Abstract
Objective
To evaluate the hazardous effects of fried potato chips upon the retina of two developmental stages of the albino rats aged 7 and 14 days from parturition.
Methods
Pregnant rats were arranged into two groups: control pregnant rats and consequently their delivered newborns until reaching 7 and 14 days old from parturition and fried potato chips group in which pregnant rats at the 6th day of gestation maintained on diet formed of fried potato chips supplied from the market mixed with standard diet at a concentration of 50% per each till 7 and 14 post-partum. Three fold integrated approaches were adopted, namely, histological, ultrastructural and proteomic analysis.
Results
Histological examination of the retina of the experimental offsprings revealed many histopathological changes, including massive degeneration, vacuolization and cell loss in the ganglion cell layer, as well as general reduction in retinal size. At the ultrastructural level, the retina of experimental offsprings exhibited number of deformities, including ill differentiated and degenerated nuclear layer, malformed and vacuolated pigment epithelium with vesiculated and fragmented rough endoplasmic reticulum, degenerated outer segment of photoreceptors, as well as swollen choriocapillaris and loss of neuronal cells. Proteomic analysis of retina of the two experimental developmental stages showed variations in the expressed proteins as a result of intoxication which illustrated the adverse toxic effects of fried potato chips upon the retina.
Conclusions
It can be concluded that the effect of fried potato chips on the development of retina in rats may be due to the presence of acrylamide or its metabolite.
Keywords: Fried potato chips, Retina, Development, Histology, Ultrastructure, Albino rats , Hazardous effect, Proteomic analysis, Acrylamide, Metabolite
1. Introduction
Potato chips are common parts of children's menus in fast food restaurants all over the world and these familiar foods are reported to contain high levels of toxic and carcinogenic byproducts which are not found in the uncooked foods[1]–[3]. Among these components are acrylamide (ACR). High levels of ACR were unexpectedly detected in widely consumed food items, notably potato chips and bread[4]. Tareke et al[5] demonstrated high levels of ACR in heat-processed commercial foods and in foods cooked at temperatures, especially in carbohydrate-rich foods.
It has been reported that ACR induced varieties of neurotoxicities[6]–[8] and it has been suggested that ACR manifested toxicity principally toward growth and development[9],[10]. Moreover, ACR may affect the visual system in both animals[11],[12] and humans[5] following absorption via dermal, oral or respiratory routes. Restricted red and green colors visual field, and hypertensive retinopathy were observed in an Italian road tunnel worker exposed to ACR containing grouts for 3 months during work until shortly prior to a health examination[5]. The effects persisted for 4 months after the first examination, although white color visual field was normal after 20 days. No group studies have later addressed such ACR-related effects in humans.
Neuropathologic studies have described axonal swelling and later degeneration in the more central optic tract, lateral geniculate nucleus and superior colliculus of ACR-intoxicated animals[13]. Vidyasagar[14] has reported that X-like cells in the lateral geniculate nucleus of the rat are especially susceptible to inactivation in acute ACR poisoning. Other visual effects of ACR such as altered evoked responses in rats[15] and impaired vision in humans[13] may have a similar basis. Wild and Kulikowski[16] reported selective disrupted X-like cells in the lateral geniculate nucleus of rats treated with ACR. The diameter of the optic tract fibers of all sizes is affected by ACR intoxication.Following a herd of cattle was accidentally exposed to ACR and N-methyloacrylamide, Godin et al[3] found abnormal papillary light reflexes in one cow. Ophthamoscopic examination showed progressive retinal degeneration and degenerative changes in the optic nerves head. Light and electronic microscopic examination revealed pathologic changes in the retina and optic nerves consistent with chronic stages of ACR-toxicity. The present study aimed therefore to study the development of retina of postnatal young rats aged 7 and 14 days maternally fed on fried potato chips using two approaches: firstly, light and transmission electron microscopy and secondly, SD-SPAGE for the developing retina of the same stages.
2. Materials and methods
2.1. Animals, housing and treatment
Thirty fertile virgin females and ten fertile males of albino rats (Rattus norvegius) weighing (100±5) g were obtained from Hellwan Animal Breeding Farm, Ministry of Health, Cairo, Egypt and used for experimentation. Rats were housed in individual cages and maintained in a room with good ventilation at 23 °C. The housing room was maintained on a 12:12 h light: dark cycle. Standard rodent diet composed of 20% casein, 15% corn oil, 55% corn starch, 5% salt mixture and 5% vitaminzed starch (Egyptian Company of Oils and Soap Kafr-Elzayat Egypt) was supplied. Free excess of water was provided ad libitum. All the experiments were done in compliance with the Guide for the Care and Use of Laboratory animals. Females were made pregnant by keeping them at a ratio of 1 male: 3 females with healthy fertile males for 12 hour between 8 pm till 8 am. During the next morning, the prospective pregnants were examined for the presence of vaginal plugs. Vaginal smears were carried out to give a precise determination of the onset of gestation.
The pregnant rats were arranged into two groups, each was composed of 15 individuals as follows: control pregnant rats and consequently their delivered newborns until reaching 7 and 14 days old from parturition, fried potato chips group in which the pregnant rats at the 6th day of gestation maintained on diet formed of fried potato chips supplied from the market mixed with standard diet at a concentration of 50% per each till 7 and 14 days post-partum.
2.2. Light microscopic examination
The retina of postnatal animals aged 7 and 14 days from parturition of both control and experimental mothers were separated, and immediately fixed in 10% neutral buffered formalin. The specimens were dehydrated in ascending grades of ethyl alcohol, cleared in xylol and mounted in molten paraplast at 58-62 °C. 5 µm serial histological sections were cut, stained with Harris hematoxylin, counter stained with eosin and observed under bright field Leitz microscope.
