Abstract
Objective
To determine the chemical profile and steroids composition of the medicinally important plant Aerva lanata (A. lanata) L.
Methods
Preliminary phytochemical screening was done by the method as Harborne described. HPTLC studies were carried out as Harborne and Wagner et al described. The Ethyl acetate-ethanol-water (8: 2: 1.2) was employed as mobile phase for glycosides.
Results
The desired aim was achieved using Chloroform-acetone (8: 2) as the mobile phase. The methanolic extract of stem, leaves, root, flower and seeds of A. lanata showed the presence of 30 different types of steroids with 30 different Rf values from 0.04 to 0.97. Maximum number (11) of steroids has been observed in leaves followed by root (10).
Conclusions
HPTLC profile of steroids has been chosen here to reveal the diversity existing in A. lanata. Such finger printing is useful in differentiating the species from the adulterant and act as biochemical markers for this medicinally important plant in the pharma industry and plant systematic studies.
Keywords: Steroids, HPTLC profile, Fingerprint, Aerva lanata L.
1. Introduction
Polpala [Aerva lanata (A. lanata) L.] is an important medicinal plant, found throughout tropical India as a common weed in fields and wasteland[1]. Even now, wild collection of the species continues to be a source of raw drug in Ayurvedic preparations. Because of its popularity in folk medicine, A. lanata has become the subject of intense pharmacological and chemical studies for the last 30 years. Numerous studies have proven its versatile pharmacological activities: anthelmintic, demulcent[2], anti-inflammatory[3], diuretic[4], expectorant, hepatoprotective[5], nephroprotective[6], anti-diabetic activity, anti-hyperglycaemic activity in rats[7],[8], anti-microbial, cytotoxic[9], urolithiatic[10], hypoglycemic, anti-hyperlipidaemic[11], anti-parasitic and anti-helmentic activities[12]. In order to identify the bioactive compounds responsible for the above pharmacological activities phytochemical studies have been carried out by several workers with different kinds of bioactive compounds particularly alkaloids such as: canthin-6-one and beta-carboline, aervine (10-hydroxycanthin-6-one), methylaervine (10-methoxycanthin-6-one), aervoside (10-β-Dglucopyranosyloxycanthin-6-one) and aervolanine (3-(6-methyoxy-β-carbolin-1-yl) propionic acid) from leaves of A. lanata[13]. The plants are distinct not only in their therapeutic properties but also in a variety of morphological characters, including those of root, stems, leaves, flower, pollen, etc. The main limitation in the use of traditional remedies is the lack of standardization of raw material, manufacturing process and the final product. A biomarker on the other hand is a group of chemical compounds which are not only unique for that plant material but also correlates with biological efficacy. So the need arises to lay standards by which the right material could be selected and incorporated into the formulation. HPTLC is a valuable tool for reliable identification. It can provide chromatographic fingerprints that can be visualized and stored as electronic images[14]–[19]. Many scientific documentations are available on crude drugs extracts, but promoting these herbal drugs in international / national market is difficult due to lack of reproducible biological reports, selection of wrong plants, lack of data on the time, area of collection and identity of the botanical source. In certain cases, the identity of the source material is confused, because the origin of particular drug is assigned to more than one plants, and sometimes has different morphological and taxonomical characters. Development and validation of analytical methods play important roles in the discovery, development and manufacture of pharmaceuticals. Significant exo-morphology, histo-morphology and physicochemical evaluation of the leaf and stem and micro-morphological studies of A. lanata have been worked out[20]. However, more work needs to be undertaken to fully characterize these compounds, identify the molecules with bioactive roles. Therefore, the current study was aimed to determine the chemical profile and steroids composition of A. lanata L, which will be useful for the proper identification of commercial samples.
2. Materials and methods
A. lanata was collected from natural habitats, Coimbatore District, Tamil Nadu, India, and authenticated by Dr. E.G. Wesely and the specimens voucher were deposited in the St. Xavier's College Herbarium for further reference. The fresh materials were shade dried and powdered using the electric homogenizer. The powdered samples were extracted with 150 mL of solvent methanol for 8 - 12 h by using the soxhlet apparatus. Preliminary phytochemical screening was done following the method of Harborne[21]. HPTLC studies were carried out following Harborne and Wagner et al[22]. For the present study CAMAG HPTLC system equipped with Linomat V applicator, TLC scanner 3, reprostar 3 with 12 bit CCD camera for photo documentation, controlled by WinCATS-4 software were used. All the solvents used for HPTLC analysis were obtained from MERCK. A total of 100 mg extract was dissolved in 5 mL of methanol and the solution was centrifuged at 3 000 rpm for 5 min and used for HPTLC analysis as test solution. The samples (5 µL) were spotted in the bands of width 5 mm with a Camag microlitre syringe on pre-coated silica gel glass plate 60F-254 (20 cm ×10 cm ×250 µm (E. Merck, Darmstadt, Germany) using a Camag Linomat IV (Switzerland). The plates were pre-washed by methanol and activated at 60 °C for 5 min prior to chromatography. The sample loaded plate was kept in TLC twin trough developing chamber (after saturated with Solvent vapor) with respective mobile phase (steroids) and the plate was developed up to 90 mm in the respective mobile phase. The Chloroform-acetone (8: 2) was employed as mobile phase for steroids. Linear ascending development was carried out in 20 cm x 10cm twin trough glass chamber (Camag, Mutenz, Switzerland) saturated with the mobile phase and the chromatoplate development for two times with the same mobile phase to get good resolution of phytochemical contents. The optimized chamber saturation time for mobile phase was 30 min at room temperature [(25± 2) °C]. The developed plate was dried by hot air to evaporate solvents from the plate. The developed plate was sprayed with anisaldehyde sulphuric acid reagent as spray reagent and dried at 100 0C in hot air oven for 3 min. The plate was photo-documented at UV 366 nm and daylight using Photo-documentation (CAMAG REPROSTAR 3) chamber. Finally, the plate was fixed in scanner stage and scanning was done at 366 nm. The plate was kept in photo-documentation chamber (CAMAG REPROSTAR 3) and captured the images under White light, UV light at 254 and 366 nm. Densitometric scanning was performed on Camag TLC scanner III and operated by CATS software (V 3.15, Camag).
