Abstract
Objective
To generate life table characteristics for the dengue vector Aedes albopictus (A. albopictus) under uncontrolled conditions, incorporating both the aquatic and the adult stages.
Methods
Ten females derived from wild pupae were allowed to fully blood-feed on restrained mice. 774 eggs were hatched in seasoned water. F1 larvae were followed for development until their F2 counterparts emerged as adults. Some population parameters were monitored (F1) or estimated (F2).
Results
A. albopictus exhibited increased fecundity and egg hatch success. Immature development was quick. Immature survival was high, with lowest rate in the pupal stage. Adult emergence was about 81% and sex ratio was close to 1:1. Generational mortality (K) was about 28%. A high proportion of females completed a reproductive cycle and the obtained parity rate was predicted to lead to higher fecundity in the next generation.
Conclusions
It can be concluded that natural A. albopictus populations in Penang seem largely determined by quick development in combination with low immature loss and increased oviposition.
Keywords: Aedes albopictus, Biology, Population parameters, Life table, Life budget
1. Introduction
Aedes albopictus (A. albopictus) is a daytime biting mosquito[1] that transmits a variety of viruses[2]. It has also been reported that it is capable of ovarian[3] transmission, but in most Aedes vectors, the general route that A. albopictus transmits arboviruses to humans is through a female bite. Upon feeding on an infected host, the ingested virus needs a certain period of time to enter the midgut epithelium, replicate in it and after a while its viral particles are transferred to the salivary glands via the haemolymph[4]. In A. albopictus, dengue virus particles reach the salivary glands 10 days after an infectious blood meal is ingested[5]. In case the female does not live long enough, the virus will not complete development and cannot be transferred to a new host. Thus, the longevity of the female is a crucial factor for vector competence. Many factors come into play when the vectorial competence of a mosquito is considered. The ability to be a competent vector depends on various physical and physiological components associated with life history and demographic traits. In particular, stage specific survivorship[7], fecundity[8] and abundance[9] are critical to disease transmission.
According to some mosquito workers[10], establishment of the demographic parameters of pest species in the form of life tables, provides a basic foundation for developing effective control strategies. Demography is the study of population statistics, including births, deaths, immigration and emigration[11]. A life table depicts the development, survival, and fecundity of a given population and supplies basic data on population increase parameters[12]. Due to the crucial importance of vector demographics on mosquito-borne diseases, many studies have been done addressing the use of life-table models to determine survivorship and population growth potential of mosquitoes[13],[14]. Most of studies have used laboratory data collected under controlled conditions, known to provide maximal growth potentials that may not occur in nature[15]. Recently, a few entomologists have attempted utilizing field data for the development of life tables of Anopheles mosquitoes[16].
In Malaysia, a recent study[17] has developed life table statistics of the dengue vector A. albopictus using data collected from a wooded area, but the authors ignore the adult stage. Adult survivorship is central to demographics of any animal population. To address this issue, Morrison and Pollock[11] argued for the need to estimate adult survival as this provides a preliminary and basic indication of the status of the population. He also claimed that quantifying female survivorship has the potential to give information regarding population persistence. This information is also important in dengue epidemics, as dengue viruses can be transmitted transovarially[3].
A. albopictus has been occasionally implicated in dengue epidemics in many parts of Asia[18],[19]. In Malaysia, as per September 2010, dengue viruses have caused 35 533 people to get sick and this resulted in 107 deaths[20]. This epidemic may be a preliminary indicator of more serious disease outbreaks, particularly involving A. albopictus, which now has established populations within peoples' houses in Penang[21]. An effective strategy to reverse this trend would be the capability to predict Aedes vector population size in time. Therefore, our goal is to generate life table statistics of A. albopictus under uncontrolled conditions.
