Abstract
Objective
To study detail microscopic evaluation and physiochemical analysis of Dillenia indica (D. indica) leaf.
Methods
Fresh leaf sample and dried power of the leaf were studied macroscopically and microscopically. Preliminary phytochemical investigation of plant material was done. Other WHO recommended parameters for standardizations were also performed.
Results
The detail microscopy revealed the presence of anomocytic stomata, unicellular trichome, xylem fibres, calcium oxalate crystals, vascular bundles, etc. Leaf constants such as stomatal number, stomatal index, vein-islet number and veinlet termination numbers were also measured. Physiochemical parameters such as ash values, loss on drying, extractive values, percentage of foreign matters, swelling index, etc. were also determined. Preliminary phytochemical screening showed the presence of steroids, terpenoids, glycosides, fatty acids, flavonoids, phenolic compounds and carbohydrates.
Conclusions
The microscopic and physiochemical analysis of the D. indica leaf is useful in standardization for quality, purity and sample identification.
Keywords: Dillenia indica, Fibre, Leaf constant, Microscopy, Physiochemical, Stomata, Xylem
1. Introduction
Dilenia indica (D. indica) Linn. (Family: Dilleniaceae) grows in moist and evergreen forests of India. It has been grown in gardens for its handsome foliage and attractive flower as an ornamental plant. The fruit is said to possess tonic laxative properties and used for relieving abdominal pain. The bark and leaves are astringent[1],[2]. The mixed juices of leaves bark and fruits are given orally for the treatment of cancer and diarrhea[3]. The alcoholic extract of D. indica leaves is reported to possess central nervous system (CNS) depressant activity[4]. The methanolic leaf extract shows anti-inflammatory activity in carrageenan induced paw edema and acetic acid-induced capillary permeability methods[5]. Phytochemical studies have shown the presence of the lupeol group of triperpene like betulinic acid and betulin and flavonol such as myricetin. Flavonoids such as kaempferol, quercetin, isorhamnetin, naringenin, and phenolic materials are also present[6],[7]. Four compounds namely, lupeol, betulinaldehyde, betulinic acid and stigmasterol can be isolated from the stem extract of the plant[8]. The crude methanol extract of the roots shows analgesic, antidiarrhoeal activities and reduced GI motility in animal models[9]. For standardization and quality assurance purposes, the following three attributes must be verified: authenticity, purity and assay[10]. Hence, in this work we report an attempt for the standardization of D. indica leaf by microscopic evaluation and physiochemical analysis.
2. Materials and methods
2.1. Chemicals
Phloroglucinol, glycerin, hydrochloric acid, chloral hydrate, potassium hydroxide and all other chemicals used in the study were of analytical grade.
2.2. Plant material
D. indica leaves were collected from the campus of Kurukshetra University, Kurukshetra, India and identified by Dr. HB Singh, Scientist F & Head, Raw Material Herbarium & Museum, NISCAIR, New Delhi, India. A voucher specimen of the plant was preserved in the herbarium for reference (NISCAIR/RHMD/Consult/-2009-10/1381/182/1).
2.3. Macroscopic and microscopic analysis
The macroscopy and microscopy of the plant were studied according to the method of Brain et al[11]. For the microscopic studies, cross transverse sections of fresh leaves were mounted in glycerin as well as stained with phloroglucinol-HCl and studied per standard procedures[12],[13]. Coarse powder was used to study microscopical characters of leaf powder.
2.4. Physiochemical analysis
Physiochemical parameters such as ash and extractive values were performed according to the official method prescribed and the WHO guidelines on quality control methods for medical plants material[14]–[16].
2.5. Preliminary phytochemical screening
Preliminary screening was carried out using the standard procedure Kokate[10].
3. Result
3.1. Macroscopic characteristics
D. indica is a handsome evergreen tree, 30-80 feet. in height and 6 feet. in girth, with a dense round crown (Figure 1a). The leaf is oblong-lanceolate 8-14 inch long and 2-4 inch broad with pointed end and toothed. The upper part of the leaf as well as vein beneath is covered with hairs (Figure 1b).