3. Results
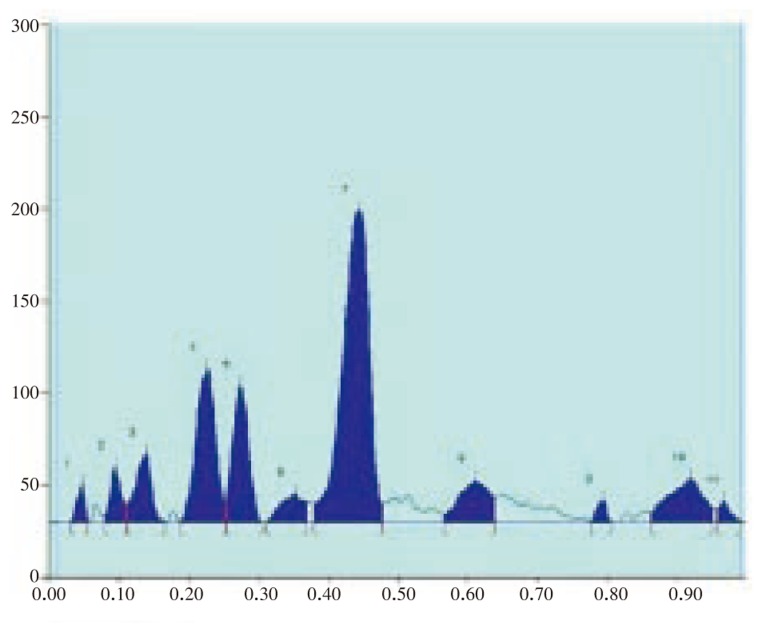
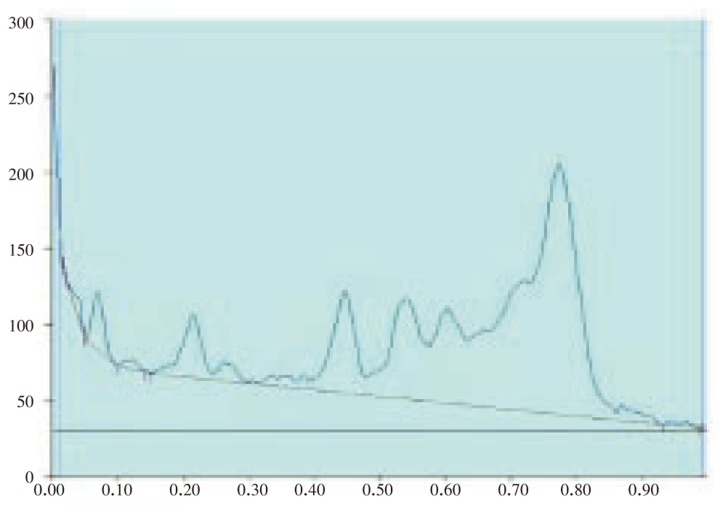
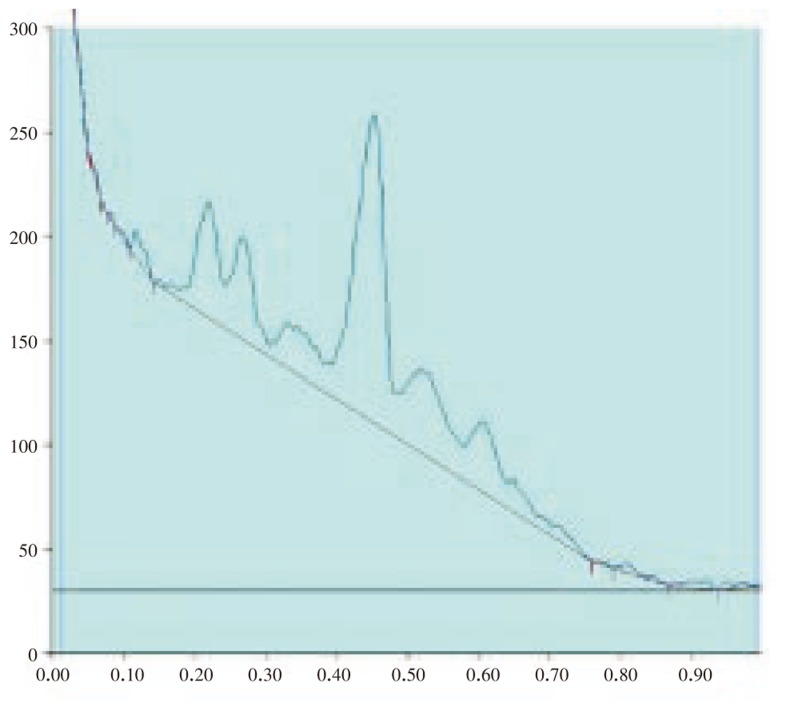
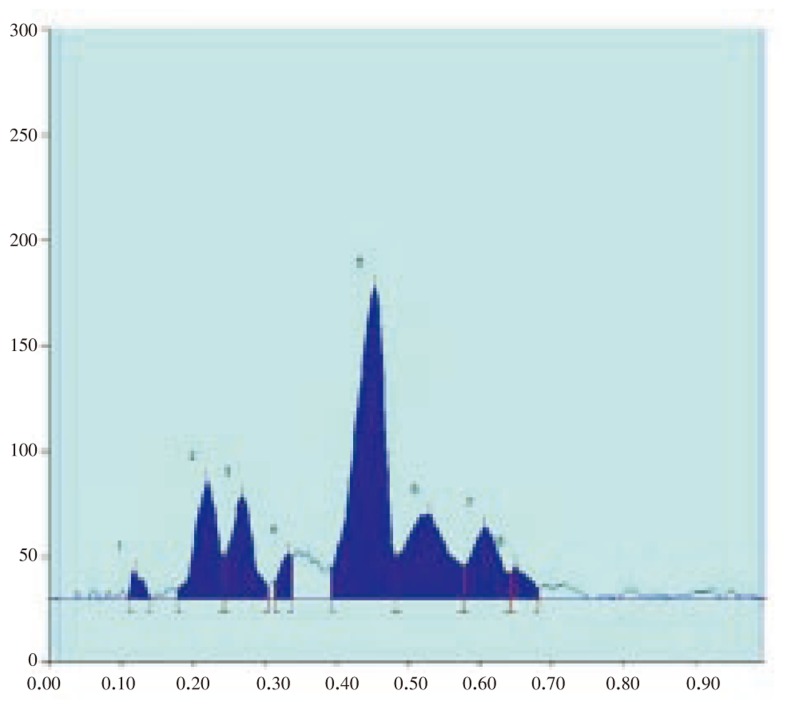
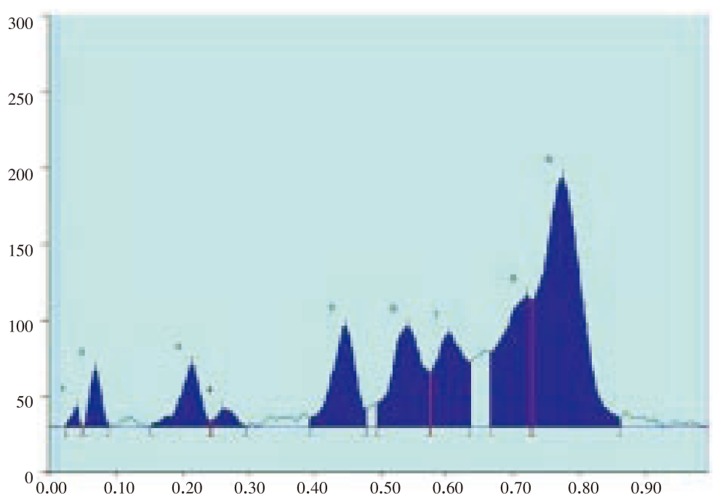
The results of the preliminary phytochemical studies confirmed the presence of steroids, alkaloids, glycosides, terpenoids and flavoniods in the methanolic extracts of A. lanata stem, leaves, root and reproductive parts. Different compositions of the mobile phase for HPTLC analysis were tested in order to obtain high resolution and reproducible peaks. The desired aim was achieved using chloroform-acetone (8: 2) as the mobile phase (Table 1–5; Figure 1–12). The methanolic extract of stem, leaves, root, flower and seeds of A. lanata showed the presence of 30 different types of steroids with 30 different Rf values from 0.04 to 0.97 (Table 1–5). In general more degree of steroids diversity has been observed in vegetative parts when compared with the reproductive part. Maximum number (11) of steroids has been observed in leaves followed by root (10). Among nine different steroids of reproductive parts (flowers and seeds), five steroids with Rf values 0.04, 0.07, 0.54, 0.72 and 0.77 were unique to reproductive parts only (Table 3). Eleven different types of steroids have been observed in leaves of A. lanata. Among eleven different steroids of leaves, 0.10, 0.14, 0.23, 0.28, 0.35, 0.80, 0.92 and 0.97 were unique to the leaves and they were not present in other vegetative and reproductive parts of the plant. The steroids with Rf values 0.12, 0.27 and 0.65 showed their unique presence only in the stem of A. lanata. Steroids with Rf value 0.11, 0.20, 0.25, 0.44, 0.48 and 0.71 were in stem. The steroids with the Rf value 0.33 and 0.53 are present commonly in root and stem of the plant. The Rf values 0.45 and 0.61 were in stem and leaves of A. Lanata, And the steroids with the Rf values 0.05 was expressed jointly in root and leaves of A. lanata.