2. Materials and methods
2.1. Survey of A. albopictus
In this study, the A. albopictus were collected as pupae in Penang State, Malaysia. Sampling was conducted within an urban type premise of University Sains Malaysia (USM) (between latitude 5°8′N - 5°35′N and longitude 100°8′E - 100°32′E). The climate of this region is typically equatorial, with a mean annual rainfall of about 2 600 mm. The mean maximum daily ambient temperature and relative humidity are 35 °C and 96%, respectively, while minimum values are 23 °C and 60%[22]. The premise comprises administrative buildings, parking sites, restaurants and is associated with wooded areas formed of trees and herbaceous plants. The area has been used for population studies of A. albopictus[23]. Following a preliminary survey, which has revealed that A. albopictus makes up the majority of the mosquito populations, we therefore set up ovitraps (plastic containers) throughout the area at shaded sites to maximize attractiveness to ovipositing females. Four weeks later, pupae were collected from ovitraps, placed into plastic bags holding seasoned tap water and brought to the insectarium of the School of Biological Sciences, USM. The insectarium has 5 rooms each with a large window (3.0 m×3.7 m×3.0 m). Here, the usual environmental conditions are: temperature (29±3) °C T, (75±10)% RH and photoperiod 13 D: 10 L, 1 h dusk.
2.2. The obtaining of experimental mosquitoes
A sample of one hundred pupae were divided into four groups, each placed in porcelain bowl (diameter 10 cm and height 5 cm). The four bowls holding pupae were singly transferred into standard mosquito rearing cages (30 cm × 30 cm × 30 cm). F1 adults (males and females) that had emerged within a three-day age range were given access to a sucrose source supplied by a cotton wick soaked in a mixture of 10% glucose and vitamin B-complex solution. To avoid any fungal or bacterial growth, cotton wicks were replaced every three days. Two-day-old F1 females were blood-fed overnight on restrained mice starting from around 6-7 pm.
2.3. Experimental design
This experiment was carried out using procedures modified from Chadee[24] in the insectarium, but the windows were maintained opened to allow natural fluctuations of its environmental conditions. Twenty F1 gravid females were placed singly in oviposition cages consisting of glass jars (diameter 10 cm and height 15 cm) covered with thin nylon clothes. Each cage was equipped with a small bottle filled with cotton wick soaked in the sucrose solution and an oviposition apparatus. The apparatus consisted of a small plastic container (diameter 3 cm and height 2 cm) half-filled with seasoned water and holding a cone-shaped piece of filter paper as oviposition substrate.
Filter papers were removed from glass jars and allowed to dry up under insectarium conditions. Immediately after filter paper retrieval, jars were covered with thin clothes to prevent undesired egg depositions. The ten dried filter papers holding eggs were transferred each into a new jar half filled with seasoned water and followed for larval eclosion and pupations. Pupae from each of the ten jars were transferred into a plastic container (15 cm × 8 cm× 7 cm) half-filled with seasoned tap water. The ten containers with pupae were each placed into mosquito rearing cages and followed for development. F2 males and females that eclosed within a three-day period were provided a source of carbohydrates as done with their F1 counterparts. Upon the end of pupation, data on survival of immature stages and adult (males and females) emergence in each of the populations derived from the ten jars were monitored.
We dissected ovaries under microscope (Meiji EMZ; Meiji Techno Co. Ltd, Tokyo, Japan) from F1 females as follows. The ends of the seventh abdominal segments were incised and the ovaries slowly pulled out. Dissections were performed in droplets of distilled water and the dissected ovaries were allowed to dry. Dried ovaries were used for parity analysis using procedures from Detinova[14]. Females with coiled tracheolar endings (skeins) were considered as nulliparous and those with uncoiled skeins, as parous.
2.4. Data collection and analysis
The population parameters measured were oviposition, egg hatch, developmental period, mortality, pupation and fecundity. The numbers of eggs laid by each female were counted under a dissecting microscope. Egg hatch was monitored 24 h after flooding by counting the number of first instars larvae. Larval developmental period corresponded to the number of days from egg hatch till the day of adult emergence. The number of eggs deposited was used as the score of fecundity. Percent egg hatch was calculated as follows: number of eggs that hatched divided by the initial number of eggs flooded multiplied by 100. Mortality of larvae (or pupae) was calculated by dividing the total number of dead larvae (or pupae) by the initial number of larvae (first, second, third and fourth instars) (or pupae) multiplied by 100. A life budget was constructed using some data from the life table. As Aedes mosquitoes are K factor reproducing animals, physiological statuses that do not reproduce were considered as mortality factors. These include dead individuals (larvae or pupae), adult males and nulliparous females. Following calculation procedures from others e.g., Okogun[13], the logarithm total numbers of dead individuals, males and nulliparous females were considered as k1, k2 and k3, respectively. The sum of these different mortality factors was regarded as generational mortality.
