Abstract
Objective
The aim of the present study was to isolate the anti-MRSA (Methicillin Resistant Staphylococcus aureus) molecule from the Mangrove symbiont Streptomyces and its biomedical studies in Zebrafish embryos.
Methods
MRSA was isolated from the pus samples of Colachal hospitals and confirmed by amplification of mecA gene. Anti-MRSA molecule producing strain was identified by 16s rRNA gene sequencing. Anti-MRSA compound production was optimized by Solid State Fermentation (SSF) and the purification of the active molecule was carried out by TLC and RP-HPLC. The inhibitory concentration and LC50 were calculated using Statistical software SPSS. The Biomedical studies including the cardiac assay and organ toxicity assessment were carried out in Zebrafish.
Results
The bioactive anti-MRSA small molecule A2 was purified by TLC with Rf value of 0.37 with 1.389 retention time at RP-HPLC. The Inhibitory Concentration of the purified molecule A2 was 30 µg/mL but, the inhibitory concentration of the MRSA in the infected embryo was 32-34 µg/mL for TLC purified molecule A2 with LC50 mean value was 61.504 µg/mL. Zebrafish toxicity was assessed in 48-60 µg/mL by observing the physiological deformities and the heart beat rates (HBR) of embryos for anti MRSA molecule showed the mean of 41.33-41.67 HBR/15 seconds for 40 µg/mL and control was 42.33-42.67 for 15 seconds which significantly showed that the anti-MRSA molecule A2 did not affected the HBR.
Conclusions
Anti-MRSA molecule from Streptomyces sp PVRK-1 was isolated and biomedical studies in Zebrafish model assessed that the molecule was non toxic at the minimal inhibitory concentration of MRSA.
Keywords: Methicillin resistant, Staphylococcus aureus, Small molecule, Mangrove, RP-HPLC, Streptomyces, Cardiac assay, Organogenesis, Biochemial study, Zebrafish embryo, Minimal inhibitory concentration
1. Introduction
The multi-drug resistant phenotype is a particular characteristic of the methicillin-resistant Staphylococcus aureus (MRSA) strains[1],[2]. MRSA is a major pathogen associated with both nosocomial- and community-acquired infections (hospital-acquired [HA] MRSA and CA-MRSA, respectively)[3]. The homogeneous insusceptibility to all betalactams, characteristic of methicillin-resistant strains, together with the continuous accumulation and organization of many resistance genes, has made this species particularly difficult to treat. This antibiotic resistance is common for many classes of antibiotics such as aminoglycosides, macrolides, lincosamides, and fluoroquinolones [4]. In recent years, the widespread use of antibiotics has undoubtedly accelerated the evolution of Staphylococcus aureus (S. aureus), and led to the emergence of strains that have systematically acquired multiple resistance genes[5]. With the current emergence of multi-drug resistant isolates in hospitals on the one hand[1] and the dramatically increased incidence of hyper-virulent community-associated MRSA on the other[6],[7], MRSA that has been able to evolve rapidly and create new clinical problems poses a substantial threat for the hospital environment, resulting in nosocomial infections[8]. The majority of MRSA strains have been associated with hospital-acquired colonization and infections[9]. 59.5% of Staphylococcus aureus strains causing infections in hospitals were MRSA, which is not only true for Europe and United States but for India as well. It was reported that vancomycin resistant S. aureus (VRSA) were severely found in the northern India with severe infections [10]. Natural products are potential new chemical structures possessing antimicrobial activity and it was reported that three of every four antibacterial agents are related in some way to natural products[11], and a majority of these discoveries have come from filamentous bacteria of the order Actinomycetales (actinomycetes). Actinomycetes are widely distributed in the natural environment and play an important role in the degradation of organic matter and production of bioactive compounds and bioactive molecules[12]. More than 9 000 biologically active molecules have been isolated from actinomycetes, yielding more than 60 pharmaceutical agents used in the medical or agricultural fields[11]. It was also reported that in recent years the S. aureus was highly resistant to conventional and traditional antibiotic due the modification in the molecular physiology of the pathogens in clinical isolates in India[13]. Hence the search of novel therapeutic small molecule for drug resistant S. aureus is urgent. As part of our small molecule screening from mangroves we have identified an anti MRSA producing Streptomyces sp. PVRK-1. The strain was identified by 16S rRNA gene sequencing. The anti-MRSA molecule was purified by TLC and RP-HPLC. There are several experimental infection models have been set up to study human pathogens[14]. In the present study Zebrafish model have been used for toxicity evaluation of pharmaceutical agents[15],[16]. Based on the above informations in this research work, we have identified a novel anti-MRSA producing mangrove symbiont Streptomyces sp. PVRK-1 and its biomedical studies were carried out in the Zebrafish embryos.