Table 1. Steroids profile of the methanolic extracts of the root of A. lanata L.
| Peak | Rf | Height | Area | Assigned substance |
| 1 | 0.05 | 48.2 | 1 081.8 | Unknown |
| 2 | 0.11 | 29.3 | 468.6 | Unknown |
| 3 | 0.20 | 72.9 | 1 721.0 | Steroid 1 |
| 4 | 0.25 | 25.4 | 614.5 | Steroid 2 |
| 5 | 0.33 | 11.9 | 270.0 | Unknown |
| 6 | 0.44 | 97.5 | 4 684.9 | Steroid 3 |
| 7 | 0.48 | 44.5 | 1 088.8 | Steroid 4 |
| 8 | 0.53 | 70.7 | 2 868.2 | Steroid 5 |
| 9 | 0.60 | 39.9 | 1 754.2 | Unknown |
| 10 | 0.71 | 45.0 | 3 069.3 | Steroid 6 |
Table 5. Steroids profile of aerial and underground parts of A. lanata L.
| Rf | Root | Stem | Leaves | Flower | No. of Bands |
| 0.04 | + | 1 | |||
| 0.05 | + | + | 2 | ||
| 0.07 | + | 1 | |||
| 0.10 | + | 1 | |||
| 0.11 | + | 1 | |||
| 0.12 | + | 1 | |||
| 0.14 | + | 1 | |||
| 0.20 | + | - | 1 | ||
| 0.22 | + | + | 2 | ||
| 0.23 | + | 1 | |||
| 0.25 | + | 1 | |||
| 0.26 | + | 1 | |||
| 0.27 | + | 1 | |||
| 0.28 | + | 1 | |||
| 0.33 | + | + | 2 | ||
| 0.35 | + | 1 | |||
| 0.44 | + | 1 | |||
| 0.45 | + | + | + | 3 | |
| 0.48 | + | 1 | |||
| 0.53 | + | + | 2 | ||
| 0.54 | + | 1 | |||
| 0.60 | + | + | 2 | ||
| 0.61 | + | + | 2 | ||
| 0.65 | + | 1 | |||
| 0.71 | + | 1 | |||
| 0.72 | + | 1 | |||
| 0.77 | + | 1 | |||
| 0.80 | + | 1 | |||
| 0.92 | + | 1 | |||
| 0.97 | + | 1 |
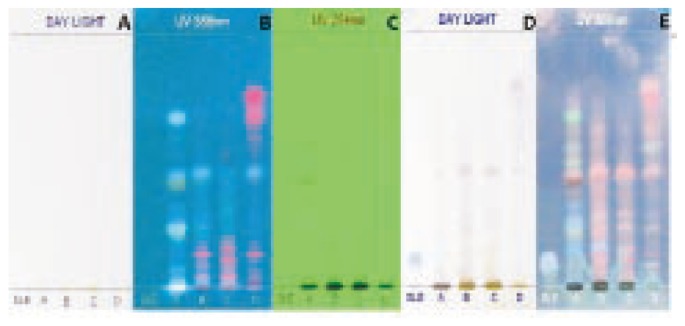
Figure 1. HPTLC profile of the methanolic extract of A. lanata.
A: under daylight; B: under UV 366; C: under UV 254; D: under daylight after derivation; E: under UV366 after derivation
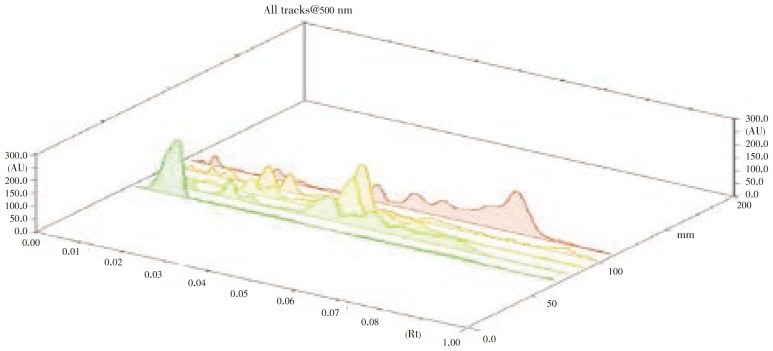
Figure 12. 3D display of HPTLC chromatogram of root, stem, leaves, flower and seeds of A. lanata.
Table 3. Steroids profile of the methanolic extracts of the leaf of A. lanata L.
| Peak | Rf | Height | Area | Assigned substance |
| 1 | 0.05 | 20.3 | 210.7 | Unknown |
| 2 | 0.10 | 30.2 | 437.2 | Unknown |
| 3 | 0.14 | 37.4 | 786.4 | Steroid 1 |
| 4 | 0.23 | 83.4 | 2 171.4 | Steroid 2 |
| 5 | 0.28 | 73.9 | 1 581.2 | Steroid 3 |
| 6 | 0.35 | 15.3 | 475.9 | Unknown |
| 7 | 0.45 | 169.0 | 6 053.6 | Steroid 4 |
| 8 | 0.61 | 22.8 | 908.8 | Unknown |
| 9 | 0.80 | 12.0 | 161.4 | Unknown |
| 10 | 0.92 | 23.5 | 1 096.3 | Unknown |
| 11 | 0.97 | 11.5 | 171.3 | Unknown |
Table 2. Steroids profile of the methanolic extracts of the stem of A. lanata L.