3. Results
3.1. Overview of the life table
Table 1 summarized the life table characteristics of A. albopictus from northern Malaysia. The study started with 774 eggs, which represented the number of eggs produced during the first gonotrophic cycle of 10 females. The different population parameters resulted from experiments were carried out under varying environmental conditions (daily mean temperature: from 27.6 °C to 32.0 °C and mean relative humidity: from 61.0% to 80.6%).
Table 1. Population parameters of A. albopictus raised under uncontrolled laboratory conditions based on the fate of 10 female ovipositions (Mean ± SDEV).
| Stage | lx | Mortality factors | dx | Mortality (%) |
| Eggs | 774 | Failed to hatch | 94 | 12.14 ± 0.80 |
| Larvae | 680 | Endogenous causes | 119 | 15.37 ± 1.40 |
| Pupae | 561 | Endogenous causes | 29 | 0.26 ± 0.90 |
| Adults | 559 | |||
| ♂ | Drowned | 2 | ||
| ♀ | Drowned | 6 | ||
| Total | 8 | 1.03 ± 0.20 | ||
| Emerged adults | 551 | |||
| ♂ | 281 | - | - | - |
| ♀ | 270 | - | - | - |
| Real mortality | 28.81 ± 2.20 |
lx stands for the number of individuals surviving to a given developmental stage x; dx indicates the number dead individuals during a given developmental.
Table 2. Fecundity of A. albopictus females maintained under uncontrolled laboratory conditions (Mean ± SDEV).
| Replicate (female) | Eggs produced | Emerged adults |
|
| ♂ | ♀ | ||
| 1 | 95.7 ± 54.5 | 33.7 ± 23.5 | 37.3 ± 27.9 |
| 2 | 57.7 ± 38.6 | 20.3 ± 16.6 | 28.3 ± 26.3 |
| 3 | 70.0 ± 54.3 | 31.7 ± 27.4 | 28.7 ± 27.6 |
| 4 | 70.7 ± 45.6 | 17.3 ± 17.0 | 16.0 ± 14.2 |
| 5 | 61.7 ± 35.2 | 27.0 ± 16.1 | 26.0 ± 15.9 |
| 6 | 112.7 ± 8.5 | 41.7 ± 16.5 | 37.7 ± 9.5 |
| 7 | 122.0 ± 5.29 | 44.0 ± 13.1 | 43.3 ± 9.0 |
| 8 | 36.0 ± 28.9 | 8.7 ± 7.6 | 9.0 ± 9.5 |
| 9 | 68.7 ± 14.0 | 28.3 ± 15.3 | 19.7 ± 7.6 |
| 10 | 78.7 ± 26.6 | 28.3 ± 10.5 | 24.0 ± 7.0 |
| Total | 773.7 ± 153.1 | 281.0 ± 58.4 | 270.0 ± 56.3 |
3.2. Population parameters
The proportion of eggs that hatched was 87.8% (94/774). A total of (15.37±1.40)% did not complete larval development. The developmental period from egg hatch to adult emergence was (6.3±0.8) days, with a maximum and minimum of 5 and 7 days, respectively. Among the 680 larvae that eclosed, a total of (0.26±0.90)% did not reach the pupal stage. The sex ratio was 1.04:1.00. Among the 270 females obtained, only 6 individuals were nulliparous.