2. Materials and methods
2.1. Isolation and identification of microorganism
S. aureus was obtained from hand-swab samples of hospitals in Colachal, by personnel. The molecular characterization of mecA gene in the MRSA was already published in the laboratory[17] and the strain was used in the present study. The Streptomyces sp. PVRK-1 was collected from the Rhizosphere soil of Rhizophora mucronata (R. mucronata), Manakkudy estuary of Arabian Sea, Tamil Nadu, India (8°6′ 12″ N 77°28′ 57″ E) at 6 feet depth. Soil samples were serially diluted in sterile water and spread plated over the medium containing soluble starch 20 g, KNO3 1 g, NaCl 0.5 g, K2HPO4 0.5 g, MgSO4 0.5 g, FeSO4 20 µM, agar 15 g, seawater from mangrove habitat 1 L, and 15 µg nalidixic acid were added to inhibit the growth of other bacteria and incubated at 28 °C for 3 days. The molecular identification of the Streptomyces sp. PVRK-1 was carried out based on the work in the laboratory[18] and submitted in the NCBI genebank (GenBank: FJ573228.1).
2.2. Antimicrobial assay
The isolated Streptomyces sp. PVRK-1 was further grown on the medium with starch 20 g, tryptone 5 g, yeast extract 5 g, KNO3 1 g, NaCl 0.5 g, K2HPO4 0.5 g, MgSO4 0.5 g, distilled water 1 L. Antagonistic activity of the isolated strain Streptomyces sp. PVRK-1 was performed by double layer agar method [19]. The antibiotic production was optimized by Solid State Fermentation (SSF) supplemented with various nutritional sources and physical parameters by agar overlay method.
2.3. Culture conditions production and purification
A 100 mL flask containing 25 mL of the seed culture NDYE medium (Nitrate Defined Yeast Extract) was inoculated with Streptomyces sp. PVRK-1 at 30 °C for 3 days on a rotary shaker incubator for 140 rpm. The seed culture was transferred into 5 L conical flask containing 2.5 L of modified fermentation medium [20]. The fermentation medium consist of glucose 25 g, yeast extract 10 g, tryptone-100 12.5 g, Fe2(SO4)3.4 H2O 20 µg, KBr 25 µg with pH-7. The temperature for antibiotic production in SSF was optimized at 37 °C. The fermentation was carried out at 7 days with shaking at 180 rpm. After 7 days, the culture broth was centrifuged at 2 000 rpm for 10 min at 4 °C and filtered using 0.2 µm membrane filters (Himedia). The cell free supernatant was added with 250 mL of hexane, chloroform, acetone and methanol, successively shaken well in a separation funnel for extraction. Thin layer chromatography was employed to separate the compounds present in the crude methanolic extracts. 10 µg of crude methanol extract was spotted on the TLC plate with the mobile phase methanol/n-butanol /acetic acid/water = 5:3:1:1. The spots were visualized by keeping the plates in Iodine chamber[21]. 250 mg of the TLC scrapped spots were dissolved in 1 000 µL of methanol, vortexed for 5 min and centrifuged at 8 000 rpm for 5 min. The supernatant was concentrated in the vaccuum concentrator 5301 (Eppendorf) at 30 °C for 4-6 hours. The absorbance microtitre of the TLC resolved spots of methanolic extract of Streptomyces sp. PVRK-1 were determined spectrophotometrically (Shimadzu). 1 mg of concentrated A2 resolved in TLC was dissolved in 1 mL of HPLC mobile phase [Methanol: double distilled water (3:2)] and centrifuged at 8000 rpm for 5 min. 100 µL was made up to 1 mL with the HPLC mobile phase and the absorbance was scanned using UV-Vis spectrophotometer (Shimadzu). The HPLC mobile phase was used as blank. 10−1 dilution was prepared and 25 µL was injected in the HPLC (Shimadzu) system using reverse-phase C18 with an isocratic elution.
2.4. Minimal inhibitory concentration
TLC purified molecule A2 was assayed to determine the MIC of the compound against MRSA by the microtiter broth dilution method[22]. Dilutions of the test compound dissolved in 1% DMSO were added to each well of the 96 well microtiter plate containing fixed volume of Nutrient agar broth. Each well was inoculated with bacteria (105 CFU mL−1) and incubated at 37 °C for 24 h. The MIC was calculated at which no growth of bacteria was observed.
2.5. Breeding and maintenance of Zebrafish embryos
Zebrafishes were maintained in 30 L tanks at 28 °C with 14 h: 10 h light/dark cycle in Fish Culture Facility of International Centre for Nanobiotechnology (ICN), Centre for Marine Science and Technology. Following successful breeding, eggs fell through the mesh, and were subsequently collected from the bottom of tanks. Zebrafish were maintained according to Westerfield 1989[23]. Zebrafish embryos are raised in E3 medium (5 mM NaCl, 0.17 mM KCl, 0.4 mM CaCl2 and 0.16 mM MgSO4).
2.6. Organogenesis effect of anti MRSA molecule A2 in Zebrafish embryos
1µL of the pathogenic culture MRSA were treated in the 2 dpf (days post fertilization) embryos in 48 well microtitre plates. 1µL of the culture was inoculated in the wells of the titre plates and incubated for 24 hours to monitor pathological effects in the sterile laminar flow chamber. The TLC purified small molecule A2 along with bacterial culture were studied for its pathological effect. Compounds were added to the wells in 1% DMSO vehicle and the DMSO alone was used as control. Heart Beat Rate (HBR) were studied in the developing embryo from 3-5 dpf for the anti MRSA molecule A2. The embryos were anesthetized by 1% tricaine (Sigma) and the heart beat rates were recorded in digital camera (Canon Ixus) in the Light microscope (Motic). The heart beat rates were counted by Windows moviemaker. Anti MRSA molecule was tested at 10 - 100 µg/mL, and the highest, nonlethal concentration was reported. The LC50 values of anti MRSA molecule was calculated by probit analysis using SPSS software.