2.3. Transmission electron microscopic (TEM) examination
The retina at 7 and 14 days of both control and experimental groups were separated and immediately fixed in 2.5% glutaraldehyde and 2% paraformaldhyde in 0.1 M cacodylate buffer (pH 7.4). After rinsing in 0.1 M cacodylate buffer, samples were post fixed in a buffered solution of 1% osmium tetraoxide at 4 °C for 1.5 h. This was followed by dehydration in ascending grades of ethyl alcohol and embedded in epoxy-resin[8]. Ultrathin sections were cut with a glass knife on a LKB microtome and mounted on formvar-coated grids, stained with uranyl acetate and lead citrate and finally examined at Joel transmission electron microscope.
2.4. Sodium dodecyl polyacrylamides gel electrophoresis (SDS-PAGE)
A set of four individuals of both control and experimental groups were sacrificed at the end of the treatment and biopsies of retina were taking and processed for SDS-PAGE. Electrophoresis was carried out with constant volt at 200v. The separated proteins on polyacrylamides gel were stained with coomassie blue R-250[17].
3. Results
3.1. Histological examination
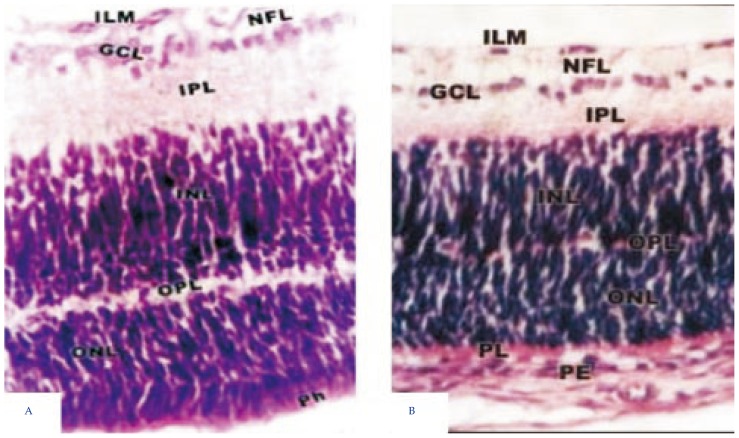
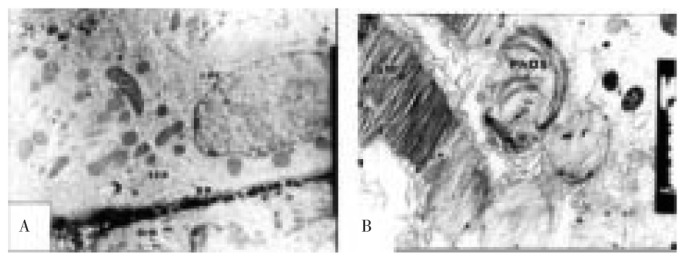
Light microscopic examination of retina of 7 days old offsprings of control mothers showed that the retina at this developmental stage is composed of four definite cell layers, namely, nuclear, inner plexiform, ganglion layer and pigment epithelial cell layer. The outer plexiform layer appeared, separating the inner from the outer nuclear layer. Internally adjacent to the vitreous humor, the nerve fiber layer attained highly organization and possessed newly formed blood capillaries (Figure 1A). However, in offsprings maternally fed on diet containing fried potato chips, a thin outer plexiform layer was developed and both inner and outer nuclear layers showed some degree of differentiation. The photoreceptor layer showed abundant spread of vacuoles (Figure 1B).
Figure 1. Light microscopic examination of retina of 7 days old rats.
A: control retina showing normal differentiation of retinal cell layers including inner limiting membrane (ILM), nerve fiber layer (NFL), ganglionic cell layer (GCL), thick inner plexiform layer (IPL), inner nuclear layer (INL), thin outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor cell layer (Ph) and pigmented epithelium (PE); B: experimental retina showing ill differentiated of nuclear layer associated with massive decrease of nuclear cells. H&E stain (×250).
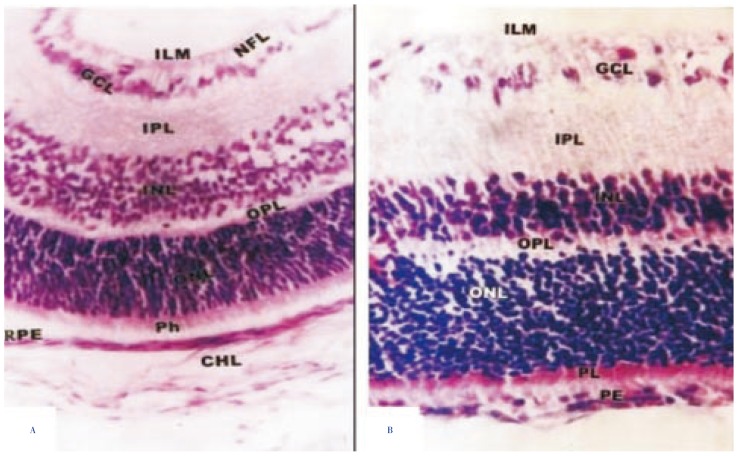
The retina of 14 days old offsprings of control mothers is composed of eight cell layers and two limiting membranes arranged from the choroid to the vitreal side as follows: cubical pigmented epithelium, rod and cone cell layers, outer nuclear cell layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer, nerve fiber layer (Figure 2A). On the other hand, in young rats fed on fried potatoes chips, the retinal histopathology possessed massive degeneration, vacuolization and cell loss in the ganglionic layer. Moreover, the retina was comparatively reduced with detected massive vacillation and degeneration of nerve fiber and thinning of ganglion cell layer. The outer plexiform layer was distorted in investigated specimens. Both outer and inner nuclear cell layer showed numerical increase of pyknotic nuclei (Figure 2B).