| Peak | Rf | Height | Area | Assigned substance |
| 1 | 0.12 | 12.9 | 192.9 | Unknown |
| 2 | 0.22 | 55.7 | 1 534.9 | Steroid 1 |
| 3 | 0.27 | 48.4 | 1 363.2 | Steroid 2 |
| 4 | 0.33 | 21.3 | 352.7 | Unknown |
| 5 | 0.45 | 147.7 | 5 567.2 | Steroid 3 |
| 6 | 0.53 | 40.5 | 2 152.5 | Steroid 4 |
| 7 | 0.61 | 33.8 | 1 219.2 | Steroid 5 |
| 8 | 0.65 | 15.5 | 344.1 | Unknown |
Table 4. Steroids profile of the methanolic extracts of flower and seeds of A. lanata L.
| Peak | Rf | Height | Area | Assigned substance |
| 1 | 0.04 | 13.3 | 132.2 | Unknown |
| 2 | 0.07 | 37.3 | 579.3 | Steroid 1 |
| 3 | 0.22 | 40.9 | 1 141.9 | Steroid 2 |
| 4 | 0.26 | 12.4 | 307.1 | Unknown |
| 5 | 0.45 | 67.3 | 2 201.8 | Steroid 3 |
| 6 | 0.54 | 66.6 | 2 832.6 | Steroid 4 |
| 7 | 0.60 | 62.2 | 2 506.4 | Steroid 5 |
| 8 | 0.72 | 87.0 | 3 515.0 | Unknown |
| 9 | 0.77 | 164.8 | 8 986.6 | Steroid 6 |
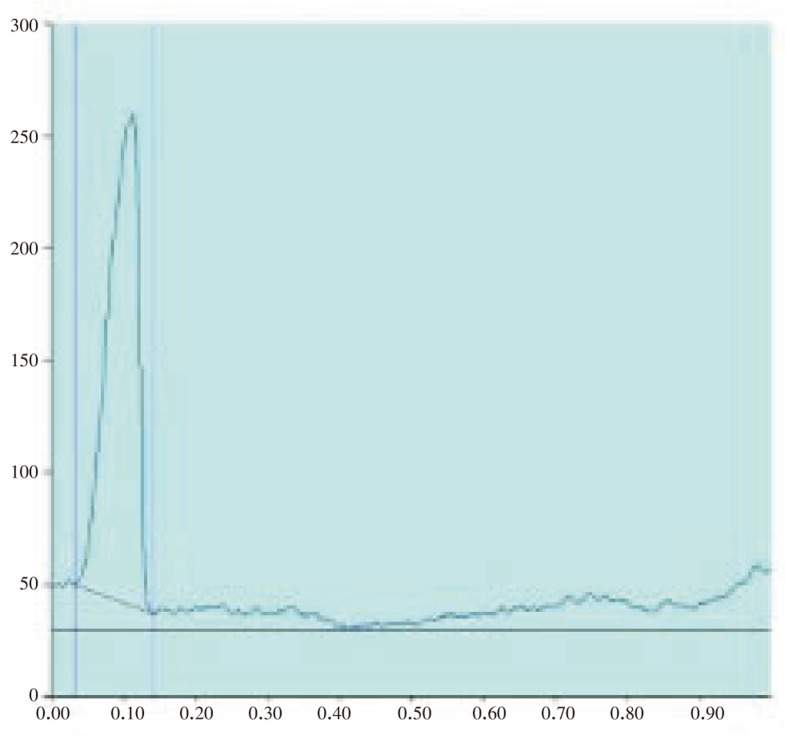
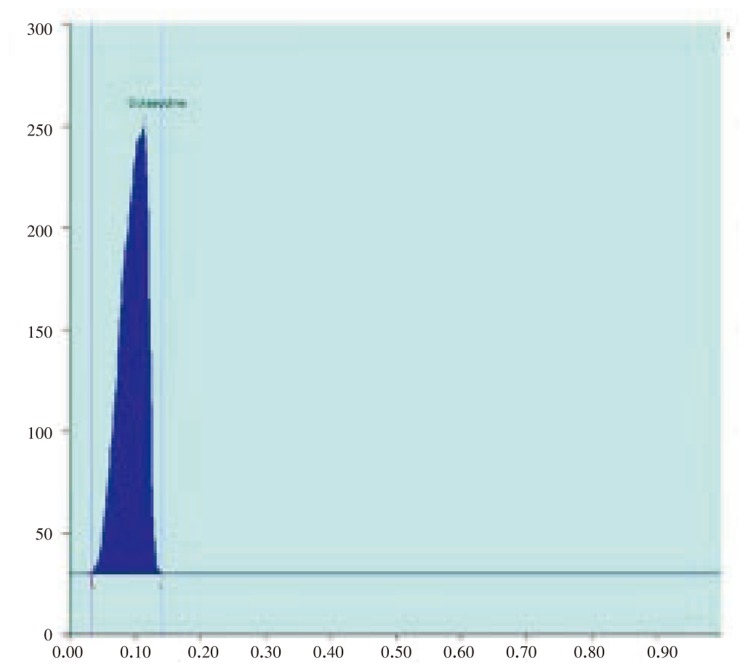
Figure 2. HPTLC chromatogram of standard solasodine (Scanned at 500 nm).
