Abstract
Objective
To evaluate the antitumor activity of Manilkara zapota (M. zapota) L. stem bark against Ehrlich ascites carcinoma (EAC) in Swiss albino mice.
Methods
The in vivo antitumour activity of the ethyl acetate extract of stem bark of M. zapota L. (EASM) was evaluated at 50, 100 and 200 mg/kg bw against EAC using mean survival time. After administration of the extract of M. zapota, viable EAC cell count and body weight in the EAC tumour hosts were observed. The animal was also observed for improvement in the haematological parameters (e.g., heamoglobin content, red and white blood cells count and differential cell count) after EASM treatment.
Results
Intraperitoneal administration of EASM reduced viable EAC cells, increased the survival time, and restored altered haematological parameters. Significant efficacy was observed for EASM at 100 mg/kg dose (P<0.05).
Conclusions
It can be concluded that the ethyl acetate extract of stem bark of M. zapota L. possesses significant antitumour activity.
Keywords: Manilkara zapota, Antitumour activity, Stem bark, Ehrlich ascites carcinoma, Haematological parameter
1. Introduction
The majority of the world's population in developing countries still relies on herbal medicines to meet their health needs in cases when synthetic medicine could not relieve patients who suffer from painful illnesses like cancer[1]. In the modern system of medicine, several chemotherapeutic agents have been developed as a result of screening of the medicinal plants in various parts of the world[2]. So there is a growing interest in the pharmacological evaluation of medicinal plants.
Manilkara zapota (M. zapota) (L.) P. Royen, which belongs to the family Sapotaceae, is an evergreen, glabrous tree, 8-15 m in height. It is cultivated throughout Indian subcontinent including Bangladesh, though it is native to Mexico and Central America[3]. The seeds of M. zapota are aperients, diuretic tonic and febrifuge. Stem bark is astringent and febrifuge[4]. The leaves and bark are used to treat cough, cold, dysentery and diarrhoea[5]. Antimicrobial and antioxidant activities are also reported from the leaves of M. zapota[6],[7]. The major constituents isolated from fruits of M. zapota are polyphenols (methyl chlorogenate, dihydromyricetin, quercitrin, myricitrin, (+)-catechin, (-)-epicatechin, (+)-gallocatechin, and gallic acid)[8]. However, no studies to date have been conducted to demonstrate the antitumour activity of M. zapota. The present study was carried out to evaluate the in vivo antitumour activity of ethyl acetate extract of the stem bark of M. zapota (EASM) against Ehrlich ascites carcinoma (EAC) in mice.
2. Materials and methods
2.1. Plant materials
Stem bark of M. zapota (Family: Sapotaceae) were collected in August, 2010 from Rajshahi district of Bangladesh. The plant material was taxonomically identified by Professor ATM Naderuzzaman, Department of Botany, University of Rajshahi and a voucher specimen was deposited under the accession number DACB-23801 at the Bangladesh National Herbarium.
2.2. Extraction
The collected stem bark were cleaned and shade-dried. The dried bark were then pulverized into a coarse powder by a grinding machine (FFC-15, China). The powdered stem bark (450 g) were extracted with ethyl acetate at room temperature. These two extracts were then filtered through filter papers and filtrates were evaporated under reduced pressure at 40 °C using a rotary evaporator to get 5.5 g EASM.
2.3. Animals
Male Swiss albino mice (25-30 g) were procured from the Animal Research Branch of the International Centre for Diarrhoeal Diseases and Research, Bangladesh (ICDDRB). They were used throughout the study and housed in iron cages in a controlled environment [temperature (25±2) °C and 12 h dark/light cycle] with standard laboratory diet and water ad libitum. Experiments were carried out in accordance with the Ethical Committee Guidelines laid down by the local committee regarding the care and use of animals for experimental procedures.
2.4. Tumour cells
EAC cells were obtained from the Courtesy of Indian Institute for Chemical Biology (IICB), Kolkata, India and maintained by weekly intraperitoneal (ip) inoculation of 105 cells/mouse in the laboratory.
2.5. Acute toxicity studies
An acute toxicity study relating to the determination of median lethal dose (LD50) was performed by the method of Lorke[9]. Mice were randomly divided into EASM-treated ‘test’ groups and vehicle-treated ‘control’ group consisting of 7 groups with 5 mice per cage. EASM (100, 200, 400, 800, 1 600, and 3 200 mg/kg) was separately administered intraperitonealy to the mice in each of the test groups. Each mouse in the control group was treated with vehicle alone [2% dimethylsulfoxide (DMSO)]. Then after 24 h, the mortality number caused by the extract was observed from which the LD50 of EASM was determined.