Figure 2. Light microscopic examination of retina of 14 days old rats.
A: control retina showing normal differentiation pattern of retinal cell layers including inner limiting membrane (ILM), nerve fiber layer (NFL), ganglionic cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor cell layer (Ph) and pigmented epithelium (PE); B: experimental retina showing retina with ill-differentiated outer plexiform layer and loss of neuronal cells. H&E stain (×250).
3.2. TEM results
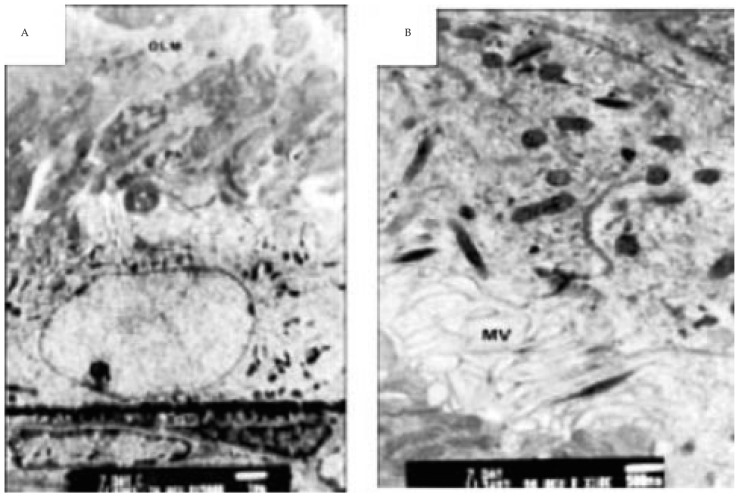
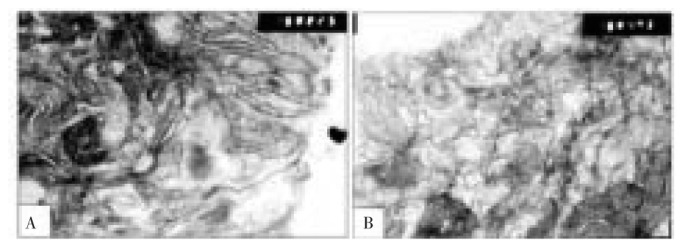
TEM examination of retina of 7 days old offsprings of control mothers revealed that the pigment epithelium consists of a single layer of cubical cells with branched microvilli. They contain large oval-shaped nuclei located at the base of the cell. The nuclei contain a remarkable abundant euchromatin except for fine occasional clumping of the chromatin about the nuclear edges. The basal surface of the pigment epithelial cell rests on a prominent basement membrane adjacent to Burch's membrane, which separates the pigment epithelial cell from the vascular choroid tissue. Burch's membrane appears as an irregular band of relatively low density containing a few loosely arranged fibers exhibiting the banding of collagen. Highly organized more homogeneous lamina is usually seen in the middle of Burch's membrane. Precursor of electron-dense granules semi-like lysosome was detected sparsely distributed in the apical cytoplasmic margin of pigmented cells. Mitochondria attained marked differentiation as the ribosomes and smooth endoplasmic reticulum. Inner segment of photoreceptor made their first appearance and the outer segment was still undifferentiated. Numerous blood capillaries were detected within the nerve fiber layer and consisted of endothelial and mural cells, separated from one another by extra cellular spaces which were often enclosed by basement membrane lamellae. The capillary walls are surrounded by an outer basement membrane, which is completely unsheathed in a glial tunnel composed of the processes of Miller cells. (Figure 3).
Figure 3. Transmission electron micrographs of control retina of 7 days old offspring rats.
A: showing retinal pigmented epithelial cells with underlying basement membrane. The cytoplasm is rich in mitochondria, ribosomes and smooth endoplasmic reticulum. The apical part is characterized by radial arranged microvilli adjacent to macrophagosome and newly formed inner segment of photoreceptors (×75 00). B: showing branched microvilli (MV) of pigmented epithelium, phagosome and newly formed inner segment of photoreceptors (×13 000).