3.3. Life budget
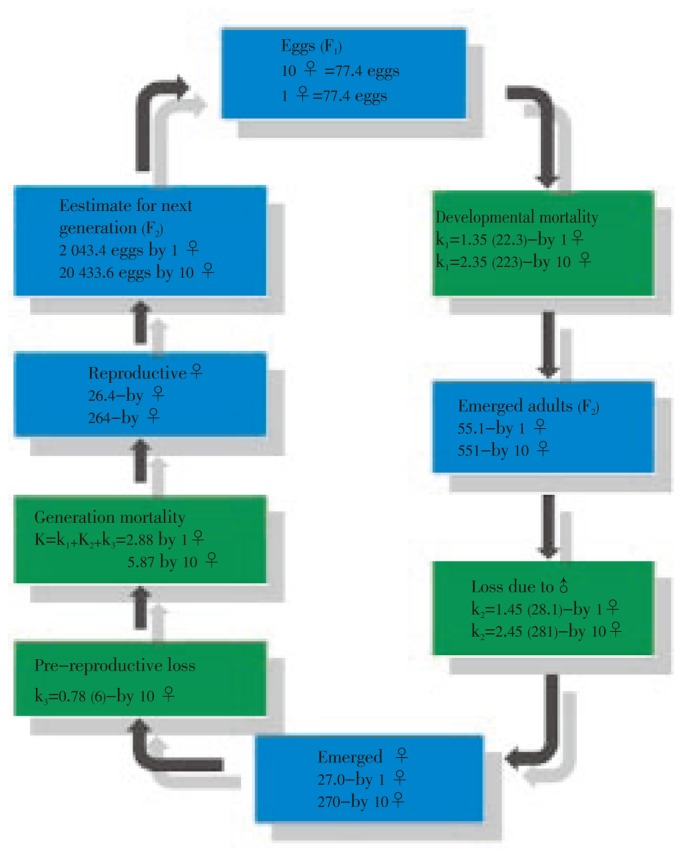
Information taken directly from the life table was used to construct a cyclic budget (Figure 1). The mortality (k1) from egg hatch to the pupal stage was 2.35 (F1). The loss of fecundity due to male mosquito emergence (k2) was 2.45. The pre-reproductive loss or (k3) was 0.78. Considering all causes of mortality, we obtained a generation mortality (K) of 2.88 for a given female and of 5.87 for the ten female used. Mortality during larval development was the factor that most contributed to to generational mortality. According to the information that a proportion of (97.8±2.4)% (264/270) of F1 was parous, we deducted that one F1 parous, would produce on average (77.4±26.0) eggs. Assuming that the same parity rate will occur in the next generation, the total number of eggs produced by would be 2 043.4. Therefore, 264 F2 parous females have the potential to produce 20 433.6 new eggs at their first gonotrophic cycles.
Figure 1. Life budget for A. albopictus in Penang using logarithmetic values at each stage.
4. Discussion
The mean number of eggs laid per female A. albopictus was 77.4. In this species, lower fecundity has been antecedently reported. Gubler[25] assessed fecundity of this species using four different host animals (chicken, guinea pig, mouse and rat). He found that the mean number of eggs laid per A. albopictus female in the first gonothrophic cycle ranged from 51.8 to 71.8 depending on the blood source. He also found that more eggs resulted from females blood fed on a mouse. This fecundity variation due to blood meal source has been well studied by Xue et al[26] who obtained a fecundity of 67, 80, 82 eggs/female when blood meals were taken from chicken, guinea pig and human, respectively. Recently, fecundity was found to be about 67 eggs per female in A. albopictus fed on mice[21]. Referring to these reports, it seems likely that A. albopictus in the present study has increased fecundity.