3. Results
3.1. Detection of mecA gene by PCR amplification
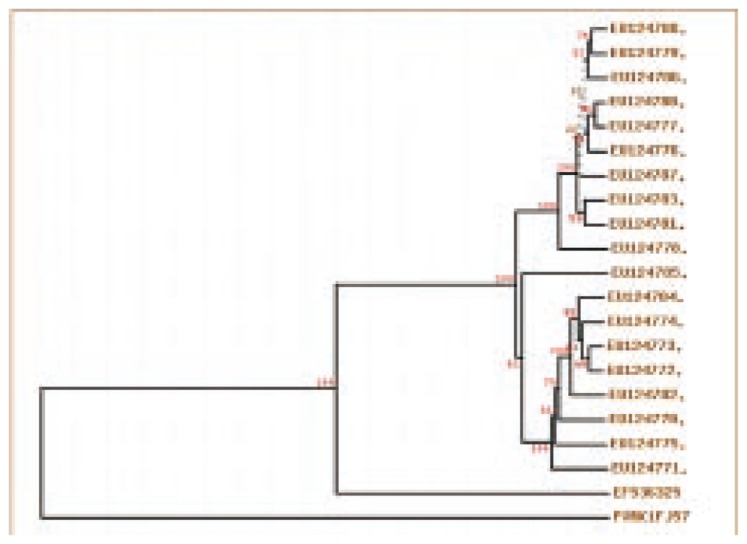
The mecA gene which is responsible for methicillin resistance in S. aureus confirmed the presence of MRSA in Colachal hospitals[17] and was the first report in Kanyakumari District, Tamilnadu, India. This confirmation of MRSA insisted to identify an antibiotic from the MRSA from the mangroves associated symbiont in Kanyakumari District and already anti MRSA molecule from Rhizophora mucronata Lamk was reported[17]. In present study we have screened around 60 Streptomyces for antimicrobial properties and 20 bioactive compound producing microbes were deposited in genebank (Figure 1)[18].
Figure 1. Phylogeny analysis of Streptomyces PVRK-1 with the other mangrove symbionts Streptomyces isolated from the Manakkudy mangrove.
EU124788.1 Streptomyces sp., EU124787.1 Streptomyces sp., EU124786.1 Streptomyces roseus, EU124785.1 Streptomyces sp., EU124784.1 Streptomyces griseoruber, EU124783.1 Streptomyces bikiniensis, EU124782.1 Streptomyces sp., EU124781.1 Streptomyces showdoensis, EU124780.1 Streptomyces sp., EU124779.1 Streptomyces venezuelae, EU124778.1 Streptomyces sp., EU124777.1 Streptomyces roseoviridis, EU124776.1 Streptomyces sp., EU124775.1 Streptomyces caelestis, EU124774.1 Streptomyces caelestis, EU124773.1 Streptomyces caelestis, EU124772.1 Streptomyces caelestis, EU124771.1 Streptomyces olivoverticillatus, EU124770.1 Streptomyces olivoverticillatus, EF536325.1 Streptomyces sp., FJ573228.1 Streptomyces sp. PVRK-1
3.2. Determination of inhibitory concentration and production
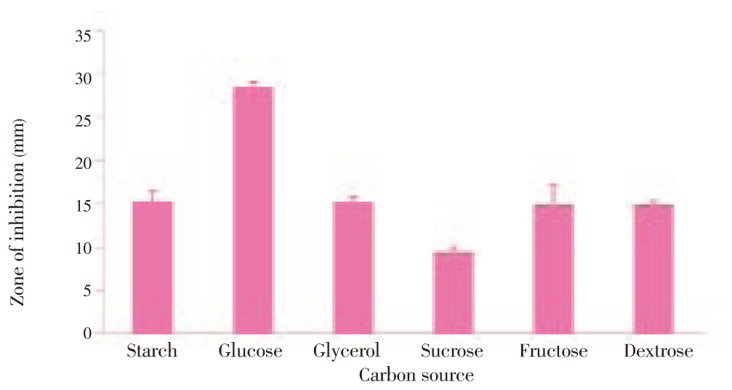