2.6. Cell growth inhibition
In vivo tumour cell growth inhibition was carried out by the method as described by Sur et al[10]. 2×105 EAC cells were inoculated into 5 groups of mice (6 in each) on day 0. The control group 1 was treated with vehicle (2% DMSO). Mice in group 2, 3 and 4 were administered (ip) with EASM at 50, 100 and 200 mg/kg/day doses and group 5 received bleomycin (0.3 mg/kg/day). Treatment was continued for 5 days and on the 6th day after tumour transplantation, animals were sacrificed. Tumour cells were collected by repeated washing with 0.9% saline and viable tumour cells in the treated groups were compared with those of the control.
2.7. Studies on survival time and hematological parameters
Swiss albino mice were divided into six groups (n = 6). All animals were injected with EAC cells (2×105 cells/mouse) intraperitoneally except for the normal group. This was taken on day 0. Group 1 served as the normal control and group 2 served as the tumour control. These two groups received 2% DMSO. Group 3, 4 and 5 were treated with EASM at 50, 100 and 200 mg/kg bw, respectively. Group 6 which served as the positive control was treated with bleomycin at 0.3 mg/kg bw. All these treatments were given 24 h after the tumour inoculation, once daily for 10 days. On the 14th day after tumor inoculation, hematological parameters (hemoglobin, RBC, WBC and differential count of WBC) were measured from freely flowing tail vein blood of each mice of each group under sterilize condition[11]. Then mean survival time (MST) of each EAC cell inoculated group was noted.
2.8. Statistical analysis
All values were expressed as mean±SEM (Standard Error of Mean). Statistical analysis was performed with one way analysis of variance (ANOVA) followed by Dunnett's ‘t’ test using SPSS statistical software of 14 version. P<0.05 was considered to be statistically significant when compared with control.
3. Results
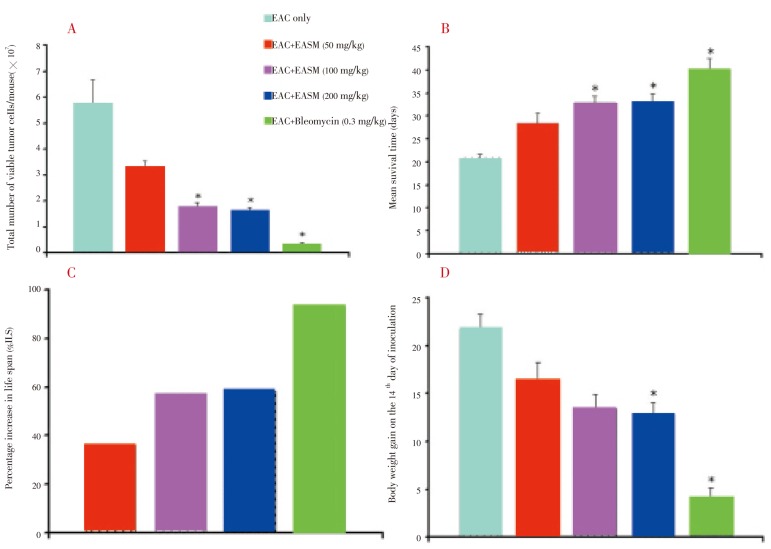
The LD50 value of EASM was evaluated in Swiss albino mice and found to be 3 025 mg/kg bw. Antitumour activity of EASM against EAC cell bearing mice was assessed by the parameters such as viable EAC cell (% inhibition in cell growth), mean survival time (MST), percentage (%) increase of life span (% ILS) and body weight gain. The average number of viable tumour cells per mouse of tumour control group was found to be (5.73±0.95)×107 cells/mL. Treatment with EASM (50, 100 and 200 mg/kg) reduced the viable cells significantly (P<0.05) (Figure 1A).
Figure 1. Effect of EASM on EAC cell bearing mice.
A: Viable EAC cells on the 6th day after tumor cell inoculation; B: Mean survival time; C: % increase in life span; D: Body weight gain on the 14th day. Data are expressed as mean ± SEM (n = 6). *P<0.05, between EAC control and EASM-treated group.
The effect of EASM on the survival of EAC bearing mice was shown in Figure 1B. The MST of the control group was (20.80±0.73) days, whereas it was (28.43±2.10), (22.80±1.46), (33.00±1.78) and (40.20±2.25) for the groups treated with EASM (50, 100 and 200 mg/kg) and bleomycin (0.3 mg/kg), respectively. The increase in the life span of EAC cell bearing mice treated with EASM (50, 100 and 200 mg/kg) and bleomycin (0.3 mg/kg) was found to be 36.5%, 57.6%, 58.7% and 93.2%, respectively (Figure 1C). On the 14th day of tumour cell inoculation, the average weight gain of only EAC cell bearing mice was (21.74±1.55) g whereas it was (16.57±1.76), (13.48±1.57), (12.86±1.20) and (4.28±1.00) g for the groups treated with EASM (50, 100 and 200 mg/kg) and bleomycin (0.3 mg/kg), respectively. EAMS at 200 mg/kg dose significantly reduced the weight gain (P<0.05).
