Abstract
Objective
To develop a simple rapid procedure for bioreduction of silver nanoparticles (AgNPs) using aqueous leaves extracts of Catharanthus roseus (C. roseus).
Methods
Characterization were determined by using UV-Vis spectrophotometry, scanning electron microscopy (SEM), energy dispersive X-ray and X-ray diffraction.
Results
SEM showed the formation of silver nanoparticles with an average size of 67 nm to 48 nm. X-ray diffraction analysis showed that the particles were crystalline in nature with face centered cubic geometry.
Conclusions
C. roseus demonstrates strong potential for synthesis of silver nanoparticles by rapid reduction of silver ions (Ag+ to Ag0). This study provides evidence for developing large scale commercial production of value-added products for biomedical/nanotechnology-based industries.
Keywords: Nanoparticles, Nanotechnology, Silver, Catharanthus roseus
1. Introduction
Nanotechnology is expected to be the basis of many main technological innovations in the 21st century. Research and development in this field is growing rapidly throughout the world. A major output of this activity is the development of new materials in the nanometer scale, including nanoparticles. These are usually defined as particulate materials with at least one dimension of less than 100 nanometers (nm), even the particles could be zero dimension in the case of quantum dots. Metal nanoparticles have been of great interest due to their distinctive features such as catalytic, optical, magnetic and electrical properties[1],[2].
Nanoparticles exhibit completely new or improved properties compared with larger particles of the bulk materials and these novel properties are derived due to the variation in specific characteristics such as size, distribution and morphology of the particles. Nanoparticles present a higher surface area to volume ratio with decrease in the size of the particles. Specific surface area is relevant to catalytic activity and other related properties such as antimicrobial activity of AgNPs[3]–[5]. As the specific surface area of nanoparticles is increased, their biological effectiveness can also increase on the account of a rise in surface energy. Nanoparticles of noble metals, such as silver, gold and platinum are widely applied in products that directly come in contact with the human body, such as shampoos, soaps, detergent, shoes, cosmetic products, and toothpaste, besides medical and pharmaceutical applications.
A number of approaches are available for the synthesis of silver nanoparticles for example facile method[6], thermal decomposition of silver compounds[7], electrochemical[8], sonochemical[9], microwave assisted process[10] and recently via green chemistry route[11]. Unfortunately, many of the nanoparticle synthesis or production methods involve the use of hazardous chemicals, low material conversions, high energy requirements, difficult and wasteful purifications. Therefore, there is a growing need to develop environmentally friendly processes for nanoparticle synthesis without using toxic chemicals. Biosynthetic methods employing either microorganisms or plant extracts have emerged as a simple and viable alternative to chemical synthetic procedures and physical methods.
Biological approaches using microorganisms and plants or plant extracts for metal nanoparticle synthesis have been suggested as valuable alternatives to chemical methods[12],[13]. The use of plant materials for the synthesis of nanoparticles could be more advantageous, because it does not require elaborate processes such as intracellular synthesis and multiple purification steps or the maintenance of microbial cell cultures. The above synthetic protocol by plant extract or biomass exemplifies the promising application of the green synthesis of metal nanoparticles. Recent research reported that silver nanoparticles have been synthesized using various natural products like green tea (Camellia sinensis)[14], neem (Azadirachta indica) leaf broth[15], natural rubber[16], Aloe vera plant extract[17], latex of Jatropha curcas[1], etc.
Catharanthus roseus (Apocynaceae) (C. roseus) is a traditionally used medicinal plants. It is an erected procumbent herb or under shrub containing latex. It is widely growing to 1 m tall at subtropical area. It possesses known antibacterial, antifungal, antibiotic, antioxidant, wound healing and antiviral activities[18]–[20]. Herein, we report for the first time synthesis of silver nanoparticles, reducing the silver ions present in the solution of silver nitrate by the aqueous extract of C. roseus leaf. Further, these biologically synthesized nanoparticles are found highly toxic against different pathogenic bacteria.
2. Materials and methods
2.1. Materials
All chemicals used in this experiment were of highest purity and obtained from Sigma (Bangalore, India) and Merck (Mumbai, India). C. roseus leaves were harvested from Villupuram, Tamilnadu, India for silver nanoparticle synthesis.
2.2. Plant extract and synthesis of silver nanoparticles
Plant leaf extract was prepared by mixing 10 g of dried powder with 100 mL deionized water in 500 mL of Erlenmeyer flask and boiled for 10 min. For the reduction of Ag+ ions, 10 mL of leaf extract was mixed to 90 mL of 1 mM aqueous of AgNO3 and then, heated at 80 °C for 15 min. A change from brown to reddish color was observed.