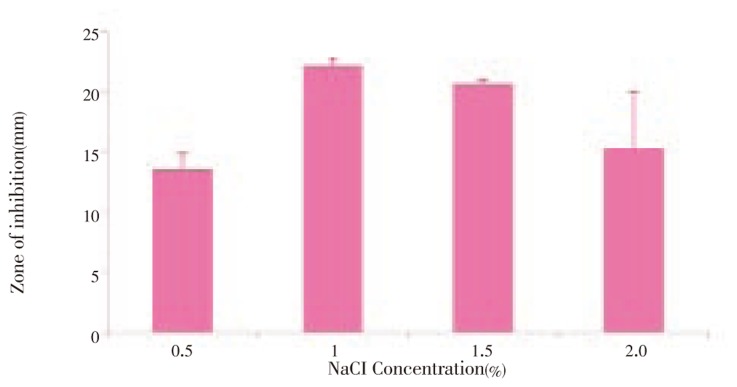
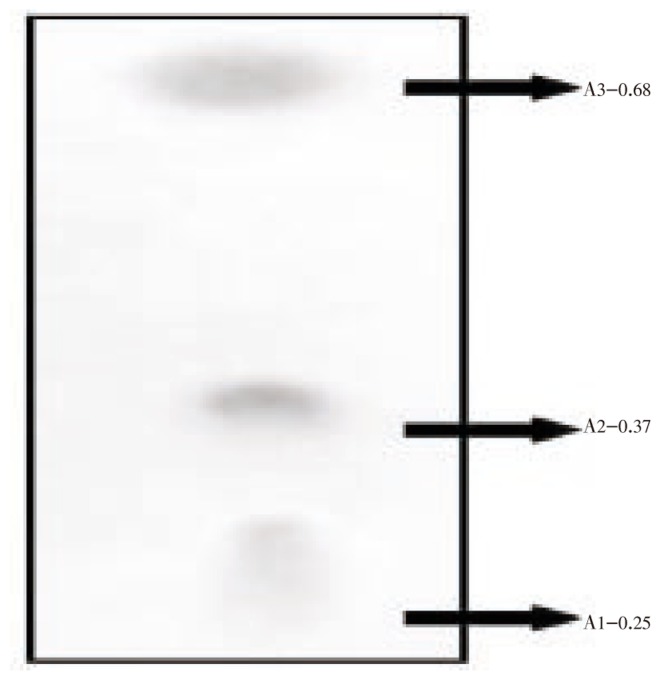
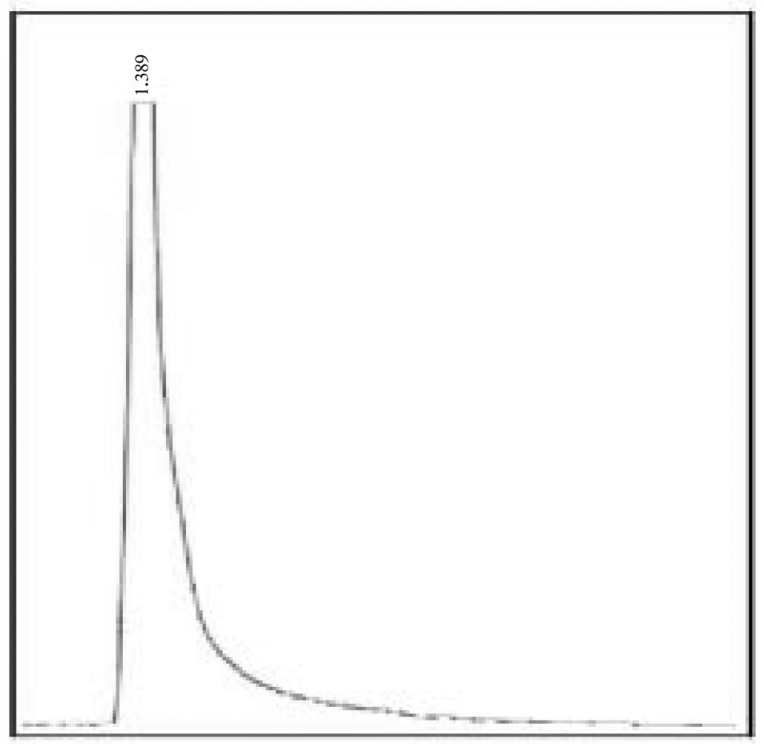
The optimum production was standardized in the Solid State fermentation and analyzed for the activity by double layer agar method[22] (Figure 2–6). After supplementing the essential nutrients in the fermentation medium for anti MRSA compound production, the compound was extracted and the activity was found only in the methanolic extract. 600 mg of crude anti MRSA compound was obtained in the 2.5 L fermentation medium of Streptomyces sp. PVRK-1. UV-Vis spectrophotometric analysis was carried out to find the wavelength to be set in HPLC analysis and the absorption maximum of the active spot A2 was 270 nm. The HPLC analysis showed that the retention time of TLC resolved molecule A2 was 1.389 min. The TLC pattern of methanolic extract of Streptomyces sp. PVRK-1 was shown in Figure 7. The chromatogram of A2 HPLC is shown in the Figure 8. The growth of the MRSA was entirely inhibited in 32-34 µg/mL of MRSA culture broth and 30 µg/mL was considered as the inhibitory concentration of the purified molecule A2 in broth dilution method in which no bacterial growth was observed. The 30 µg/mL of methicillin as the inhibitory concentration of MSSA (Methicillin susceptible Staphylococcus aureus) was reported[17].
Figure 2. Anti MRSA activity of Streptomyces sp. PVRK-1 supplemented with different carbon sources.
Figure 6. Anti MRSA activity of Streptomyces sp. PVRK-1 supplemented with different concentration of NaCl.
Figure 7. TLC analysis of methanol extract of anti MRSA molecule A2.
Figure 8. RP-HPLC analysis of TLC eluted anti MRSA molecule A2.