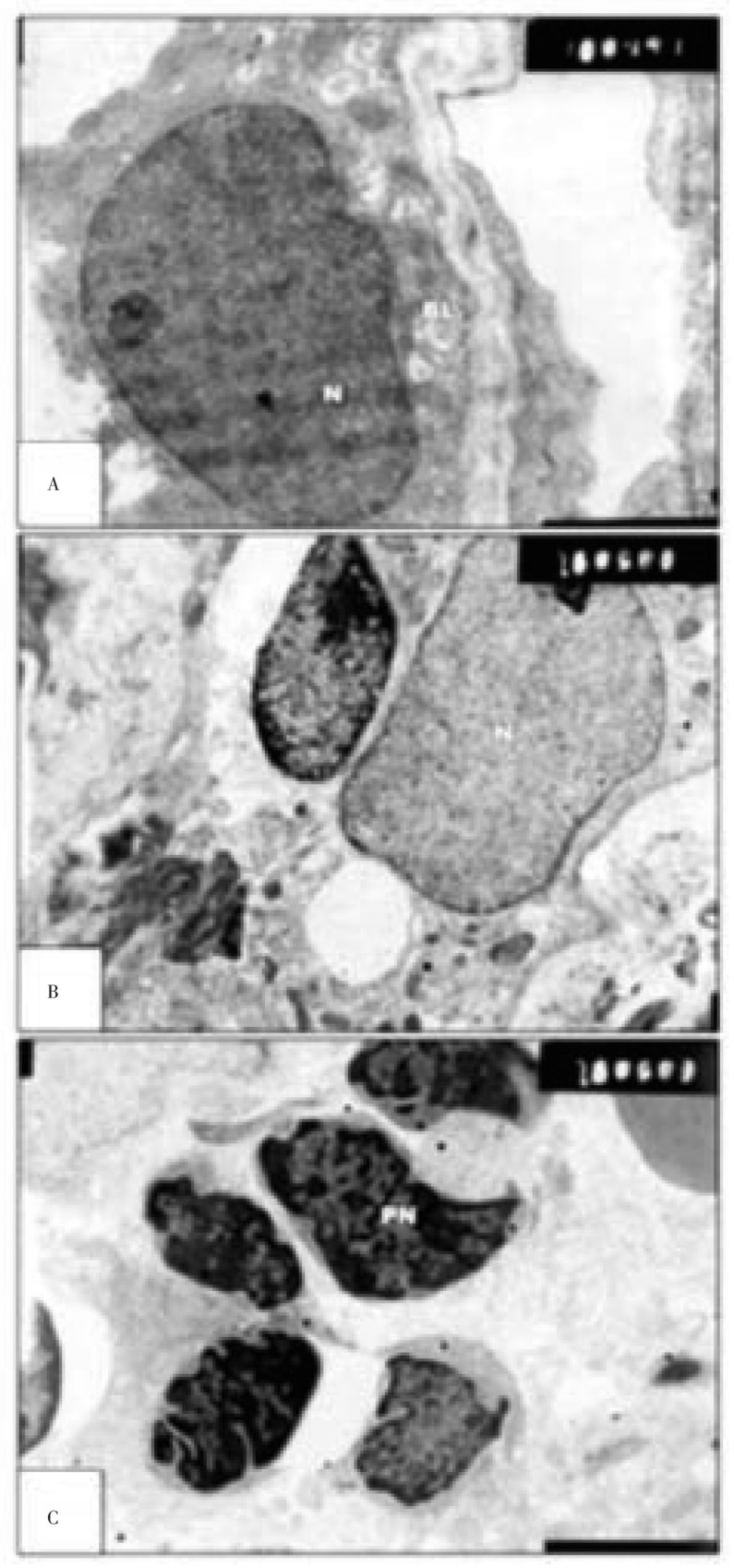
The retina of offsprings maternally fed on fried potato chips possessed altered pigment epithelium characterized by increased accumulation of heterochromatin at the nuclear envelop. The cytoplasm enclosed by vacuoles of different sizes. Numerous electron-dense particles were dispersed within the cytoplasm. There was a considerable folding of the basal lamina of pigmented epithelium. The choriocapillaris were disturbed and lacked regular structural pattern of blood vessel. Different phases of degeneration were detected within the retinal cell layers including both the nuclear cell layers and ganglion cells. These included pyknosis, nuclear convolution and karyolysis of the nuclei of many of the cells (Figure 4).
Figure 4. Transmission electron micrographs of experimental retina of 7 day old rats.
A&B: showing malformed pigment cell. Their cytoplasm enclosed by numerous vacuoles (V) and phagosomes. Inner segment appears degenerated in many of them. C: showing massive degeneration of nuclear cells building nuclear layer. The nuclear cells appeared with convoluted and pyknotic nuclei (PN) and electron-dense chromatin material (A&B×13 000; C×7 500).
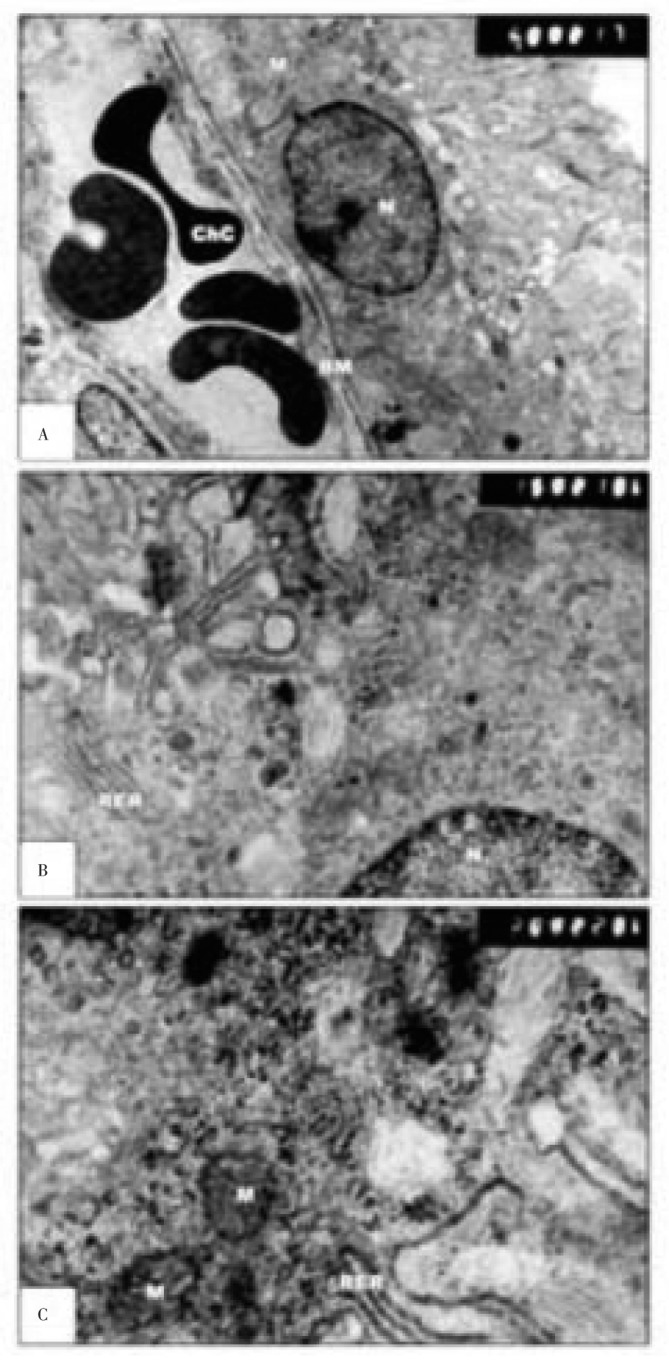
TEM examination of retina of 14 days old offsprings of control mothers revealed that the pigment epithelium of retina attained a considerable development compared with the previous stage. Underlying the basement membrane of the pigment epithelium is firmly attached to the Burch's membrane with more differentiated choriocapillaris lined by flattened endothelial cells. While it's inner aspect has a rather fragile attachment to the rods and cones of the retina by means of cilia-like microvilli. The nuclei become spindle shaped with thin peripheral arrangement of chromatin and widespread of euchromatin. The cytoplasm is rich with cytoplasmic organelles. The mitochondria of these cells are typical, and their cristae often extending completely across their width. The mitochondria have a very characteristic distribution, being found primarily just below the edge of the convoluted basal margin and along the lateral boundaries of the cell. Throughout the bulk of the pigment epithelial cytoplasm, many particles with the density and dimensions of ribosomes are detected. The photoreceptor of outer segments is interdigitated with microvilli of the pigment epithelium. The rod proper is composed of a slender inner segment filled with mitochondria, ribosomes and rosette-shaped glycogen particles. The mitochondria in the rod inner segments appear arranged in a circular pattern adjoining the plasma membrane and extending along the whole length of the inner segment. The cone proper has an inner (ellipsoid) segment with numerous mitochondria. The outer segment is wider near its inner portion than the corresponding area in the rod. Above the