2.3. UV-VIS spectra analysis
UV-VIS spectroscopy measurements (Shimadzu UV -2450) were carried out as a function of time of the reaction at room temperature operated at a resolution of 1 nm. The reduction of silver ions was confirmed by qualitative testing of supernatant obtained after centrifugation with a pinch of NaCl.
2.4. SEM analysis of silver nanoparticles
Scanning electron microscopic (SEM) analysis was done using Hitachi S-4500 SEM machine. Thin films of the sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid. Extra solution was removed using a blotting paper and then the films on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 min.
2.5. Energy dispersive X-ray (EDX)
In order to carry out EDX analysis, the leaves extracts reduced silver nanoparticles were dried and drop coated onto carbon film and performed on Hitachi S-4500 SEM instrument equipped with a Thermo EDX attachments.
2.6. X-ray diffraction measurements
X-ray diffraction (XRD) measurements of the bioreduced silver nitrate solution drop-coated onto glass substrates were done for the determination of the formation of Ag by an X'Pert Pro P Analytical X-ray diffractometer instrument with X'Pert high score plus software operating at a voltage of 45 kV and a current of 40 mA with Cu Kα radiation.
2.7. Antibacterial activity study
Antibacterial activities of the synthesized Ag nanoparticles were determined using the agar well diffusion assay method[21]. Approximately 20 mL of molten and cooled media (Nutrient agar) was poured in sterilized Petri dishes. The plates were left overnight at room temperature to check for any contamination to appear. The bacterial test organisms were grown in nutrient broth for 24 h. A 100 mL nutrient broth culture of each bacterial organism (1×105 cfu/mL) was used to prepare bacterial lawns. Agar wells of 5 mm diameter were prepared with the help of a sterilized stainless steel cork borer. Two wells were prepared in the agar plates. The wells were labeled as A, B. ‘A’ well was loaded with 30 µL of Ag nanoparticles suspended ‘hydrosol’ and ‘B’ well loaded with 30 µL of positive control drugs (chloromphenical) was used as positive control. The plates containing the bacterial and Ag nanoparticles were incubated at 37 °C. The plates were examined for evidence of zones of inhibition, which appeared as a clear area around the wells. The diameter of such zones of inhibition was measured using a meter ruler and the mean value for each organism was recorded and expressed in millimeter.
3. Results
3.1. UV-VIS spectra analysis
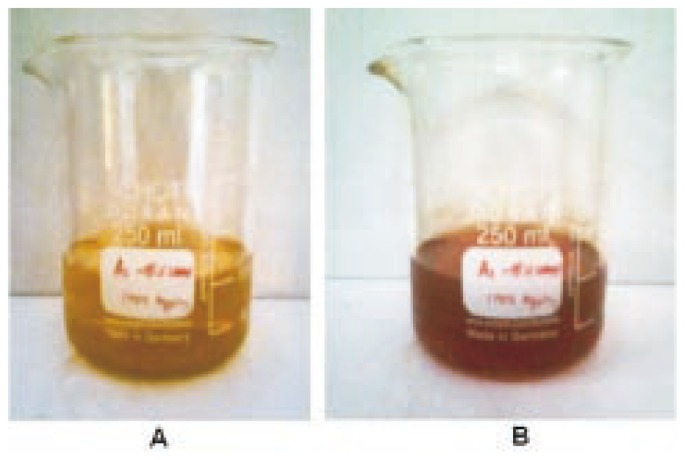
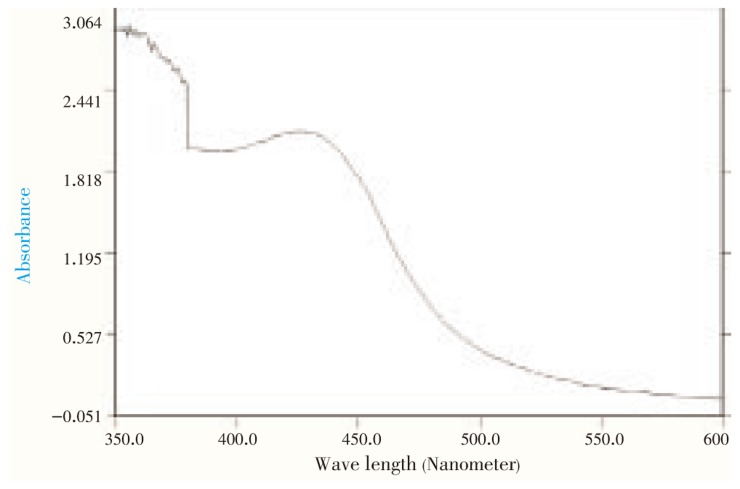
The reaction mixture, C. roseus leaf extract with aqueous solution of the silver nitrate, started to change its color from yellowish brown to reddish brown (Figure 1). It indicated the formation of silver nanoparticle with the reduction of silver ion. The characteristic surface plasmon absorption bands were observed at 440 nm. Extinctions spectra of silver synthesized from AgNO3 were shown in Figure 2.
