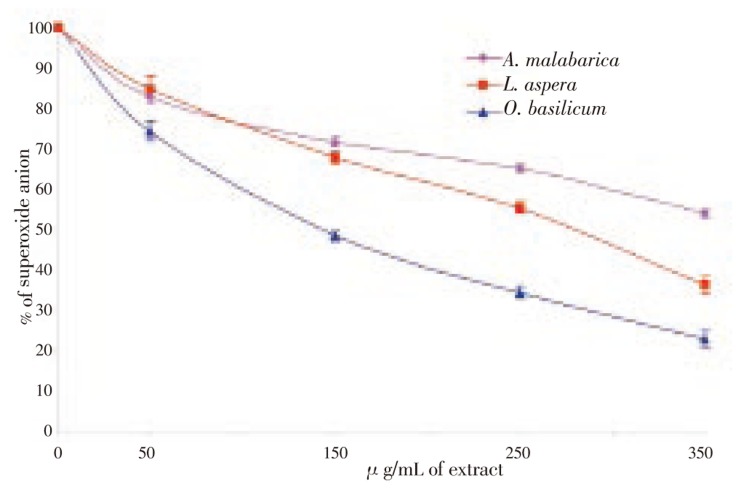
The nitric oxide generated by cadmium chloride was measured using Griess reagent. Nitric oxide generated in cells treated with 150 µg/mL of cadmium chloride was considered as 100%. The cells pretreated with the three plant extracts showed a dose dependent inhibition of nitric oxide. The maximal nitric oxide inhibitory effect was exhibited by O. basilicum which inhibited 79% of nitric oxide at 350 µg/mL of extract. An inhibition of 67% and 45% of nitric oxide was observed in cells treated with A. malabarica and L. aspera extracts (Figure 2). The presence of superoxide anion was analyzed using the NBT. A statistically significant inhibition of superoxide was observed in all concentration of the three plants. At 350 µg/mL, O. basilicum inhibited 78% of superoxide anion produced by cadmium chloride, whereas L. aspera and A. malabarica inhibited 64% and 46% of nitric oxide (Figure 3). Among the three plants tested, O. basilicum contains higher polyphenols and flavonoids than L. aspera and A. malabarica. It also exhibited maximal levels of DNA protecting and free radical scavenging activity against cadmium chloride induced toxicity.
Figure 2. Nitric oxide scavenging effect of three lamiaceae plants.
* denotes that data significantly (P<0.05) different from the control.
Figure 3. Super oxide anion scavenging effect of three lamiaceae plants.
* denotes that data significantly (P<0.05) different from the control.
4. Discussion
DNA damage by free radicals is an important contributor for cancer development. Cadmium is an occupational and environmental pollutant that has been associated with renal, skeletal, vascular, reproductive and respiratory disorders[21]. Cd-induced oxidative stress has been associated with production of reactive oxygen species consisting mainly of superoxide anion radical (O2−), hydrogen peroxide (H2O2) and the hydroxyl radicals (OH−)[22]. These reactive oxygen species play a crucial role in the development of tissue damage in various human diseases such as cancer, aging, neurodegenerative disease, malaria and arteriosclerosis[23],[24]. Epidemiological studies suggest that people exposed to cadmium have an increased risk of bladder and prostate cancer[25],[26].
Medicinal plants and herbs are promising and diverse sources of natural antioxidants. Most of the members of Lamiaceae family have been found to be very effective with regard to natural antioxidants[27]–[29]. Phenolic compounds like flavonoids from L. aspera showed significant DPPH radical scavenging activity[30]–[33]. A linear positive relationship existed between the antioxidant activity and total phenolic content of the O. bascillicum[34]. In the present study the anticarcinogenic potential exhibited by A. malabarica, L. aspera and O. bascillicum may be due to the presence of high levels of phenols. Free radical scavenging activity of phenolic compounds may act as potential anticarcinogens. According to Aherne et al[35], Rosmarinus officinalis, Origanum vulgare and Salvia officinalis showed significant antioxidant activity and DNA protecting ability which may be due to the presence of phenols. The antioxidant activity of phenols is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers. In the present study, O. bascillicum showed high level of phenols and exhibited DNA protecting activity against cadmium chloride induced genotoxicity. This plant also showed maximal free radical scavenging activity when compared with the other two plants tested in this study. The findings of the present study suggest that the proper use of herbal products is safer and may provide beneficial health effects to humans.