Figure 1. Macroscopic characteristic of D. indica.
a: Uniform look of D. indica; b: Oblong-lanceolate leaves of D. indica.
3.2. Microscopical characteristics
3.2.1. Leaf microscopy
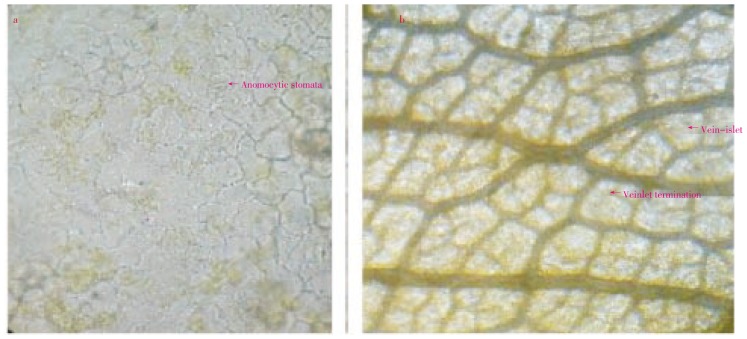
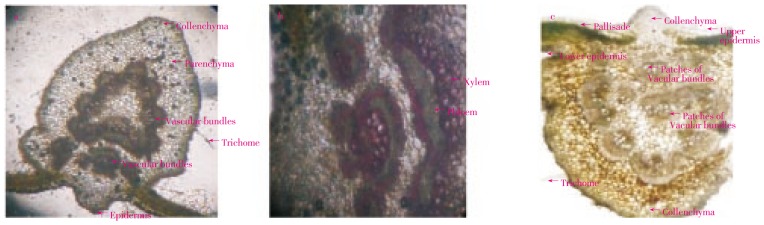
D. indica leaf surface shows the anomocytic types of stomata which is characteristics of family Dilleniceae (Figure 2a). Leaf surface also shows the presence of veins, vein- islets and vein terminations (Figure 2b). Transverse section of leaf (Figure 3a) shows the epidermis layer, and patches vascular bundles (xylem and phloem), collenchymas, etc. The vascular bundles were stained pink with phloroglucinol and conc. HCl (Figure 3b) Trichomes are unicellular and lignified. Strips of collenchyma are present below and upper layer of epidermis (Figure 3c). Leaf constants such as stomatal number, stomatal index, veinlet terminations and vein-islet number were measured. The results were shown in Table 1.
Figure 2. Leaf surface of D. indica.
a: Stomata; b: Veins, veinlet termination & vein-islet.
Figure 3. Transverse section of D. indica leaf.
a: T.S. (stained) of D. indica leaf (100 ×); b: Vascular bundles (400 ×); c: T.S. (unstained) of D. indica leaf (100 ×).
Table 1. Leaf constants.
| No | Parameters | Value (in 1mm2 area) |
| 1 | Average stomatal number in 25 different fields (400×) | 130 |
| 2 | Stomatal index (4000×) lower suface | 15.6-18.5 |
| 3 | Vein-islet number (50×) | 16-18-20 |
| 4 | Vein-termination number (50×) | 10-12-14 |
3.2.2. Powder microscopy
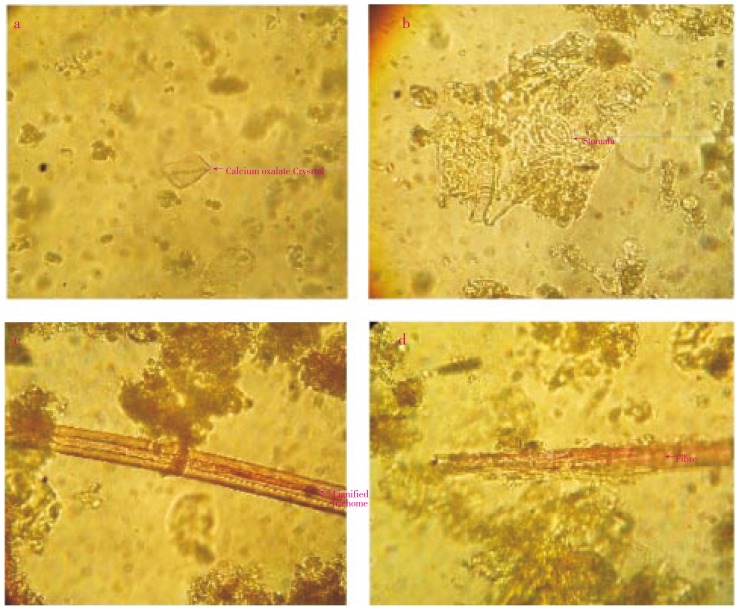
The fine powder was mounted in glycerin as well as stained (phlorogucinol + conc. HCl). After observation under microscope, it showed presence of unicellular lignified trichomes, anomocytic stomata, calcium oxalate crystals, epidermal cells, xylem vessels, etc. (Figure 4).