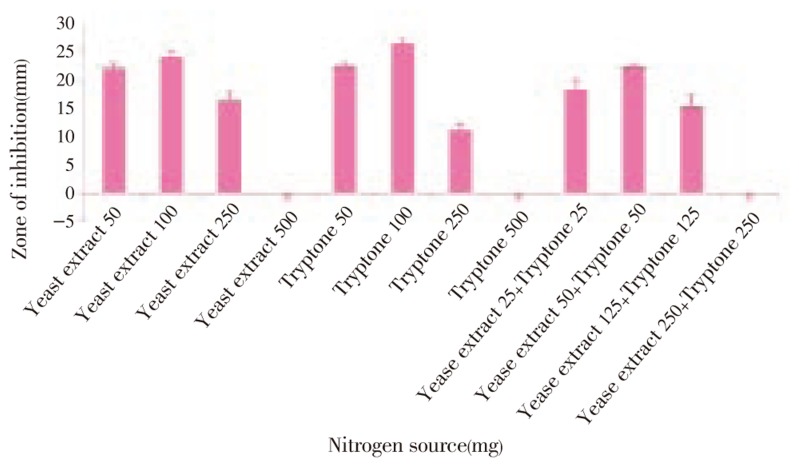
Figure 3. Anti MRSA activity of Streptomyces sp. PVRK-1 supplemented with different Nitrogen sources.
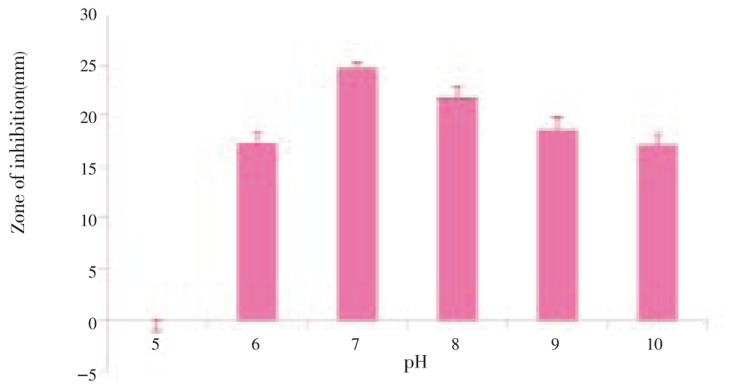
Figure 4. Anti MRSA activity of Streptomyces sp. PVRK-1 supplemented with different pH.
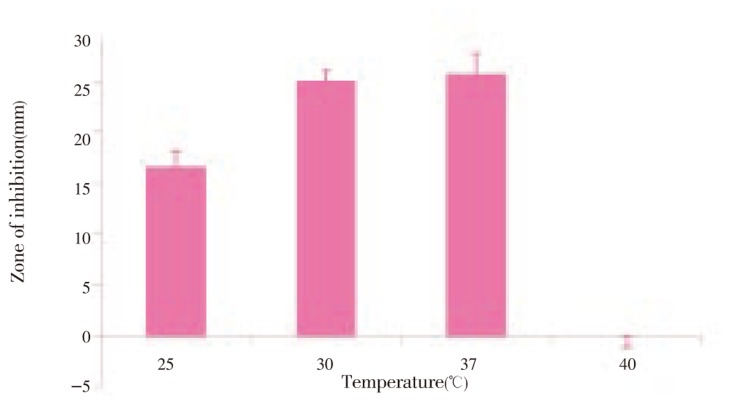
Figure 5. Anti MRSA activity of Streptomyces sp. PVRK-1 supplemented with different temperature.
3.3. Compound toxicity assessment in Zebrafish embryos
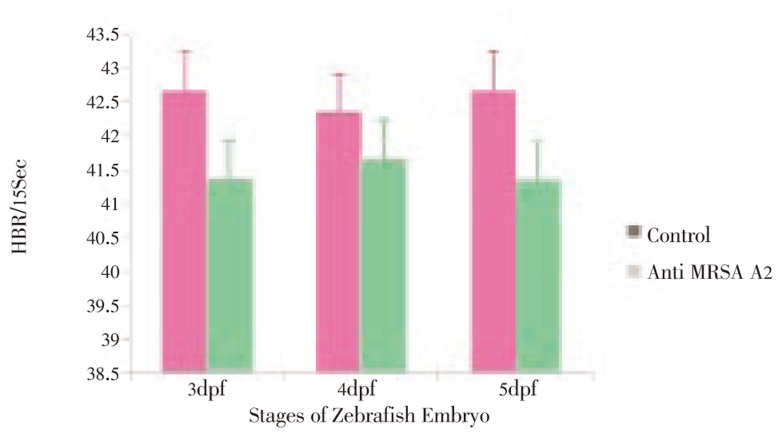
The phenotypic deformities observed in 48-60 µg/mL of the molecule A2 in 3 dpf of the developing embryo, showing the muscular deformities, pericardial edema, trunk flexure, protruded mouth and bulging (Figure 9). In the cardiac assay, Zebrafish embryos were exposed to compounds for 3 h before measurement of the Heart Beat Rates (HBR). HBR were recorded and analyzed in 3-5 dpf embryos for 40 µg/mL of anti MRSA molecule, the HBR value ranging from 41.33-41.67 HBR for 15 seconds in the molecule treated fish was similar 42.33-42.67 to control (Figure 10). There was no notable differences found in the HBR assay. The LC50 mean value of the purified small molecule A2 is 61.504 µg/mL. The inhibitory concentration of the MRSA in the embryo was 32-34 µg/mL for the purified molecule in which the toxicity was not observed and MRSA was inhibited. All developmental deformities and the physiological effects were shown in Figure 9 A-K. The purified molecule A2 as a potential antibiotic for methicillin resistance and the molecule was stable with less toxic effects in the Zebrafish embryos, was found in 48-60 µg and the inhibition of the MRSA in the infected embryos was in 32-34 µg/mL.