Footnotes
Foundation Project: Supported by PG and Research Department of Biotechnology, Kongunadu Arts and Science College, GN Mills, India.
Conflict of interest: We declare that have no conflict of interest.
References
- 1.Parida AK, Das AB. Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf. 2004;60:324–349. doi: 10.1016/j.ecoenv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Waisberg M, Joseph P, Hale B, Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/s0300-483x(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 3.Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19:529–535. [PubMed] [Google Scholar]
- 4.Kawata K, Shimazaki R, Okabe S. Comparison of gene expression profiles in HepG2 cells exposed to arsenic, cadmium, nickel, and three model carcinogens for investigating the mechanisms of metal carcinogenesis. Environ Mol Mutagen. 2009;50:46–59. doi: 10.1002/em.20438. [DOI] [PubMed] [Google Scholar]
- 5.Chung AS, Maines MD. Differential effect of cadmium on GSH-peroxidase activity in the Leydig and the Sertoli cells of rat testis. Suppression by selenium and the possible relationship to heme concentration. Biochem Pharmacol. 1987;36:1367–1372. doi: 10.1016/0006-2952(87)90096-7. [DOI] [PubMed] [Google Scholar]
- 6.Whiteside JR, Box CL, McMillan TJ, Allinson SL. Cadmium and copper inhibit both DNA repair activities of polynucleotide kinase. DNA Repair (Amst) 2010;9:83–89. doi: 10.1016/j.dnarep.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Waalkes MP, Anver MR, Diwan BA. Chronic toxic and carcinogenic effects of oral cadmium in the Noble (NBL/Cr) rat: induction of neoplastic and proliferative lesions of the adrenal, kidney, prostate and testes. J Toxicol Environ Health A. 1999;58:199–214. doi: 10.1080/009841099157296. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira H, Spano M, Santos C, Pereira Mde L. Adverse effects of cadmium exposure on mouse sperm. Reprod Toxicol. 2009;28:550–555. doi: 10.1016/j.reprotox.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez I, Alegre L, Van Breusegem F, Munne-Bosch S. How relevant are flavonoids as antioxidants in plants? Trends Plant Sci. 2009;14:125–132. doi: 10.1016/j.tplants.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Prakash D, Singh BN, Upadhyay G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa) Food Chem. 2007;102:1389–1393. [Google Scholar]
- 11.Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. [Google Scholar]
- 12.Barros L, Heleno SA, Carvalho AM, Ferreira ICFR. Lamiaceae often used in Portuguese folk medicine as a source of powerful antioxidants: vitamins and phenolics. LWT - Food Sci Technol. 2010;43:544–550. [Google Scholar]
- 13.Akkol EK, Goger F, Kosar M, Baser KHC. Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chem. 2008;108:942–949. doi: 10.1016/j.foodchem.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 14.Kivrak I, Duru ME, Ozturk M, Mercan N, Harmandar M, Topcu G. Antioxidant, anticholinesterase and antimicrobial constituents from the essential oil and ethanol extract of Salvia potentillifolia. Food Chem. 2009;116:470–479. [Google Scholar]
- 15.Reichling J, Nolkemper S, Stintzing FC, Schnitzler P. Impact of ethanolic lamiaceae extracts on herpesvirus infectivity in cell culture. Forsch Komplementmed. 2008;15:313–320. doi: 10.1159/000164690. [DOI] [PubMed] [Google Scholar]
- 16.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 17.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 18.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 19.Grover P, Danadevi K, Mahboob M, Rozati R, Banu BS, Rahman MF. Evaluation of genetic damage in workers employed in pesticide production utilizing the comet assay. Mutagenesis. 2003;18:201–205. doi: 10.1093/mutage/18.2.201. [DOI] [PubMed] [Google Scholar]