Figure 1. Color changes before (A) and after (B) the process of reduction of Ag+ to Ag nanoparticles.
Figure 2. UV-visible absorption spectra of reduction of silver ions to silver nanoparticles.
3.2. SEM and EDX studies
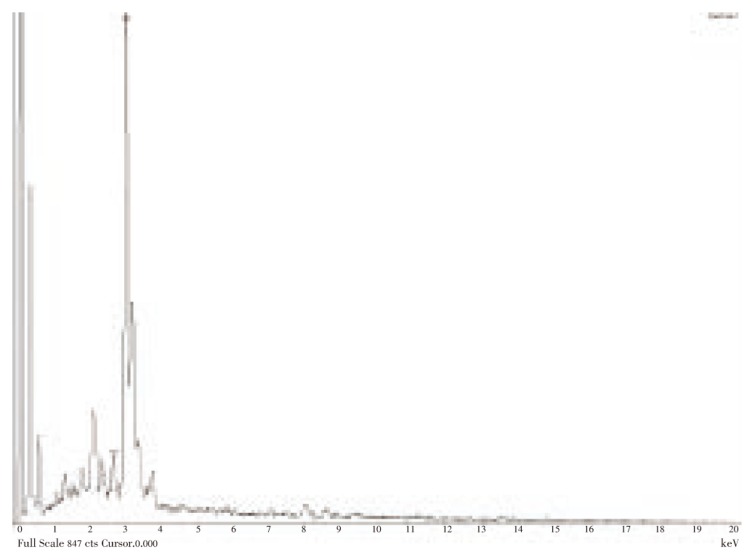
Figure 3 represents the SEM image recording from drop-coated films of the silver nanoparticles synthesized with C. roseus leaf extract. The SEM image showed cubical and relatively uniform shape of nanoparticle formation with diameter range 48-67 nm. Analysis through EDX spectrometers confirmed the presence of elemental silver signal of the silver nanoparticles (Figure 4). The vertical axis displays the number of X-ray counts whilst the horizontal axis displays energy in KeV.
Figure 3. SEM image of Ag nanoparticles formed by C. roseus.
Figure 4. EDX spectrum recorded from a film, after formation of silver nanoparticles with different X-ray emission peaks labeled.
3.3. XRD Studies
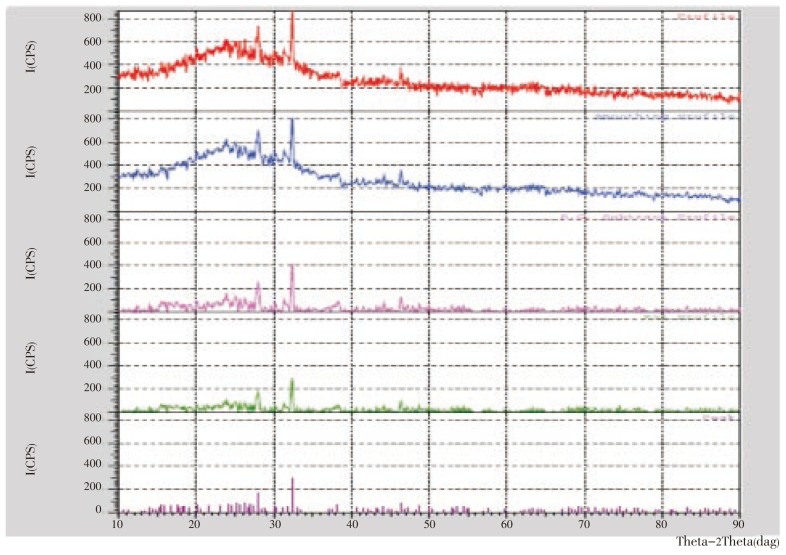
The studies with XRD pattern obtained in Figure 5 showed number of Braggs reflections. There are three intense peaks in the whole spectrum of 2θ values ranging from 24 to 90. A comparison of our XRD spectrum with the standard confirmed that the silver particles formed in our experiments were in the form of nanocrystals, as evidenced by the peaks at 2θ values of 26.2°, 27.9°, and 32.3°, and integrated intensity values of (125), (226), and (264), respectively, for silver.