Hematological parameters of EAC cell bearing mice on the 14th day showed significant changes when compared with normal mice (P<0.05) (Table 1). The total WBC count was found to increase with a reduction in the hemoglobin content and total RBC count. The differential count of WBC showed that the percentage of neutrophils was increased while that of lymphocytes was decreased when compared with normal mice. At the same time interval, treatment of EASM (50, 100 and 200 mg/kg) could change these parameters near to normal. Maximum and significant alteration occurred in the EASM treatment at the dose of 100 mg/kg (P<0.05). The differential counts were found to be similar to that of EAC cell bearing mice and treatment of EASM could not normalize the differential count.
Table 1. Effect of EASM on hematological parameters of EAC cell bearing mice (Mean±SEM) (mg/kg bw).
| Parameters | Treatment |
|||||
| Normal | EAC +Vehicle | EAC + EASM (50) | EAC + EASM (100) | EAC + EASM (200) | EAC + Bleomycin (0.3) | |
| Hgb (g/dL) | 15.48 ± 0.22 | 7.38 ± 0.18* | 7.73 ± 0.25 | 8.38 ± 0.19t | 7.13 ± 0.36 | 14.37 ± 0.25t |
| RBC(×109 cells/mL) | 5.67 ± 0.10 | 2.80 ± 0.27* | 3.27 ± 0.15 | 4.06 ± 0.16t | 3.12 ± 0.08 | 4.90 ± 0.09t |
| WBC(×106 cells/mL) | 8.75 ± 0.53 | 15.40 ± 1.19* | 13.60 ± 1.47 | 11.00 ± 0.78t | 12.40 ± 1.05 | 9.37 ± 0.59t |
| Lymphocytes (%) | 75.50 ± 1.36 | 37.80 ± 1.35* | 38.60 ± 1.13 | 34.30 ± 1.09 | 40.30 ± 0.91 | 68.20 ± 0.90t |
| Neutrophils (%) | 19.60 ± 1.38 | 60.70 ± 1.04* | 58.20 ± 1.05 | 61.70 ± 1.10 | 55.10 ± 0.72 | 28.80 ± 0.93t |
| Monocytes (%) | 1.87 ± 0.40 | 1.20 ± 0.38 | 1.65 ± 0.25 | 1.75 ± 0.31 | 1.52 ± 0.26 | 2.00 ± 0.27 |
*P<0.05, against normal group; tP<0.05, against EAC control group.
4. Discussion
The results of the present study clearly demonstrate the tumour inhibitory activity of EASM against EAC. The reliable criteria for evaluating an anticancer drug are prolongation of lifespan of the animal and decrease in WBC count of blood. Our results have shown an increase in lifespan accompanied by a reduction in WBC count in EASM treated mice. It had significant effect on increasing the life span of ascities tumour bearing animals and also found to reduce the viable EAC cells in animal models. These results clearly demonstrate the antitumour effect of EASM against EAC. During the process of cancer chemotherapy the major problems are myelosuppression and anaemia[12]. The anaemia encountered in tumour bearing mice is mainly due to reduction in RBC and hemoglobin and this may occur either due to iron deficiency or hemolytic or myelopathic conditions[13]. Treatment with EASM restored the hemoglobin content, RBC and WBC cell count to normal values. This indicates that EASM possesses protective effect on the haematopoietic system.
A regular and rapid increase in ascetic fluid volume was observed in EAC bearing mice. Ascetic fluid is the direct nutritional source for tumour growth because it meets the nutritional requirements of tumor cells[14]. EASM treatment reduced the number of viable cancer cell count and increased the lifespan. It may be suggested that EASM can reduce the nutritional fluid volume and thereby arrests tumour growth and increases the life span.
Preliminary phytochemical screening indicated the presence of triterpenoids, flavonoids and glycosides in EASM. These compounds are known to possess potent antitumor properties[15]–[19]. In addition, flavonoids could also induce mechanisms that may kill cancer cells and inhibit tumor invasion[20],[21]. The antitumour properties of EASM may be due to these compounds. Further research work is needed to establish the exact antitumour mechanism action of EASM as well as identify the main active phytochemicals responsible for the inhibition of EAC.
Acknowledgments
The authors are thankful to Indian Institute for Chemical Biology (IICB), Kolkata, India for providing Ehrlich ascites carcinoma (EAC) cells to carry out the research.