Figure 3. HPTLC chromatogram of standard solasodine: Peak densitogram display (Scanned at 500 nm).
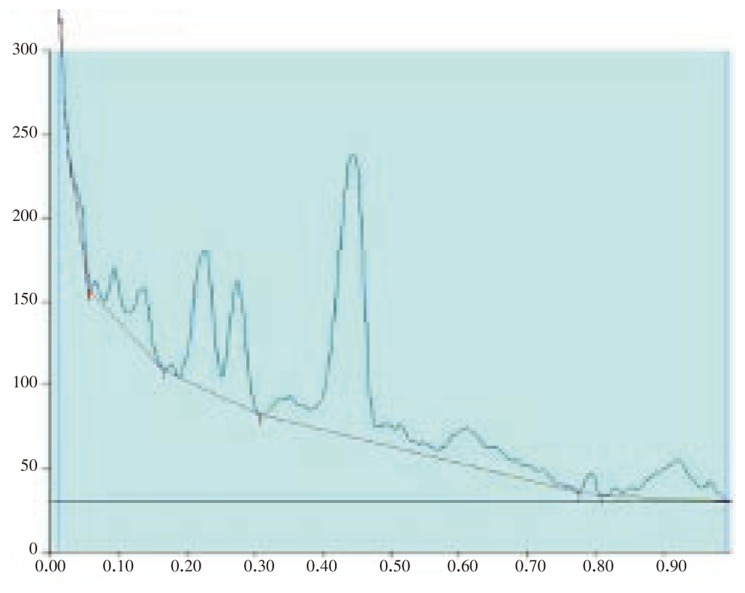
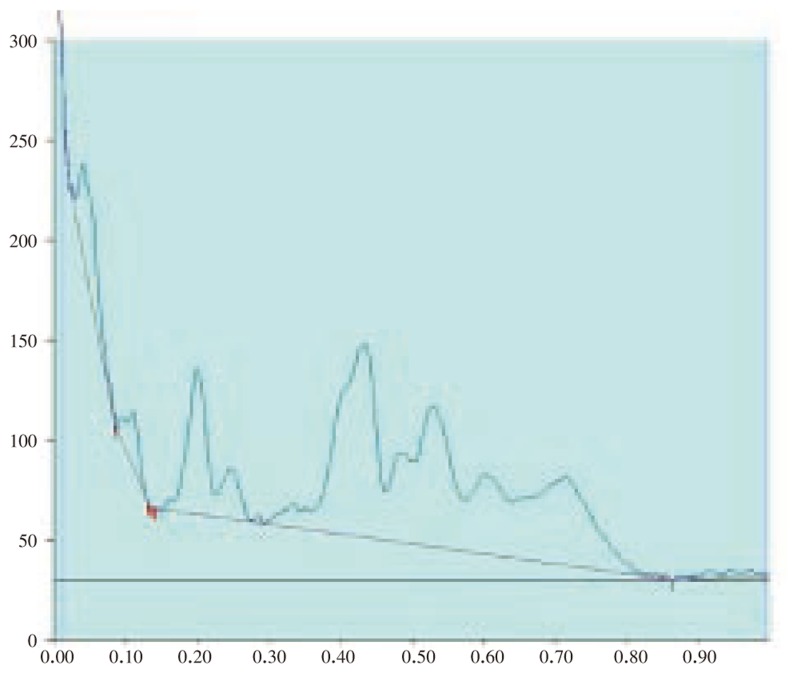
Figure 4. HPTLC chromatogram of A. lanata root - Baseline display (Scanned at 500 nm).
Figure 5. HPTLC chromatogram of A. lanata root - Peak densitogram display (Scanned at 500 nm).
Figure 6. HPTLC chromatogram of A. lanata stem - Baseline display (Scanned at 500 nm).
Figure 7. HPTLC chromatogram of A. lanata stem - Peak densitogram display (Scanned at 500 nm).
Figure 8. HPTLC chromatogram of A. lanata leaf - Baseline display (Scanned at 500 nm).
Figure 9. HPTLC chromatogram of A. lanata leaf - Peak densitogram display (Scanned at 500 nm).