- 20.Siwik DA, Tzortzis JD, Pimental DR, Chang DLF, Pagano PJ, Singh K, et al. et al. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res. 1999;85:147–153. doi: 10.1161/01.res.85.2.147. [DOI] [PubMed] [Google Scholar]
- 21.Thompson J, Bannigan J. Cadmium: toxic effects on the reproductive system and the embryo. Reprod Toxicol. 2008;25:304–315. doi: 10.1016/j.reprotox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Bertin G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88:1549–1559. doi: 10.1016/j.biochi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Gutteridge JM. Biological origin of free radicals and mechanisms of antioxidant protection. Chem Biol Interact. 1994;91:133–140. doi: 10.1016/0009-2797(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 24.Méndez-Armenta M, Ríos C. Cadmium neurotoxicity. Environ Toxicol Pharmacol. 2007;23:350–358. doi: 10.1016/j.etap.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Kellen E, Zeegers MP, Hond ED, Buntinx F. Blood cadmium may be associated with bladder carcinogenesis: the Belgian case-control study on bladder cancer. Cancer Detect Prev. 2007;31:77–82. doi: 10.1016/j.cdp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Vinceti M, Venturelli M, Sighinolfi C, Trerotoli P, Bonvicini F, Ferrari A, et al. et al. Case-control study of toenail cadmium and prostate cancer risk in Italy. Sci Total Environ. 2007;373:77–81. doi: 10.1016/j.scitotenv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Cuvelier ME, Richard H, Berset C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J Am Oil Chem Soc. 1996;73:645–652. [Google Scholar]
- 28.Orhan I, Aslan M. Appraisal of scopolamine-induced antiamnesic effect in mice and in vitro antiacetylcholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. J Ethnopharmacol. 2009;122:327–332. doi: 10.1016/j.jep.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Kivilompolo M, Hyotylainen T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: characterisation and quantification of antioxidant phenolic acids. J Chromatogr A. 2007;1145:155–164. doi: 10.1016/j.chroma.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 30.Sadhu SK, Okuyama E, Fujimoto H, Ishibashi M. Separation of Leucas aspera, a medicinal plant of Bangladesh, guided by prostaglandin inhibitory and antioxidant activities. Chem Pharm Bull. 2003;51:595–608. doi: 10.1248/cpb.51.595. [DOI] [PubMed] [Google Scholar]
- 31.Devi GK, Manivannan K, Thirumaran G, Rajathi FAA, Anantharaman P. In vitro antioxidant activities of selected seaweeds from Southeast coast of India. Asian Pac J Trop Med. 2011;4(3):205–221. doi: 10.1016/S1995-7645(11)60070-9. [DOI] [PubMed] [Google Scholar]
- 32.Viswanatha GLS, Vaidya SK, Ramesh C, Krishnadas N, Rangappa S. Antioxidant and antimutagenic activities of bark extract of Terminalia arjuna. Asian Pac J Trop Med. 2010;3(12):965–970. [Google Scholar]
- 33.Kannan RRR, Arumugam R, Anantharaman P. In vitro antioxidant activities of ethanol extract from Enhalus acoroides (L.F.) Royle. Asian Pac J Trop Med. 2010;3(11):898–901. [Google Scholar]
- 34.Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83:547–550. [Google Scholar]
- 35.Aherne SA, Kerry JP, O'Brien NM. Effects of plant extracts on antioxidant status and oxidant-induced stress in Caco-2 cells. Br J Nutr. 2007;97:321–328. doi: 10.1017/S0007114507250469. [DOI] [PubMed] [Google Scholar]