Footnotes
Foundation Project: Supported by Faculty of Science, Rajshahi University, Bangladesh (No. 662-5/52/UGC/Science(2)/2010).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Nadkarni AK. Indian materia medica. Bombay: Popular Prakashan; 2005. pp. 972–973. [Google Scholar]
- 2.Radha R, Kavimani S, Ravichandran V. Antitumour activity of methanolic extract of Plumeria alba L. leaves against Dalton lymphoma ascites in mice. Int J Health Res. 2008;1:79–85. [Google Scholar]
- 3.Ghani A. Medicinal plants of Bangladesh: chemical constituents and uses. Dhaka: Asiatic Society of Bangladesh; 2003. p. 292. [Google Scholar]
- 4.Patricia LDM, Maria RAM, Luis COL, José DA, Ricardo EA, José DS. Cell wall biochemistry of sapodilla (Manilkara zapota) submitted to 1-methylcyclopropene. Braz J Plant Physiol. 2008;20:85–94. [Google Scholar]
- 5.Chanda SV, Nagani KV. Antioxidant capacity of Manilkara zapota L. leaves extracts evaluated by four in vitro methods. Nat Sci. 2010;8:260–266. [Google Scholar]
- 6.Nair R, Chanda S. Antimicrobial activity of Terminalia catappa, Manilkara zapota and Piper betel leaf extract. Indian J Pharm Sci. 2008;70:390–393. doi: 10.4103/0250-474X.43012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaneria M, Baravalia Y, Vaghasiya Y, Chanda S. Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra region, India. Indian J Pharm Sci. 2009;71:406–412. doi: 10.4103/0250-474X.57289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma J, Luo XD, Protiva P, Yang H, Ma C, Basile MJ, et al. et al. Bioactive novel polyphenols from the fruit of Manilkara zapota L. (Sapodilla) J Nat Prod. 2003;66:983–986. doi: 10.1021/np020576x. [DOI] [PubMed] [Google Scholar]
- 9.Lorke DA. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 10.Sur P, Ganguly DK. Tea plant root extract (TRE) as an antineoplastic agent. Planta Med. 1994;60:106–109. doi: 10.1055/s-2006-959427. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee KL. Medical laboratory technology. New Delhi: Tata Mcgraw Hill Publishing Company Limited; 1988. pp. 218–280. [Google Scholar]
- 12.Hirsch J. An anniversary for cancer chemotherapy. JAMA. 2006;296:1518–1520. doi: 10.1001/jama.296.12.1518. [DOI] [PubMed] [Google Scholar]
- 13.Hogland HC. Heamatological complications of cancer chemotherapy. Semin Oncol. 1982;9:95–102. [PubMed] [Google Scholar]
- 14.Shimizu M, Azuma C, Taniguchi T, Murayama T. Expression of cytosolic phospholipase A2α in murine C12 cells, a variant of L929 cells, induces arachidonic acid release in response to phorbol myristate acetate and Ca2+ ionophores, but not to tumor necrosis factor-α. J Pharm Sci. 2004;96:324–332. doi: 10.1254/jphs.fpj04033x. [DOI] [PubMed] [Google Scholar]
- 15.Kintzios SE. Terrestrial plant derived anticancer agents and plants used in anticancer research. Crit Rev Plant Sci. 2006;25:79–113. [Google Scholar]
- 16.Viswanatha GLS, Vaidya SK, Ramesh C, Krishnadas N, Rangappa S. Antioxidant and antimutagenic activities of bark extract of Terminalia arjuna. Asian Pac J Trop Med. 2010;3(12):965–970. [Google Scholar]
- 17.Kannan RRR, Arumugam R, Anantharaman P. In vitro antioxidant activities of ethanol extract from Enhalus acoroides (L.F.) Royle. Asian Pac J Trop Med. 2010;3(11):898–901. [Google Scholar]
- 18.Kumar BSA, Lakshman K, Jayaveera KN, Shekar DS, Kumar AA, Manoj B. Antioxidant and antipyretic properties of methanolic extract of Amaranthus spinosus leaves. Asian Pac J Trop Med. 2010;3(9):702–706. [Google Scholar]
- 19.Melinda KP, Rathinam X, Marimuthu K, Diwakar A, Ramanathan S, Kathiresan S, et al. et al. A comparative study on the antioxidant activity of methanolic leaf extracts of Ficus religiosa L, Chromolaena odorata (L.) King & Rabinson, Cynodon dactylon (L.) Pers. and Tridax procumbens L. Asian Pac J Trop Med. 2010;3(5):348–350. [Google Scholar]
- 20.De Sousa RR, Queiroz KC, Souza AC, Gurgueira SA, Augusto AC, Miranda MA, et al. et al. Phosphoprotein levels, MAPK activities and NFkappaB expression are affected by fisetin. J Enzyme Inhib Med Chem. 2007;22:439–444. doi: 10.1080/14756360601162063. [DOI] [PubMed] [Google Scholar]
- 21.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon. Free Radic Biol Med. 2006;41:1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]