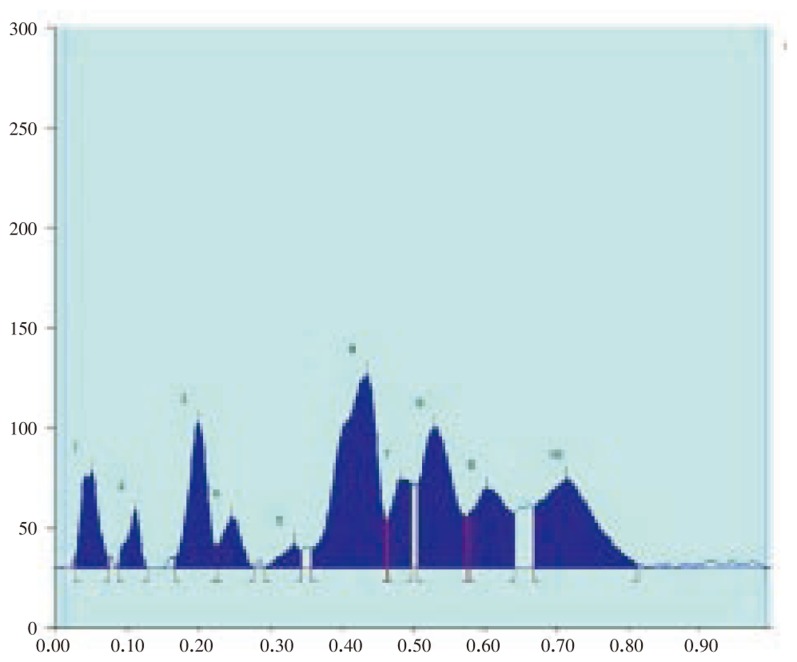
Figure 10. HPTLC chromatogram of A. lanata flowers and seeds - Baseline display (Scanned at 500 nm).
Figure 11. HPTLC chromatogram of A. lanata flowers and seeds - Peak densitogram display (Scanned at 500 nm).
4. Discussion
A large number of plants produce secondary metabolites such as alkaloids, flavanoids, phenols, terpenes, steroids and quinines that are used in pharmaceuticals, cosmetics and pesticide industries. Steroids (naturally occurring or synthetic) such as methylprednisolone, hydrocortisone, gluco-cortisteroids, corticosteroids, squalamine, oestrogens, androgens, are also used for the treatment of various diseases such as allergic reactions, arthritis, some malignancies, and diseases resulting from hormone deficiencies or abnormal production. In addition, synthetic steroids (e.g., mifepristone) that mimic the action of progesterone are widely used as oral contraceptive agents. Other synthetic steroids (e.g., oxandrolone) are designed to mimic the stimulation of protein synthesis and muscle-building action of naturally occurring androgens. The results of the present study revealed 30 different types of steroids in the different parts of A. lanata. Thus the present study confirms the traditional medical practice and previous pharmacological observations and supplement treatment for other health problems such as allergic reactions, arthritis, some malignancies, and diseases resulting from hormone deficiencies or abnormal production etc[23],[24]. The results of the present study also supplement the folkloric usage of the studied plants which possess several known and unknown bioactive compounds with bio-activity. By isolating and identifying these bioactive compounds new drugs can be formulated to treat various diseases and disorders. Authentication of medicinal plants as genetic and chemical level is a critical step in the use of these botanical materials for both research purposes and commercial preparations. For any living organism, identity is very important in order to distinguish itself from other organisms within the population and other populations. In plant taxonomy, during this molecular era, the morphological characters also play a vital role in plant systematic study and are used as a tool for the classification of a taxon. In recent times, in addition to morphological markers, anatomical, cytological, biochemical and molecular markers are also being used to classify the organisms. HPTLC profile (Chemical profile) of the methanolic extracts of A. lanata also confirms and supplements the previous observation and strengthen the identification of A. lanata using HPLTC profile in this study. HPTLC is useful as phytochemical markers and also as good estimators of genetic variability in plant populations. The presence or absence of chemical constituent has been found useful in the placement of the plant in taxonomic categories. HPTLC profile differentiation is one such important and powerful procedure which has often employed for this purpose. Each and every metabolite has a specific role and functions in harmony with other metabolites within the organizational framework of cells in the defense mechanism of the plants. Metabolites often exhibit tissue or cell specificity. The results of present study showed the presence of 30 different types of steroids with 30 different Rf values in different parts of A. lanata, the profile of root, stem, leaves and reproductive parts (flowers and seeds) showed unique profile and variation in steroid composition among the parts of the same plant. HPTLC profile of steroids has been chosen here to reveal the diversity existing at biochemical level in A. lanata. HPTLC is an invaluable assessment tool for the evaluation of botanical materials. It is efficient and economic for the analysis of a broad number of compounds.