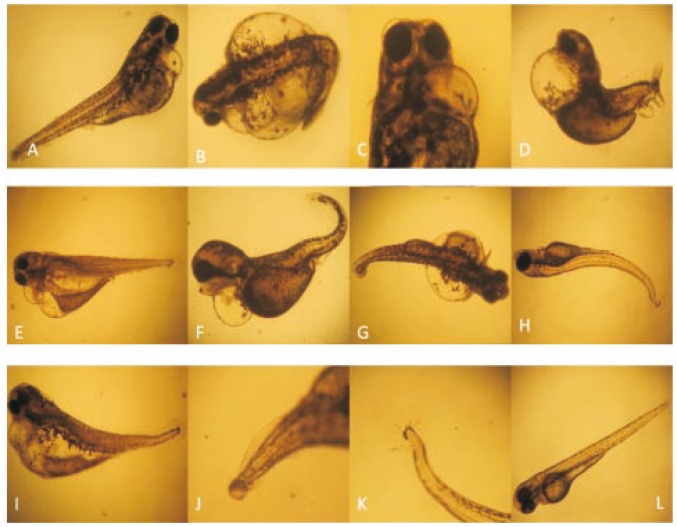
Figure 9. Organogenesis effect and compound toxicity in Zebrafish embryos.
A, B, C: Pericardial bulging, protruded mouth and abnormal eyes at 35-40 µg/mL in the 3dpf embryos; D: Pericardial bulging,Eye damage, trunk flexure, spinal cord flexure, tail damage 40-45 µg/mL at 3dpf; E, F: Pericardial bulging, gut deformities and trunk flexure; G, H, I: Damage in the cardiac, trunk and tail (45-50 µg/ml at 3dpf); J, K: Tail and caudal fin deformities at (40-50 -3 µg/ml in 3dpf embryos; L: control Zebrafish at 3dpf. (has mutation and small was observed in A, B, C, D, E & F)
Figure 10. Cardiac assay of anti MRSA molecule A2 from Streptomyces sp. PVRK-1.
4. Discussion
The search for newer sources of antibiotics is a global challenge for preoccupying research institutions, pharmaceutical companies and academia, since many infectious agents are becoming resistant to synthetic drugs[24]–[28]. Recently published guidelines have recommended the use of vancomycin in the management of severe MRSA infection[29], but it was reported that the vancomycin resistant S. aureus was emerging in northern India[10]. So that the search of novel anti-MRSA molecules is needed globally. The Streptomyces sp. PVRK-1 was isolated from the manakkudy mangrove forest and the the phylogeny analysis was compared with the twenty Streptomyces isolated from Manakkudy mangrove sediments[18]. The Streptomyces sp. PVRK-1 showed unique differences when compared to the already submitted 20 Streptomyces from Manakkudy mangrove sediment. All the twenty Streptomyces from the mangrove comparable to the Streptomyces sp. PVRK-1 showed novel antimicrobial properties and reported[18]. Generally, terrestrial (soil) actinomycetes were the main stay for antibiotic discovery efforts in the previous five decades. In contrast, the marine environment, covering more than three-fourths of the world's surface, represents a vast and relatively untapped source of novel scaffolds with unique antimicrobial properties[30], [31]. In present study we have screened a novel Anti-MRSA molecule producing Streptomyces sp. PVRK-1 from the Manakkudy, mangroves. Many small molecules identified from new marine actinomycete species have been shown to exhibit potent anti-MRSA activity with desirable cross activity against other bacterial pathogens[32],[33]. Zebrafish is an ideal organism for small molecule studies. The ability to use the whole organism allows complex in vivo phenotypes to be assayed and combines animal testing with screening. During the last decade, this technology has been extended to the generation of high-value knowledge on safety risks of novel drugs[34]. Streptogramin antibiotic, etamycin, for the first time from a newly discovered marine actinomycete characterized its activity against a panel of HA- and CA-MRSA strains according to studies in murine infection models[35]. The inhibitory concentration of anti-MRSA molecule A2 from Streptomyces sp. PVRK-1 was less than the inhibitory concentration of the etamycin accordign to Haste et al[35]. In the present work the anti MRSA molecule toxicity was assessed in the Zebrafish embryos. Similar studies were carried out in the anti MRSA compound from Rhizophora mucronata[18]. Embryos are easily treatable by waterborne exposure[36]. The Zebrafish model organism is increasingly used for assessing drug toxicity and safety and numerous studies confirm that mammalian and Zebrafish toxicity profiles are strikingly similar[37]. The anti-MRSA molecule A2 did not showed any physiological deformities and organogenesis in the drug dose (in vitro) of the purified small molecule A2 which is coincide with earlier reports[17]. Zebrafish development has been well characterized[38] and the heart beat rates were counted in the Purified Molecule A2 treated embryos which did not show notable deviation in the HBR. Cardiotoxicity is a major problem that can result in drug withdrawal. In 2004, rofecoxib (Vioxx), Merck's blockbuster antiarthritic drug was removed from the market because of increased risk of heart attack and stroke[37]. However the function of the Zebrafish and mammalian heart are similar, the contractility, rhythmicity and morphology can be visually assessed[39] and there is no notable physiological toxic effects in the heart development, hence it could be concluded that the purified compound may not show any cardiotoxic effects when compare to the existing antibiotics. But the formation of pericardial edema, organogenesis and developmental deformities were observed in higher concentration (48-60 µg/mL) in the 3 dpf embryos. The pericardial bulging and small heart of the 3 dpf embryos showed the phenocopy of heart and soul mutation under the genetic screen[40]. Furthermore it was proved that small molecule concentramide also showed heart and soul mutation in