outer limiting membrane, the outer nuclear cell layers, is the layer formed by the closely packed nuclei of the photoreceptors, rods and cones. The nuclei of photoreceptors are characterized by their unique rosette shaped dense heterochromatin. The inner plexiform layer formed of a layer of synapses of the cells bipolar layer with the next ganglion cell layers. The inner plexiform separated the inner nuclear layer from inward ganglion, nerve fiber and inner limiting membrane. The ganglion cells are disposed, together with some few neuralgia cells. Ganglion cells are larger on average than most of the preceding retinal interneuron, but they are not all of the same size. Nuclei are relatively large euchromatin and contained prominent nucleoli. The ganglion cells have relative abundance of cytoplasm that contains rough endoplasmic reticulum grouped in masses. Other prominent organelles can be distinguished such as mitochondria, Golgi apparatus and lysosome. Dendrite cell processes make synaptic junctions with bipolar neurons and amacrine cells in the inner plexiform layer. The layer of nerve fibers consists of the axons of ganglion cells running parallel to the inner surface of the retina (Figure 5).
Figure 5. Transmission electron micrographs of control retina of 14 days old rat.
A: showing pigmented epithelial cells having cytoplasm rich in mitochondria (M), smooth endoplasmic reticulum (SER), free ribosomes with the base showing well development basement (BM) and brush membrane (BrM); B: showing outer segment (OS) of photoreceptors with regular arrangement of stacked membranes ( A&B×7 500).
The pigmented epithelium of the experimental group exhibited the following features: electron-dense heterochromatin material, reduced mitochondria, cytoplasmic vacuolation, electron-dense lysosomes, vesiculated, rough endoplasmic reticulum, thickened basement membrane, swollen blood capillaries, deteriorated Microvilli, as well as degenerated photophores of the outer segments (Figure 6&7).
Figure 6. Transmission electron micrographs of experimental retina of 14 days old rats.
A&B: showing pigmented epithelium with vacuolated cytoplasm, vesiculated rough endoplasmic reticulum and fragmented rough endoplasmic reticulum. The choriocapillaris appear swollen. C: showing degenerated outer segment of photoreceptors (A&B×13 000; C×20 000).
Figure 7. Transmission electron micrographs of experimental retina of 14 days old rats showing degeneration of outer segment of photoreceptors. The stacked membrane loosely separated from each other( ×13 000).
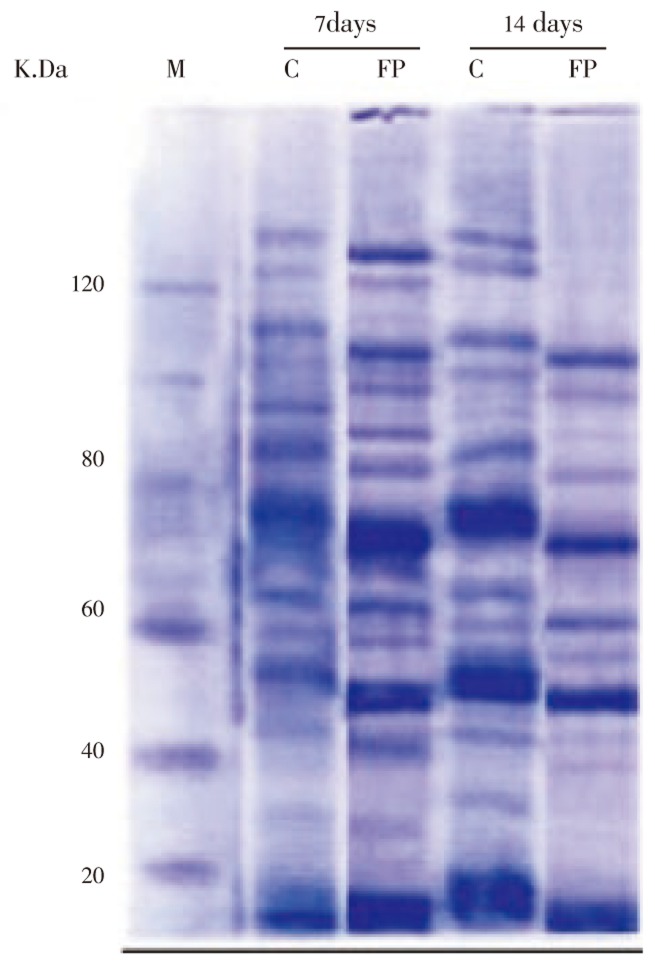
3.3. Proteomic (SDS-PAGE) analysis of retina
Total protein bands concentrations and identifications are illustrated in Tables 1&2 and Figure 8. After fractionation by polyacrylamide gel electrophoresis marked differences in pattern were observed between experimental and control groups.