Among the eggs laid, a proportion of about 12% failed to hatch. There has been an ample body of works examining the causes of egg hatch failure in mosquitoes. Immaturity due to a non-insemination of a teneral female has been shown to have a major effect. Flooding patterns have also been reported influential to egg hatch. In fact, the newly laid eggs of A. albopictus as any other Aedes mosquitoes, remain in a state of dormancy until flooding and environmental stimuli activate the pharate larvae[27]. Upon first flooding, only a fraction of the eggs hatch. Some require further subsequent hatch stimuli inundations before hatching. This variability in hatching time has been documented in the species studied here[28],[29]. This pattern of larval eclosion, known as erratic hatching is suggested as an evolutionary response to potential competitive interactions[30] and environmental adversities[31]. In fact, egg hatching patterns in container-breeding Aedes are modulated by the interactions temperature-larval environment and this has been well developed by Khatchikian et al[32]. According to these authors, in populations facing low desiccation risks, where rainfall is abundant and/or consistent, most of the egg hatching occurs rapidly, in few stimuli, disregarding temperature effects. Under high temperature conditions, where few eggs hatch in the first stimulus, the majority do hatch on few subsequent stimuli. On the other hand, in desiccation prone habitats, high temperature could play a more relevant role as it would increase evaporation rates, and thus, risk. In the present study, eggs were flooded only once and these eggs came from A. albopictus from Penang Island, an area known to have high levels of rainfall associated with high temperatures. With reference to the previous reports and our experimental settings, it is likely that A. albopictus egg hatch success is relatively high in this Island. It is possible that the increased larval eclosion occurs in nature to compensate for larval population loss that may result from unpredictable adversities i.e., rainfall and warmness. The increased larval eclosion will tend to result in high larval population density.
The developmental period of A. albopictus from egg to adult was about 6.3 days. It is well-known that following eclosion, larvae enter a phagoperiod during which they grow by converting ingested nutrients into biomass[33]. The duration of this period depends on many environmental factors, with food and temperature playing major roles[34],[35]. In our experiment food was supplied in optimum amounts, and evidence exists that under such nutritional conditions, larval developmental period vary from 5 to 10 days[36]. Monteiro et al[37] recorded no adult emergence when immature stages of A. albopictus were maintained at 35 °C and concluded that the limit temperature for the development of its larval stages is close to 35 °C. Our result is consistent with a similar previous study in Penang[17] in indicating a short larval period of A. albopictus, although the environmental conditions are not identical. This study acquired data from laboratory settings where environmental conditions were controlled. Thus, our result adds to the body of knowledge that relates to A. albopictus development under field conditions. The short mean time of 6.3 days for development of the A. albopictus larvae has important epidemiological implications. Clearly, a rapid development will favor high pupal population size. With a quick development, larvae are less subjected to parasite infections and predation as well as desiccation risks. The fast development is also likely to taint body size of pupae, because larvae may not have enough time to accumulate sufficient nutrients. A quick larval development is habitually supposed to be advantageous since it may result in low levels of water-borne substances, probably excretory products, toxic waste and crowding chemicals[38].
The mortality of larvae was high compared with that of the pupal stage. A similar observation has been reported by previous studies. Working with the Penang strain of A. albopictus, Hashim et al[17] observed a lower mortality during larval development when compared with the pupal period. Although this discrepancy in survival between the larval and pupal stages of A. albopictus is not yet clear, they could be explained on the basis of a difference in physiological traits of the two developmental stages. As in all mosquito species, A. albopictus pupae do not feed, while its larvae eat. In container ecosystems, food particles (i.e., micro-organisms and organic matters), the source of all larval nutritional requirements, are scattered throughout the water body. To survive, larvae must accumulate enough nutrients, whereas pupae do not need to do so. Therefore, the greater mortality of larvae could be related to food availability during larval development. The increased pupal survival is also likely to boost the population density of the mosquito.
The parity rate of A. albopictus during this study was about 87%, which means that some females did not complete one gonotrophic cycle. As this mosquito is anautogenous, females need a blood meal to produce eggs. The initial flight to find a blood source does not occur just after emergence. Newly emerged females must wait for a certain period of time before expressing host-seeking behaviors. The length of this waiting period is closely associated with the nutritional status of the females. Those with poor nutrient reserves take a longer time to initiate blood meal search[39]. With reference to these statements, it is likely that the parous females in the current study had better nutritional features, since all females were given the same blood feeding opportunities. The parity rate was considerably high, which suggests that there will be a relatively high population density in the next generation. In support of this suggestion, it has been estimated that 264 F2 parous females may produce 20 433.6 new eggs at their first gonotrophic cycles.