the cardiogenesis of the Zebrafish embryos at 3 dpf by disrupting the heart patterning in the embryos[41] and such based mutations were also observed in the higher concentration of the anti MRSA molecule A2 thus it can be concluded that the anti-MRSA small molecule A2 was also affecting the cardiac patterning in higher concentration, but not in the inhibitory concentration. Also it can be concluded that the anti-MRSA molecule A2 did not affecting the embryos in its drug dose concentration level in in vitro and in vivo. These basic preclinical studies suggest that the molecule is very novel and studies showed that the mangrove associated Streptomyces sp. PVRK-1 shows potential for the production of anti MRSA molecule due to a novel structure for antibiotic resistance. The biomedical and toxicity studies prove that the molecule is potent against antibiotic resistant Staphylococcus aureus, which did not, showed any toxic effects or organogenesis effects in the minimal inhibitory concentration. Hence this molecule could be further structurally characterized to get anti-MRSA drugs for the medicament of MRSA infected patients all over the world.
Footnotes
Foundation Project: Supported by Xpression Biotek Ltd. and International Centre for Nanobiotechnology (ICN), Manonmaniam Sundaranar University.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Sakoulas G, Moellering RC., Jr Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis. 2008;46(Suppl 5):S360–S367. doi: 10.1086/533592. [DOI] [PubMed] [Google Scholar]
- 2.Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008;8(6):747–763. doi: 10.1016/j.meegid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Bilthoven: earess; 2009. European antimicrobial resistance surveillance system. EARSS annual report 2008. [Google Scholar]
- 4.Campanile F, Bongiorno D, Borbone S, Stefani S. Hospital-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) in Italy. Ann Clin Microbiol Antimicrob. 2009;8:22. doi: 10.1186/1476-0711-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hope R, Livermore DM, Brick G, Lillie M, Reynolds R. BSAC working parties on resistance surveillance: non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001-06. J Antimicrob Chemother. 2008;62(Suppl 2):ii65–74. doi: 10.1093/jac/dkn353. [DOI] [PubMed] [Google Scholar]
- 6.Valentini P, Parisi G, Monaco M, Crea F, Spanu T, Ranno O, et al. et al. An uncommon presentation for a severe invasive infection due to methicillin-resistant Staphylococcus aureus clone USA300 in Italy: a case report. Ann Clin Microbiol Antimicrob. 2008;7:11. doi: 10.1186/1476-0711-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefani S, Bongiorno D, Cafiso V, Campanile F, Crapis M, Cristini F, et al. et al. Pathotype and susceptibility profile of a community-acquired methicillin-resistant Staphylococcus aureus strain responsible for a case of severe pneumonia. Diagn Microbiol Infect Dis. 2009;63(1):100–104. doi: 10.1016/j.diagmicrobio.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Asad UK, Ayesha S, Anju T, Shazia Z, Mohd A, Sukhminderjit K, et al. et al. Amplification of mecA gene in multi-drug resistant Staphylococcus aureus strains from hospital personnel. J Infect Dev Ctries. 2007;1(3):289–295. [PubMed] [Google Scholar]
- 9.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiwari HK, Sen MR. Emergence of vancomycin resistant Staphylococcus aureus (VRSA) from a tertiary care hospital from northern part of India. BMC Infect Dis. 2006;6:156. doi: 10.1186/1471-2334-6-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demain AL. Antibiotics: Natural products essential to human health. Med Res Rev. 2009;29(6):821–842. doi: 10.1002/med.20154. [DOI] [PubMed] [Google Scholar]
- 12.Yoo JC, Kim JH, Ha JW, Park NS, Sohng JK, Lee JW, et al. et al. Production and biological activity of Laidomycin, Anti MRSA/VRE antibiotic from Streptomyces sp. CS684. J Microbiol. 2007;45(1):6–10. [PubMed] [Google Scholar]
- 13.Chakraborty SP, Mahapatra SK, Roy S. Biochemical characters and antibiotic susceptibility of Staphylococcus aureus isolates. Asian Pac J Trop Biomed. 2011:212–216. doi: 10.1016/S2221-1691(11)60029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Astrid MV, Appelmelk BJ, Christina MJE, Vandenbroucke G, Bitter W. A star with stripes: Zebrafish as an infection model. Trends in Microbiology. 2004;12(10):451–457. doi: 10.1016/j.tim.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Bretaud S, Lee S, Guo S. Sensitivity of Zebrafish to environmental toxins implicated in Parkinson's disease. Neurotoxicol Teratol. 2004;26:857–64. doi: 10.1016/j.ntt.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 17.Rajaretinam RK, Vincent SGP. Isolation of a novel bioactive compound from Rhizophora mucronata for methicillin resistant Staphylococcus aureus (MRSA) and compound toxicity assessment in Zebra fish embryos. J Pharm Res. 2010;3(8):2000–2003. [Google Scholar]