Table 1. Protein bands densities and identifications of SDS-PAGE of retina of 7 days old offsprings of both control and experimental mothers.
| Marker (kDa) | Control (kDa) | Experimental (kDa) |
| 200.00 | 311.77 | 263.45 |
| - | 226.06 | 209.40 |
| - | 147.25 | 118.85 |
| - | 111.79 | 105.15 |
| 97.40 | 87.98 | 94.15 |
| - | 75.79 | 80.02 |
| - | - | 70.35 |
| 68.00 | 60.64 | 56.41 |
| - | 47.36 | 45.67 |
| - | - | 43.52 |
| 43.00 | 41.40 | 38.86 |
| - | 30.54 | 28.31 |
| - | - | 19.36 |
| - | - | - |
| 18.40 | 17.92 | - |
| - | 15.76 | - |
Table 2. Protein bands densities and identifications of SDS-PAGE of retina of 14 days old offspring of both control and experimental mothers.
| Marker (kDa) | Control (kDa) | Experimental (kDa) |
| 200.00 | 347.03 | 240.33 |
| - | 297.77 | 209.40 |
| - | 244.04 | 115.26 |
| - | 132.29 | 100.43 |
| - | - | - |
| - | - | - |
| 97.40 | 96.74 | 92.89 |
| - | 91.02 | 80.56 |
| - | 86.21 | 69.87 |
| 68.00 | 74.27 | 55.39 |
| - | 58.84 | 51.22 |
| - | 47.64 | 43.26 |
| 43.00 | 41.40 | 35.56 |
| - | 28.67 | 25.91 |
| - | 20.36 | 21.97 |
| - | - | - |
| - | - | - |
| - | - | - |
| 18.40 | 14.43 | 18.00 |
| - | - | - |
Figure 8. SDS-PAGE of retina of 7 and 14 days old offspring rats of both control and maternally treated with fried potatoes chips.
M: Marker; C: control; FP: fried potato chips.
Proteomic analysis of experimental retina showed variations in expressed proteins. Therefore, at 7 and 14 days old rats maternally fed on fried potatoes chips, there was lacked expression of protein or expression of new stressed proteins as a result of intoxication which illustrates the adverse toxic effects.
4. Discussion
The present results revealed that eye is sensitive to the cytotoxicity of feeding on fried potatoes chips during breast-feeding young milk after parturition. The disease causes drastic alterations in retina of 7 and 14 days offsprings. The retinal pigment epithelium exhibited massive pathological alterations. Earlier at parturition, there was a numerical reduction of pigmented neurons associated with apparent damage degeneration of undifferentiated nuclear, ganglion and nerve fiber cell layers. The partial loss of retinal cell neurons proceeded in the next rat ages, being with characteristic with a considerable atrophy of mitochondria, vesiculation of agranular endoplasmic reticulum and distortion of cytoplasm as a result of presence of large vacuoles causing a loss of its pattern structure of microvilli. These were associated with massive degeneration of photoreceptor inner segment at seven-days old rats and much more loss of both outer and inner photoreceptor segment at fourteen-days old rats. The outer segment showed internal vacuolation with marked loss of the stacked membranes. Besides, the posterior pole of pigment cell possessed abnormal cytological feature of bruch's membrane in the form of thickened electron-dense heavy concentration of an amorphous dense fibers and clumped material causing obliteration and degeneration of choriocapillaris. Besides, the blood capillaries appeared swollen with massive degeneration of both pigment cells and choriocapillaris.
The obtained findings may be attributed to the presence of ACR[4]. Reports from laboratory studies have provided insight into the biochemical mechanism of acrylamide (ACR) formation. As a neurotoxic chemical, ACR can be generated during the heating of specific foodstuffs as a result of Millard reaction between amino acids and sugars[18],[19]. In particular, ACR is formed when the amino acid asparagine in the presence of sugar is heated above 100 °C. Potatoes and cereals which had the highest measured levels of ACR in the Swedish NFA survey were found to be rich in asparagine[20].
ACR was reported to be a neurotoxic agent. LoPachin et al[21] reported that the cumulative neurotoxicity produced by ACR exposure was linked to nerve terminal damage in the central nervous system (CNS) and peripheral nervous system (PNS). At the molecular level, this presynaptic toxicity appears to be mediated by the formation of sulfhydryl adducts on the cysteine residues of many proteins[22]. Quantitative (gas chromatography/mass spectrometry) analyses of whole-brain synaptosomes isolated from ACR-intoxicated rats revealed an accumulation of the cysteine adduct, S-(2-carboxyethyl)-cysteine (CEC) that was closely correlated to the development of neurotoxicity[23]. Although the molecular mechanism of this presumed ACR effect is unknown, membrane fusion processes such as neurotransmission are highly sensitive to inhibition by sulfhydryl alkylation[24].