Figure 5. XRD patterns recorded from drop-coated films on glass substrate of silver nanoparticles synthesized by treating C. roseus leaf extract with AgNO3 aqueous solutions with the Bragg reflections indexed on the basis of the FCC silver structure.
3.4. Antibacterial studies
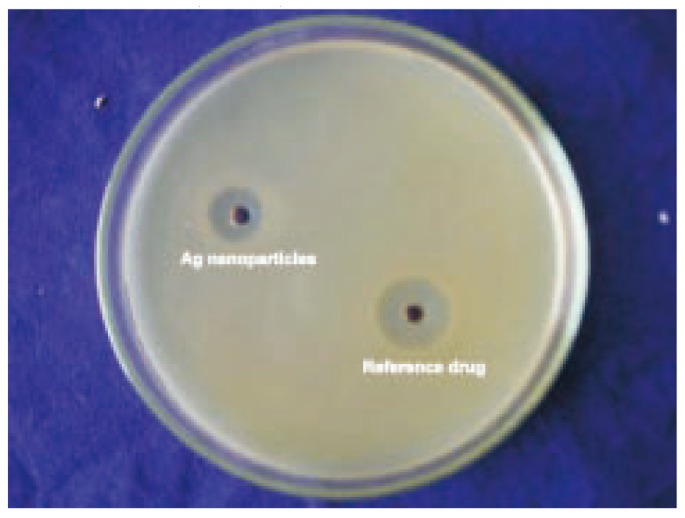
Biosynthesis of silver nanoparticles were studied for antimicrobial activity against pathogenic bacteria (clinical isolate) by using standard zone of inhibition (ZOI) microbiology assay, with a well size of 5 mm diameter and 50 µL of samples. Chloromphenical of 10 mg/mL concentration was used as a control antimicrobial agent. The silver nanoparticles synthesized showed inhibition zone against all the studied bacteria. Maximum zone of inhibition was found to be 11 mm in Bacillus cereus (Figure 6) and minimum of 6 mm in all studied bacteria (Table 1).
Figure 6. The antibacterial activity of Ag nanoparticle against Bacillus cereus.
Table 1. The antibacterial activity of Ag nanoparticle synthesized using leaves of C. roseus (mm).
| Name of the bacterial sps | Zone of inhibition |
|
| Ag nanoparticle | Reference drug | |
| Staphylococcus aureus | 10 | 18 |
| Escherichia coli | 8 | 20 |
| Klebsiella pneumoniae | 7 | 22 |
| Bacillus cereus | 11 | 19 |
| Pseudomonas aeruginosa | 6 | 22 |
4. Discussion
The synthesis of nanoparticles is in lime light of modern nanotechnology. Biosynthesis of nanoparticles by plant extracts is currently under exploitation. The development of biologically inspired experimental processes for the synthesis of nanoparticles is evolved into an important branch of nanotechnology. The present study deals with the synthesis of silver nanoparticles using leaves extracts of C. roseus and aqueous Ag+ ions. Comparative experiments were carried out to study the effect of biomass dosage and the concentration of silver nitrate on the rate of bioreduction of silver ions. The approach appears to be cost effective alternative to conventional methods of assembling silver nanoparticles.
Formation and stability of silver nanoparticles in aqueous colloidal solution are confirmed using UV-VIS spectral analysis. It is well known that silver nanoparticles exhibit yellowish brown color in aqueous solution due to excitation of surface plasmon vibrations in silver nanoparticles[22],[23]. As the C. roseus leaf extract was mixed with aqueous solution of the silver nitrate, it started to change the color from watery to reddish brown due to reduction of silver ion, which indicated the formation of silver nanoparticles. It is generally recognized that UV-VIS spectroscopy could be used to examine size and shape-controlled nanoparticles in aqueous suspensions[24]. Absorption spectra of silver nanoparticles formed in the reaction media has absorbance peak at 440 nm and broadening of peak indicated that the particles are polydispersed.