- 18.Kannan RR, Vincent SGP. Molecular characterization of antagonistic Streptomyces isolated from a mangrove swamp. Asian J Biotechnol. 2011;3:237–245. [Google Scholar]
- 19.Gauthier MJ, Shewan JM, Gibson DM. Taxonomic position and seasonal variations in marine neritic environment of some Gram-negative antibiotic-producing bacteria. J Microbiol. 1975;87:211–218. doi: 10.1099/00221287-87-2-211. [DOI] [PubMed] [Google Scholar]
- 20.Jensen PR, Williams PG, Oh DC, Zeigler L, Fenical W. Species-specific secondary metabolite production in marine actinomycetes of the genus Salinispora. Appl Environ Microbiol. 2007;73(4):1146–1152. doi: 10.1128/AEM.01891-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner H, Bladt W. 2nd edi. Birlin: Springer-Verlag; 1996. Plant Drug Analysis. A thin layer chromatography atlas. [Google Scholar]
- 22.Kim S, Oh KB. Evaluation of antimicrobial activity of farnesoic acid derivatives. J Microbiol Biotchnol. 2002;12:1006–1009. [Google Scholar]
- 23.Westerfield M. Eugene: University of Oregon Press; 1989. The Zebrafish book. A Guide for the laboratory use of Zebrafish Danio rerio. [Google Scholar]
- 24.Latha SP, Kannabiran K. Antimicrobial activity and phytochemicals of Solanum trinobatum Linn. Afr J Biotechnol. 2006;5(23):2402–2404. [Google Scholar]
- 25.Bhimba BV, Meenupriya J, Joel EL, Naveena DE, Kumar S, Thangaraj M. Antibacterial activity and characterization of secondary metabolites isolated from mangrove plant Avicennia officinalis. Asian Pac J Trop Med. 2010;3(7):544–546. [Google Scholar]
- 26.Moghadam MS, Maleki S, Darabpour E, Motamedi H, Nejad SMS. Antibacterial activity of eight Iranian plant extracts against methicillin and cefixime restistant Staphylococcous aureus strains. Asian Pac J Trop Med. 2010;3(4):262–265. [Google Scholar]
- 27.Rasheed MU, Ahmed Z. Phenotypic methods of greater accuracy to detect the mecA gene product for the recognition of MRSA in resource constraint settings. Asian Pac J Trop Med. 2010;3(9):741–744. [Google Scholar]
- 28.Jarrar N, Abu-Hijleh A, Adwan K. Antibacterial activity of Rosmarinus officinalis L. alone and in combination with cefuroxime against methicillin–resistant Staphylococcus aureus. Asian Pac J Trop Med. 2010;3(2):121–123. [Google Scholar]
- 29.Gould FK, Brindle R, Chadwick PR. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63:849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 30.Fenical W, Jensen PR. Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 31.Kwon HC, Kauffman CA, Jensen PR, Fenical W. Marinomycins A-D, antitumor-antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “marinispora”. J Am Chem Soc. 2006;128:1622–1632. doi: 10.1021/ja0558948. [DOI] [PubMed] [Google Scholar]
- 32.Hughes CC, Prieto-Davo A, Jensen PR, Fenical W. The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. Org Lett. 2008;10:629–631. doi: 10.1021/ol702952n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McArthur KA, Mitchell SS, Tsueng G, Rheingold A, White DJ, Grodberg J, et al. et al. Lynamicins A-E, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. J Nat Prod. 2008;71:1732–1737. doi: 10.1021/np800286d. [DOI] [PubMed] [Google Scholar]
- 34.Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S. Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol. 2008;154:1400–1413. doi: 10.1038/bjp.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haste NM, Perera V, Maloney KN, Tran DN, Jensen P, Fenical W, et al. Activity of the streptogramin antibiotic etamycin against methicillin-resistant Staphylococcus aureus. J Antibiot. 2010;63(5):219–224. doi: 10.1038/ja.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphey RD, Zon LI. Small molecule screening in the Zebrafish. Methods. 2006;39:255–261. doi: 10.1016/j.ymeth.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Patricia M, Chun QL. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov Today. 2008;13:9–10. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TT. Stages of embryonic development of the Zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 39.Thisse C, Zon L. Organogenesis - heart and blood formation from the Zebrafish point of view. Science. 2002;295:457–462. doi: 10.1126/science.1063654. [DOI] [PubMed] [Google Scholar]
- 40.Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, et al. et al. Mutations affecting the formation and function of the cardiovascular system in the Zebrafish embryo. Development. 1996;123:285–292. doi: 10.1242/dev.123.1.285. [DOI] [PubMed] [Google Scholar]
- 41.Peterson RT, Mably JD, Chen JN, Fishman MC. Convergence of distinct pathways to heart patterning revealed by the small molecule concentramide and the mutation heart-and-soul. Curr Biol. 2001;11:1481–1491. doi: 10.1016/s0960-9822(01)00482-1. [DOI] [PubMed] [Google Scholar]