Early morphological studies suggested that ACR neurotoxicity was mediated by nerve damage classified as a central-peripheral distal axonopathy[19]. However, other contemporary researches demonstrated that ACR can produce neurological toxicity in the absence of axonopathy, whereas equivalent neurotoxicity can be induced by intoxication over a wide range of daily dose-rates (e.g. 10-50 mg/kg per day for 28 days), axon degeneration in PNS and CNS occurred only during long-term exposure to lower ACR dose-rates (e.g. 21 mg/kg per day for 42 days)[6]. The dissociation between neurological dysfunction and the presumed underlying morphological lesion suggests that axonopathy might not be importantly involved in the pathophysiological process leading to ACR neurotoxicity. Nerve terminals have been suggested as an alternative site of ACR action based on early structural and functional changes as in PNS and CNS[21].
ACR interferes with axonal retrograde transport mechanisms essential for the survival of the axon. Completed morphological studies[6] using a contemporary amino-cupric silver staining method to detect degenerating neurons and their processes indicated that intoxication at a higher ACR dose-rate produced selective, widespread degeneration of nerve terminals in rat brain and spinal cord regions. Intoxication at a lower dose rate was associated with initial nerve terminal degeneration followed later by pre-terminal axon degeneration. These findings are consistent with other evidences suggesting that, regardless of dose-rate, nerve terminal degeneration was an initial consequence of ACR intoxication in both CNS[26] and PNS[27]. Experimental ACR-intoxication in mice[28] and rat[29] was found to produce a pronounced neuropathy characterized by flaccid paralysis and ataxia. The histopathological alterations characterized by eccentrically placed nuclei, folding of the nuclear membrane, accumulations of dense bodies, and clusters of smooth endoplasmic reticulum associated with numerous microtubules in cerebellar Purkinje cells that may contribute to the pronounced ataxia in these animals.
The obtained findings may be attributed to the increased level of ACR in the blood of treated mothers as well as in the umbilical cord blood of neonates as a result of its higher affinity of forming N-terminal haemoglobin adducts. In view of the shorter life span of neonatal erythrocytes and the lower body weight of newborn infants, the relative internal dose of ACR adducts in neonates (in microgrammes per kg. body weight) must be assumed to be at least equal to that of the mother. Because of the high cell-replication rates during foetus development, trans-placental exposure of neonates to ACR might raise concerns[30].
ACR neurotoxicity may be attributed to its higher affinity to form adducts with glutathione, proteins, and DNA directly or after metabolized to its epoxide, glycidamide (2, 3-epoxy-1-propanamide). Glycidamide was found to pass through placenta and distributed in fetal tissues and also pass through mother's milk during lactation[31]. Abou-Donia et al[32] reported that brain stem of glycimide-treated rats exhibited axonal degeneration with chromatolytic necrosis in midbrain medial and lateral reticular nuclei.
The obtained retina alterations may also be attributed to oxidative stress induced by acrylamide. In this concern, Zhu et al[33] reported that ACR-induced neurotoxicity may be associated with the enhancement of lipid peroxidation and reduction of the antioxidative capacity. Allam et al[34] reported that prenatal and perinatal acrylamide disrupts the biochemical machinery, causes oxidative stress and induces structural changes in the developing rat cerebellum. It is concluded that fried potato chips affected the developing retina in rats and this may be due to the presence of acrylamide or its metabolite.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Exon J. A review of the toxicology of the acrylamide. J Toxicol Environ Health B Crit Rev. 2006;9:397–412. doi: 10.1080/10937400600681430. [DOI] [PubMed] [Google Scholar]
- 2.Mitka M. Fear of frying: is acrylamide in foods a cancer risk? JAMA. 2002;288:2105–2106. doi: 10.1001/jama.288.17.2105. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi M, Shibutani M, Inoue K, Fujimoto H, Hirose M, Nishikawa A. Pathological assessment of the nervous and male reproductive systems of rat offspring exposed maternally to acrylamide during the gestation and lactation periods-a preliminary study. Toxicol Sci. 2008;33:11–24. doi: 10.2131/jts.33.11. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang Y. Study on reduction of acrylamide in fried bread sticks by addition of antioxidant bamboo leaves and extract of green tea. Asia Pac J Clin Nutr. 2007;16:131–136. [PubMed] [Google Scholar]
- 5.Mapp C, Mazzotta M, Bartolucci G, Fabbri L. La neuropatia da acrilamide: prime osservazioni in Italia. [Neuropathy due to acrylamide: First observations in Italy] Med Lav. 1977;68:1–12. [PubMed] [Google Scholar]
- 6.Lehning E, Balaban C, Ross J, LoPachin R. Acrylamide neuropathy: III. Spatiotemporal characteristics of nerve cell damage in rat forebrain. Neurotoxicology. 2003;24:125–136. doi: 10.1016/s0161-813x(02)00155-9. [DOI] [PubMed] [Google Scholar]