This study was carried out primarily to determine some demographic parameters of A. albopictus during the aquatic and adult stages in view of having some insights into its population potential increase in the field. Under uncontrolled conditions of temperature and humidity, A. albopictus showed increased egg hatch, a short aquatic life, increased immature survival and fecundity. These attributes may boost the population size of the adults. In Penang, A. albopictus is present in large numbers throughout the year. Therefore, it seems likely that the persistence of its populations in nature is largely sustained by these boosting attributes. In this study, we blood fed all females, as such we are not sure to state whether higher parity was a consequence of higher mosquito survivorship. Additional studies are required to survey parity in nature.
The current study was performed simulating natural settings by not controlling environmental conditions i.e., humidity and temperature. However, we did not take into consideration natural enemies and this may sound as a drawback to our study. A. albopictus commonly domiciles in the protozoan parasite Ascogregarina taiwanensis, which can cause high mortality[40]. This mosquito species has been shown to have increased escape ability against Ascogregarina taiwanensis due to the fact that the parasitism of the gregarine is low in newly colonized areas for a long period of time[41]. In the present study, no deliberate effort was made to find out whether experimental mosquitoes harbored gregarines. In nature, the dynamics of mosquito populations are also precluded by interactions with predators. Aedes larvae are naturally very active[42] and when faced with predators, they often alter their behavior to reduce the risk of being killed[42]. Immature stages of A. albopictus were commonly observed associated with Odonata larvae during surveys throughout Penang Island in 2008 and 2009, mostly in rural areas. Immature populations were heterogeneous and most developmental stages were present. Although we did not give consideration to predation, the positive associations with Odonata predators are likely to suggest limited predatory effects. Also our study was conducted in urban settings, which mimic well the absence of A. albopictus-Odonata associations in most Penang's urban sites. Additional studies are needed to examine gregarine parasitism in A. albopictus Penang strain.
Acknowledgments
Warm thanks to the team of the Vector Control Unit of the School of Biological Sciences, Universiti Sains Malaysia. This work was supported in part from by grants from the Universiti Sains Malaysia (# 07-05-16-MG1-GM15, # 1001/PBIOLOGI/842004).
Footnotes
Foundation Project: Supported by grants from Universiti Sains Malaysi (No: # 07-05-16-MG1-GM15, # 1001/PBIOLOGI/842004).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Benedict MQ, Levine RS, Hawley WA, Lounibos P. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007;7(1):76–85. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delatte H, Desvars A, Bouétard A, Bord S, Gimonneau G, Vourc'h G, et al. et al. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector Borne Zoonotic Dis. 2009;10:249–258. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- 3.Joshi V. Letter to the editor. Am J Trop Med Hyg. 2003;68:267. [Google Scholar]
- 4.Luplertlop N, Surasombatpattana P, Patramool S, Dumas E, Wasinpiyamongkol L, Saune L, et al. et al. Induction of a peptide with activity against a broad spectrum of pathogens in the Aedes aegypti salivary gland, following infection with dengue virus. PLoS Pathog. 2011;7(1):e1001252. doi: 10.1371/journal.ppat.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho BC, Chan KL, Chan YC. Aedes aegypti (L) and Aedes albopictus (Skuse) in Singapore city. 3. Population fluctuations. Bull World Health Organ. 1971;4:636–641. [PMC free article] [PubMed] [Google Scholar]