Figure 4. Powder characteristics of D. indica leaf (400 ×).
3.3. Preliminary phytochemical screening
Preliminary phytochemical screening revealed the presence of steroids, terpenoids, saponins, fatty acids, flavonoids, phenolic compounds, glycosides and carbohydrates.
3.4. Physiochemical parameters
The physiochemical parameters such as ash values, losses on drying, swelling index and percentage of foreign matters were measured and shown in Table 2. The results of extractive values of different solvents were shown in Table 3.
Table 2. Physiochemical parameters.
| No | Parameters | Value (%w/w) |
| 1 | Foreign matter | 0.45 |
| 2 | Loss on drying | 3.2 |
| 3 | Total ash value | 11.25 |
| 4 | Acid insoluble ash | 8.25 |
| 5 | Water soluble ash | 5.5 |
| 6 | Swelling index | 1.75 mL |
Table 3. Extractive values of crude drug.
| No | Parameters | Extractive values (%w/w) |
| 1 | Pet. ether soluble extractive | 9.7 |
| 2 | Chloroform soluble extractive | 7.2 |
| 3 | Methanol soluble extractive | 27.7 |
| 4 | Water soluble extractive | 31.51 |
| 5 | Alcohol soluble extractive | 26.42 |
4. Discussion
Today sophisticated modern research tools for evaluation of the plant drugs are available but microscopic method is still one of the simplest and cheapest methods to start for establishing the correct identity of the source materials[17]. In the present work microscopy evaluation and physiochemical analysis of D. indica leaf were carried out. Morphological and histological studies of the leaf will enable to identify the crude drug. The macroscopical characters of the leaf can serve as diagnostic parameters. The microscopical studies of the transverse section showed presence of unicellular lignified or non-lignified trichomes and anomocytic stomata, which is characteristic of the family Dillenaceae. The extractive values are useful to evaluate the chemical constituents present in the crude drug and also help in estimation of specific constituents soluble in a particular solvent[18]. Preliminary phytochemical analysis indicated presence of steroids, terpenoids, glycosides, fatty acids, flavonoids, phenolic compounds and carbohydrates. The information obtained from preliminary phytochemical screening will be useful in finding out the genuity of the drug. Ash values of a drug give an idea of the earthy matter or the inorganic composition and other impurities present along with the drug. The percentage of total ash, acid insoluble ash and water soluble ash are carried out. Extractive values are primarily useful for the determination of exhausted or adulterated drugs[19].
In conclusion, the present work was undertaken with a view to lay down standards which could be useful to detect the authenticity of this medicinally useful plant. Microscopic study and physiochemical standards can be useful to substantiate and authenticate the drug.
Acknowledgments
Authors are thankful to AICTE, New Delhi for honoring Sunil Kumar with Career Award for Young Teachers and financially supporting to research work [F.No. 1-51/RID/CA/4/2009-10].