- 7.Lopachin R, Barber D, Geohagen B, Gavin T, He D, Das S. Structure-toxicity analysis of type-2 alkenes: in vitro neurotoxicity. Toxicol Sci. 2007a;95:136–146. doi: 10.1093/toxsci/kfl127. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds E. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garey J, Ferguson A, Paule G. Developmental and behavioral effects of acrylamide in Fischer 344 rats. Neurotoxicol Teratol. 2005;27:553–563. doi: 10.1016/j.ntt.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Huang P, Lie T, Li J, Hutz R, Li K, et al. Reproductive toxicity of acrylamide treated male rats. Reprod Toxicol. 2010;29:225–230. doi: 10.1016/j.reprotox.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Totani N, Yawata M, Ojiri Y, Fujioka Y. Effects of trace acrylamide intake in Wistar rats. J Oleo Sci. 2007;56:501–506. doi: 10.5650/jos.56.501. [DOI] [PubMed] [Google Scholar]
- 12.Merigan W. Chromatic and achromatic vision of macaques: role of the P pathway. J Neurosci. 1989;9:776–783. doi: 10.1523/JNEUROSCI.09-03-00776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaumburg H, Spencer P. Clinical and experimental studies of distal axonopathy a frequent form of brain and nerve damage produced by environmental chemical hazards. Ann N Y Acad Sci. 1979;329:14–29. [Google Scholar]
- 14.Vidyasagar T. Optic nerve components may not be equally susceptible to damage by acrylamide. Brain Res. 1981;16(224):452–455. doi: 10.1016/0006-8993(81)90877-5. [DOI] [PubMed] [Google Scholar]
- 15.Boyes W, Laurie R, Cooper G. Acrylamide toxicity effects on cortical evoked potentials and locomotor activity in rats. Neurosci Abstr. 1980;6:727. [Google Scholar]
- 16.Wild H, Kulikowski J. Neurotoxic effects of acrylamide on rat retinogeniculate fibres. Behav Brain Res. 1984;13:201–207. doi: 10.1016/0166-4328(84)90162-1. [DOI] [PubMed] [Google Scholar]
- 17.Andrews A. Electrophoresis theory, techniques, and biochemical and clinical application. 2nd ed. Oxford: Clarendon Press; 1986. [Google Scholar]
- 18.Mottram D, Wedzicha B, Dodson A. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- 19.Tareke E, Rydberg P, Karlsson P, Eriksson S, Tornqvist M. Analysis of acrylamide, a carcinogen formed in heated food stuffs. J Agric Food Chem. 2002;50:4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- 20.Becalski A, Lau B, Lewis D, Seama S. Acrylamide in foods: occurrence, sources and modeling. J Agric Food Chem. 2003;51:802–808. doi: 10.1021/jf020889y. [DOI] [PubMed] [Google Scholar]
- 21.Lopachin R, Balaban C, Ross J. Acrylamide axonopathy revisited. Toxicol Appl Pharmacol. 2003;188:135–153. doi: 10.1016/s0041-008x(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 22.Lopachin R, Gavin T, Geohagen B, Das S. Neurotoxic mechanisms of electrophilic type-2 alkenes: soft interactions described by quantum mechanical parameters. Toxicol Sci. 2007b;98:561–570. doi: 10.1093/toxsci/kfm127. [DOI] [PubMed] [Google Scholar]
- 23.Barber D, Lopachin R. Proteomic analysis of acrylamide- protein adduct formation in rat brain synaptosomes. Toxicol Appl Pharmacol. 2004;201:120–136. doi: 10.1016/j.taap.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Lonart G, Sudhof T. Assembly of SNARE core complexes prior to neurotransmitter release sets the readily releasable pool of synaptic vesicles. J Biol Chem. 2000;275:27703–27707. [PubMed] [Google Scholar]
- 25.Lopachin R, Lehning E. Acrylamide-induced distal axon degeneration: a proposed mechanism of action. Neurotoxicology. 1994;15:247–260. [PubMed] [Google Scholar]
- 26.Cavanagh J. The pathokinetics of acrylamide intoxication: a reassessment of the problem. Neuropathol Appl Neurobiol. 1982;8:315–336. doi: 10.1111/j.1365-2990.1982.tb00300.x. [DOI] [PubMed] [Google Scholar]
- 27.De Grandchamp R, Lowndes H. Early degeneration and sprouting at the rat neuromuscular junction following acrylamide administration. Neuropathol Appl Neurobiol. 1990;16:239–254. doi: 10.1111/j.1365-2990.1990.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 28.Ko M, Chen W, Lin-Shiau S, Hsieh S. Age-dependent- acrylamide neurotoxicity in mice morphology, physiology and function. Exp Neurol. 1999;158:37–48. doi: 10.1006/exnr.1999.7102. [DOI] [PubMed] [Google Scholar]
- 29.Gold B, Schaumburg H. Acrylamide. In: Spencer PS, Schaumburg H, Ludolph A, editors. Experimental and clinical neurotoxicology. 2nd ed. New York: Oxford University Press; 2000. pp. 124–132. [Google Scholar]
- 30.Schettgen T, Kutting B, Hornig M, Bechmann M, Weiss T, Drexler H, et al. Trans-placental exposure of neonates of acrylamide a pilot study. Int Arch Occup Environ Health. 2004;77:213–216. doi: 10.1007/s00420-003-0496-8. [DOI] [PubMed] [Google Scholar]
- 31.Soergel F, Weissenbacher R, Schippers MK, Hofmann MA, Skott A, Landersdorfer C. Acrylamide increased concentrations in homemade food and first evidence of its variable absorption from food, variable metabolism and placental and breast milk transfer in humans. Chemotherapy. 2002;48:267–274. doi: 10.1159/000069715. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Donia M, Ibrahim S, Cororan J, Lack J, Friedman M, Lapadula D. Neurotoxicity of glycidamide, an acrylamide metabolite, following intraperitoneal injections in rats. J Toxicol Environ Health. 1993;39:447–464. doi: 10.1080/15287399309531764. [DOI] [PubMed] [Google Scholar]
- 33.Zhu F, Cai Y, Ke J, Corke H. Evaluation of the effect of plant extracts and phenolic compounds on reduction of acrylamide in an asparagine/glucose model system by RP-HPLC-DAD. J Sci Food Agric. 2009;89:1674–1681. [Google Scholar]
- 34.Allam A, El-Ghareeb AA, Abdul-Hamid M, Baikry A, Sabri MI. Prenatal and perinatal acrylamide disrupts the development of cerebellum in rat: biochemical and morphological studies. Toxicol Ind Health. 2011 doi: 10.1177/0748233710386412. [DOI] [PubMed] [Google Scholar]