- 7.Sumanochitrapon W, Danial S, Sithiprasana R, Kittayapong P, Innis BL. Effects of size and geographic origin of Aedes aegypti on oral infection with dengue virus-2. Am J Trop Med Hyg. 1998;58:283–286. doi: 10.4269/ajtmh.1998.58.283. [DOI] [PubMed] [Google Scholar]
- 8.Xue RD, Barnard DR, Ali A. Influence of multiple blood meals on gonotrophic dissociation and fecundity in Aedes albopictus. J Am Mosq Control Assoc. 2009;25(4):504–507. doi: 10.2987/09-5912.1. [DOI] [PubMed] [Google Scholar]
- 9.Arunachalam N, Susilowati TS, Espino F, Kittayapong P, Abeyewickreme W, Wai KT, et al. et al. Eco-bio-social determinants of dengue vector breeding: a multicountry study in urban and periurban Asia. Bull World Health Organ. 2010;88(3):173–184. doi: 10.2471/BLT.09.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seawright JA, Haile DG, Rabbani MG, Weidhaas DE. Computer simulation of the effectiveness of male-linked translocations for the control of Anopheles albimanus Weidhmann. Am J Trop Med Hyg. 1979;28:155–160. doi: 10.4269/ajtmh.1979.28.155. [DOI] [PubMed] [Google Scholar]
- 11.Morrison ML, Pollock KH. Development of a practical modelling framework for estimating the impact of wind technology on bird populations. Colorado: National Renewable Energy Laboratory; 1997. [Google Scholar]
- 12.Ma ZS, Bechinski EJ. Life tables and demographic statistics of Russian wheat aphid (Hemiptera: Aphididae) reared at different temperatures and on different host plant growth stages. Eur J Entomol. 2009;106(2):205–210. [Google Scholar]
- 13.Okogun GRA. Life-table analysis of Anopheles malaria vectors: generational mortality as tool in mosquito vector abundance and control studies. J Vector Borne Dis. 2005;42(2):45–53. [PubMed] [Google Scholar]
- 14.Detinova TS. Age grouping methods in Diptera of medical importance with special reference to some vectors of malaria. Monogr Ser World Health Organ. 1962;47:13–191. [PubMed] [Google Scholar]
- 15.Grimstad PR, Haramis LD. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus III. Enhanced oral transmission by nutrient deprived mosquitoes. J Med Entomol. 1984;21:249–265. doi: 10.1093/jmedent/21.3.249. [DOI] [PubMed] [Google Scholar]
- 16.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Life-table analysis of Anopheles arabiensis in Western Kenya highlands: effects of land covers on larval and adult survivorship. Am J Trop Med Hyg. 2007;77(4):660–666. [PubMed] [Google Scholar]
- 17.Hashim NA, Ahmad AH, Rawi CS, Tahir NA, Basari N. Life tables study of immature Aedes albopictus (Skuse) (Diptera: Culicidae) during the wet and dry seasons in Penang, Malaysia. Southeast Asian J Trop Med Public Health. 2008;39(1):39–47. [PubMed] [Google Scholar]
- 18.Lian CW, Seng CM, Chai WY. Spatial, environmental and entomological risk factors analysis on a rural dengue outbreak in Lundu district in Sarawak, Malaysia. Trop Biomed. 2006;23(1):85–96. [PubMed] [Google Scholar]
- 19.Malavige GN, Fernando S, Fernando DJ, Seneviratne SL. Dengue viral infections. Postgrad Med J. 2004;80:588–601. doi: 10.1136/pgmj.2004.019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Dengue in the Western Pacific Region. [Online] Available from: http://www.wpro.who.int/health_topics/dengue/ [Accessed on 20 Oct, 2010]
- 21.Dieng H, Saifur RGM, Hassan AA, Salmah MRC, Boots M, Satho T, et al. et al. Indoor-breeding of Aedes albopictus in Northern peninsular Malaysia and its potential epidemiological implications. PLoS ONE. 2010;5(7):e11790. doi: 10.1371/journal.pone.0011790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad F, Ahmad SY, Farooqi MA. Characterization and geotechnical properties of Penang residual soils with emphasis on landslides. Am J Environ Sci. 2006;2:121–128. [Google Scholar]
- 23.Nur Aida H, Abu Hassan A, Nurita AT, Che Salmah MR, Norasmah B. Population analysis of Aedes albopictus (Skuse) (Diptera: Culicidae) under uncontrolled laboratory conditions. Trop Biomed. 2008;25:117–125. [PubMed] [Google Scholar]