Footnotes
Foundation Project: Supported by Career Award for Young Teachers, AICTE, New Delhi. (No. 1-51/RID/CA/4/2009-10)
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Sastri BN. The wealth of India raw material. New Delhi: Council of Scientific & Industrial Research; 2003. pp. 64–65. [Google Scholar]
- 2.Kritikar KR, Basu BD. Indian medicinal plants. Dehradun: Oriental Enterprizes; 2003. pp. 75–77. [Google Scholar]
- 3.Sharma HK, Chhangte L, Dolui AK. Traditional medicinal plants in Mizoram, India. Fitoterapia. 2001;72:146–161. doi: 10.1016/s0367-326x(00)00278-1. [DOI] [PubMed] [Google Scholar]
- 4.Bhakuni DS, Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN. Screening of Indian plants for biological activity Part II. Indian J Exp Biol. 1969;7:250–262. [PubMed] [Google Scholar]
- 5.Yeshwante SB, Juvekar AR, Nagmoti DM, Wankhede SS, Shah AS, Pimprikar RB, et al. et al. Anti-inflammatory activity of methanolic extracts of Dillenia indica L. leaves. Pharmacology. 2009;1(1):63–66. [Google Scholar]
- 6.Banerji N, Majumder P, Dutta NL. A new pentacyclic triterpene lactone from Dillenia indica. Phytochemistry. 1975;12:1447–1448. [Google Scholar]
- 7.Pavanasasivam G, Suktanbawa MU. Flavonoids of some Dilleniaceae species. Phytochemistry. 1975;14:1127–1128. [Google Scholar]
- 8.Parvin MN, Rahman MS, Islam MS, Rashid MA. Chemical and biological investigations of Dillenia indica Linn. Bangladesh J Pharmacol. 2009;4:122–125. [Google Scholar]
- 9.Bose U, Gunasekaran K, Bala V, Rahman AA. Evaluation of phytochemical and pharmacological properties of Dillenia indica Linn. Leaves. J Pharmacol Toxicol. 2010;5(5):222–228. [Google Scholar]
- 10.Torey A, Sasidharan S, Yeng C, Latha LY. Standardization of Cassia spectabilis with respect to authenticity, assay and chemical constituent analysis. Molecules. 2010;15:3411–3420. doi: 10.3390/molecules15053411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brain KR, Turner TD. The practical evaluation of phytopharmaceuticals. Bristol: Wright-Scientechnica; 1975. pp. 4–9. [Google Scholar]
- 12.Kokate CK. Practical pharmacognosy. 1st ed. New Delhi: Vallabh prakashan; 1986. [Google Scholar]
- 13.Pandya DJ, Desai TR, Nadpara NP, Mehta HA, Modi AM. Pharmacognostic study and establishment of quality parameters of leaves of Bombax insigne Linn. Int J Pharmacogn Phytochem Res. 2010;2(3):1–5. [Google Scholar]
- 14.WHO . Quality control methods for medicinal plant material. Geneva: Organisation Mondiale De La Sante; 1992. pp. 22–34. [Google Scholar]
- 15.Ministry of Health and Welfare . Indian pharmacopeia. 4th ed. New Delhi: Ministry of Health and Welfare, Controller of Publications; 1996. pp. A53–A54. [Google Scholar]
- 16.Khandelwal KR. Practical pharmacognosy. 18th ed. Pune: Nirali Publication; 2007. [Google Scholar]
- 17.Singh S, Machawal L, Chauhan MG. Pharmacognostic study of male leaves of Trichosanthes dioica Roxb. with special emphasis on microscopic technique. J Pharmacogn Phytother. 2010;2(5):71–75. [Google Scholar]
- 18.Thomas S, Patil DA, Patil AG, Chandra N. Pharmacognostic evaluation and physicochemical analysis of Averrhoa carambola L. fruit. J Herb Med Toxicol. 2008;2(2):51–54. [Google Scholar]
- 19.Singhal AK, Bhati VS, Singhal VK. Pharmacognostic study of aerial parts of plant Geniosporum prostratum (L) Benth. J Sci Specul Res. 2010;1(1):19–24. [Google Scholar]