This study has revealed that the methanolic extract of A. lanata has 30 peaks in chromatogram which has been produced by HPTLC. Therefore, HPTLC fingerprinting is proved to be a linear, precise, accurate method for herbal identification and can be used further in authentication and characterization of the medicinally important plant. The developed HPTLC fingerprints will help the manufacturer for quality control and standardization of herbal formulations. Such finger printing is useful in differentiating the species from the adulterant and act as biochemical markers for this medicinally important plant in the pharma industry and plant systematic studies.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Krishnamurthi A. The wealth of India, Vol. I. A publication and information directorate. New-Delhi: Council of Scientific and Industrial Research; 2003. p. 92. [Google Scholar]
- 2.Pullaiah T, Naidu CK. New-Delhi: Regency Publications; 2003. Antidiabetic plants in India and herbal based anti-diabetic research; pp. p.68–69. [Google Scholar]
- 3.Vertichelvan T, Jegadeesan M, Senthil Palaniappan S, Murali NP, Sasikumar K. Diuretic and anti-inflammatory activities of Aerva lanata in rats. Indian J Pharm Sci. 2000;62:300–302. [Google Scholar]
- 4.Udupihille M, Jiffry MTM. Diuretic effect of Aerva lanata with water, normal saline and coriander as controls. Indian J Physiol Pharmacol. 1986;30:91–97. [PubMed] [Google Scholar]
- 5.Manokaran S, Jaswanth A, Sengottuvelu S, Nandhakumar J, Duraisamy R, Karthikeyan D, et al. et al. Hepatoprotective activity of Aerva lanata Linn. against paracetamol induced hepatotoxicity in rats. Research J Pharm Tech. 2008;1(4):398–400. [Google Scholar]
- 6.Shirwaikar A, Issac D, Malini S. Effect of Aerva lanata on cisplatin and gentamicin models of acute renal failure. J Ethnopharmacol. 2004;90:81–86. doi: 10.1016/j.jep.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 7.Vetrichelvan T, Jegadeesan M. Anti-diabetic activity of alcoholic extract of Aerva lanata (L.) Juss. Ex Schultes in rats. J Ethnopharmacol. 2002;80:103–107. doi: 10.1016/s0378-8741(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh T, Yadav BV, Badole SL, Bodhankar SL, Dhaneshwar SR. Antihyperglycaemic activity of alcoholic extract of Aerva lanata (L.) A. L. Juss. Ex J. A. Schultes leaves in alloxan induced diabetic mice. J Appl Biomed. 2008;6:81–87. [Google Scholar]
- 9.Dulaly C. Antimicrobial activity and cytotoxicity of Aerva lanata. Fitoterapia. 2002;73:92–94. doi: 10.1016/s0367-326x(01)00369-0. [DOI] [PubMed] [Google Scholar]
- 10.Rao SG. Evaluation of an experimental model for studying urolithiasis effect of Aerva lanata on urinary stones. Indian Drugs. 1985;22:640–643. [Google Scholar]
- 11.Appia Krishnan G, Rai VK, Nandy BC, Meena KC, Dey S, Tyagi PK, et al. et al. Hypoglycemic and antihyperlipidaemic effect of ethanolic extract of aerial parts of Aerva lanata Linn. in normal and alloxan induced diabetic rats. IJPSDR. 2009;1(3):191–194. [Google Scholar]
- 12.Anantha D, Israiel Kumar T, Santosh kumar M, Manohar Reddy A, Mukharjee NSV, Lakshmana Rao A. In vitro anti helmentic activity of aqueous and alcoholic extracts of Aerva lanata Seeds and leaves. J Pharm Sci & Res. 2010;2(5):317–321. [Google Scholar]
- 13.Zapesochnaya G. Canthin-6-one and beta-carboline alkaloids from Aerva lanata. Planta Medica. 1992;58:192–196. doi: 10.1055/s-2006-961427. [DOI] [PubMed] [Google Scholar]
- 14.Manikandan A, Victor Arokia Doss A. Evaluation of biochemical contents, nutritional value, trace elements, SDS-PAGE and HPTLC profiling in the leaves of Ruellia tuberosa L. and Dipteracanthus patulus (Jacq.) J Chem Pharm Res. 2010;2(3):295–303. [Google Scholar]
- 15.Tripathi AK, Verma RK, Gupta AK, Gupta MM, Khanuja SP. Quantitative determination of phyllanthin and hypophyllanthin in Phyllanthus species by high-performance thin layer chromatography. Phytochem Anal. 2006;17:394–397. doi: 10.1002/pca.936. [DOI] [PubMed] [Google Scholar]
- 16.Ramya V, Dheena Dhayalan V, Umamaheswari S. In vitro studies on antibacterial activity and separation of active compounds of selected flower extracts by HPTLC. J Chem Pharm Res. 2010;2(6):86–91. [Google Scholar]
- 17.Patil AG, Koli SP, Patil DA, Chnadra N. Phamacognostical standardization and HPTLC finger print of Crataeva tapia Linn. SSP. Odora (Jacob.) Almeida leaves. Int J Pharma Biosciences. 2010;1(2):1–14. [Google Scholar]
- 18.Santosh MK, Shaila D, Sanjeeva Rao I. Standardization study of dadimastaka and pushyannga churnas. Asian J Chemistry. 2004;16(3&4):1735–1741. [Google Scholar]
- 19.Aparna Saraf. Phytochemical and antimicrobial studies of medicinal plant Costus Speciosus (Koen.) E-J Chemistry. 2010;7(S1):S405–413. [Google Scholar]
- 20.Madhavan V, Goswami PK, Gurudeva MR, Yoganarasimhan SN. Pharmacognostical studies on the root of Nothosaerva brachiata Wt. - A botanical source of the Ayurvedic drug. Pashanabheda. Ind J Traditional Knowledge. 2010;9(4):629–634. [Google Scholar]
- 21.Gupta AK, Tandon N, Sharma M, editors. Quality standards of Indian medicinal plants. New Delhi: Medicinal plants Unit, Indian Council of Medical Research; [Google Scholar]
- 22.Harborne JB. Phytochemical methods. 3rd ed. London: Chapman and Hall; 1998. [Google Scholar]
- 23.Wagner H, Baldt S, Zgainski EM. Plant drug analaysis. Berlin: Springer; 1996. [Google Scholar]
- 24.Bhawani SA, Sulaiman O, Hashim R, Mohamad Ibrahim MN. Thin-layer chromatographic analysis of steroids: A review. Trop J Pharmaceutical Res. 2010;9(3):301–313. [Google Scholar]