- 24.Chadee DD. The life table characteristics of a selected laboratory population of Aedes aegypti (L) J Florida Mosq Control Assoc. 1992;63(2):68–72. [Google Scholar]
- 25.Gubler DJ. Comparison of reproductive potentials of Aedes (Stegomyia) albopictus Skuse and Aedes (Stegomyia) polynesiensis Marks. Mosq News. 1970;30:201–208. [Google Scholar]
- 26.Xue RD, Barnard DR, Ali A. Influence of multiple blood meals on gonotrophic dissociation and fecundity in Aedes albopictus. J Am Mosq Control Assoc. 2009;25(4):504–507. doi: 10.2987/09-5912.1. [DOI] [PubMed] [Google Scholar]
- 27.Novak RJ, Shroyer DA. Eggs of Aedes triseriatus and A. hendersoni: a method to stimulate optimal hatch. Mosq News. 1978;38:515–521. [Google Scholar]
- 28.Dieng H, Boots M, Tamori N, Higashihara J, Okada T, Kato K, et al. et al. Some technical and ecological determinants of hatchability in Aedes albopictus, potential candidate for transposon-mediated transgenesis. J Am Mosq Control Assoc. 2006;22(3):382–389. doi: 10.2987/8756-971X(2006)22[382:STAEDO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Rahman GMS, Dieng H, Hassan AA, Satho T, Miake F, Boots M, et al. et al. The effects of moisture on ovipositional responses and larval eclosion of Aedes albopictus. J Am Mosq Control Assoc. 2010;26(4):373–380. doi: 10.2987/10-6003.1. [DOI] [PubMed] [Google Scholar]
- 30.Dieng H, Boots M, Tamori N, Higashihara J, Okada T, Kato K, et al. et al. Effects of food, embryo density and conspecific immatures on hatchability in the dengue vector Aedes albopictus. House Househ Insect Pest. 2007a;28:117–127. [Google Scholar]
- 31.Khatchikian CE, Dennehy JJ, Vitek CJ, Livdahl T. Climate and geographic trends in hatch delay of the treehole mosquito, Aedes triseriatus Say (Diptera: Culicidae) J Vector Ecol. 2009;34(1):119–128. doi: 10.1111/j.1948-7134.2009.00015.x. [DOI] [PubMed] [Google Scholar]
- 32.Khatchikian CE, Dennehy JJ, Vitek CJ, Livdahl T. Environmental effects on bet hedging in Aedes mosquito egg hatch. Evol Ecol. 2010;24(5):1159–1169. [Google Scholar]
- 33.Briegel H. Physiological bases of mosquito ecology. J Vector Ecol. 2003;28:1–11. [PubMed] [Google Scholar]
- 34.Dye C. Intraspecific competition amongst larval Aedes aegypti: food exploitation or chemical interference? Ecol Entomol. 2008;7(1):39–46. [Google Scholar]
- 35.Chang LH, Hsu EL, Teng HJ, Ho CM. Differential survival of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) larvae exposed to low temperatures in Taiwan. J Med Entomol. 2007;44(2):205–210. doi: 10.1603/0022-2585(2007)44[205:dsoaaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Hawley AW. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4(Suppl 1):1–40. [PubMed] [Google Scholar]
- 37.Monteiro LC, De Souza JR, De Albuquerque CM. Eclosion rate, development and survivorship of Aedes albopictus (Skuse) (Diptera: Culicidae) under different water temperatures. Neotrop Entomol. 2007;36:966–971. doi: 10.1590/s1519-566x2007000600021. [DOI] [PubMed] [Google Scholar]
- 38.Bédhomme S, Agnew P, Sidobre C, Michalakis Y. Pollution by conspecifics as a component of intraspecific competition among Aedes aegypti larvae. Ecol Entomol. 2005;30:1–7. [Google Scholar]
- 39.Renshaw M, Service MW, Birley MH. Host finding, feeding patterns and evidence for a memorized home-range of the mosquito Aedes cantans. Med Vet Entomol. 1994;8:187–193. doi: 10.1111/j.1365-2915.1994.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 40.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aliabadi BK, Juliano SA. Escape from gregarine parasites affects the competitive impact of an invasive mosquito. Biol Invasions. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dieng H, Satho T, Miake F, Eshita Y. Copepod predation and arbovirus control: potential thinning with focus on dengue epidemics. House Househ Insect Pest. 2007b;29:1–25. [Google Scholar]