The silver nanoparticles formed were predominantly cubical with uniform shape. It is known that the shape of metal nanoparticles considerably change their optical and electronic properties[25]. The SEM image showed relatively spherical shape nanoparticle formed with diameter range 48-67 nm. Similar phenomenon was reported by Chandran et al[17].
Analysis through EDX spectrometers confirmed the presence of elemental silver signal of the silver nanoparticles. Identification lines for the major emission energies for silver (Ag) are displayed and these correspond with peaks in the spectrum, thus giving confidence that silver has been correctly identified.
Further studies using X-ray diffraction were carried out to confirm the crystalline nature of the particles, and the XRD pattern showed numbers of Braggs reflections that may be indexed on the basis of the face centered cubic structure of silver. A comparison of our XRD spectrum with the standard confirmed that the silver particles formed in our experiments were in the form of nanocrystals, as evidenced by the peaks at 2θ values of 26.2°, 27.9°, and 32.3°, corresponding to (125), (226), and (264), respectively, for silver. The X-ray diffraction results clearly show that the silver nanoparticles formed by the reduction of Ag+ ions by the C. roseus leaf extract are crystalline in nature[26]. As mentioned in the method section, the silver nanoparticles once formed were repeatedly centrifuged and redispersed in sterile distilled water prior to XRD analysis, thus ruling out the presence of any free compound/protein that might independently crystallize and giving rise to Bragg reflections.
Silver has been widely utilized for thousands of years in human history. Its applications include jewels, utensils, currency, dental alloy, photography and explosives. Among silver's many applications, its disinfectant property is being exploited for hygienic and medicinal purposes, such as treatment of mental illness, nicotine addiction and infectious disease like syphilis and gonorrhea[26],[27]. Silver nanoparticles have been demonstrated to exhibit antimicrobial properties against bacteria with close attachment of the nanoparticles themselves with the microbial cell and the activity being size dependent[28].
Lee et al[29] investigated the antibacterial effect of nanosized silver colloidal solution against Staphylococcus aureus (S. aureus) and Klebsiella pneumoniae after padding the solution on textile fabrics. Shrivastava et al[30] studied antibacterial activity against Escherichia coli (E. coli)(ampicillin resistant), E. coli, S. aureus, and Salmonella typhi (multi-drug resistant). They reported that the effect was dose dependent and was more pronounced against gram-negative organisms than gram-positive ones. They found that the major mechanism through which silver nanoparticles manifest antibacterial properties was either by anchoring or penetrating the bacterial cell wall, and modulating cellular signaling by dephosphorylating putative key peptide substrates on tyrosine residues[30]. The antibacterial efficacy of the biogenic silver nanoparticles reported in the present study may be ascribed to the mechanism described above but it still remains to clarify the exact effect of the nanoparticles on important cellular metabolism like DNA, RNA and protein synthesis.
We have developed a biological method to synthesize silver nanoparticles using the aqueous leaves extract of C. roseus, whose leaves extract is environmentally benign and renewable, act as reducing agent. Particles are mostly polydispersed in shape. Ag nanoparticles prepared in this process are quiet fast and low cost. We believe that development of eco-friendly process for the synthesis of metallic nanoparticles is an important step in the field of application of nanotechnology.
Acknowledgments
The authors would like to thank the Department of Biotechnology, MIT, Manipal for providing logistic support and their continuous encouragement and advice. Special thanks to Dr. Balaji S for critical remarks on the manuscript.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Eng Asp. 2009;339:134–139. [Google Scholar]
- 2.Rassaei L, Sillanpaa M, French RW, Compton RG, Marken F. Arsenite determination in the presence of phosphate at electro-aggregated gold nanoparticle deposits. Electroanalysis. 2008;20:1286–1292. [Google Scholar]
- 3.Bae E, Park HJ, Lee J, Kim Y, Yoon J, Park K. Bacterial cytotoxicity of the silver nanoparticle related to physicochemical metrics and agglomeration properties. Envion Toxicol Chem. 2010;29(10):2154–2160. doi: 10.1002/etc.278. [DOI] [PubMed] [Google Scholar]
- 4.Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, Muniyandi J, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces. 2009;74(1):328–335. doi: 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 5.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nidhi N, Santosh K, Ghosh T, Dutta PK. Preparation of Chitosan based silver nanocomposites by a facile method. Chandigiah: CSIO; 2009. International Conference on Optics and Photonics. [Google Scholar]
- 7.Navaladian S, Viswanathan B, Viswanath RP, Varadarajan TK. Thermal decomposition as route for silver nanoparticles. Nanoscale Res Lett. 2007;2:44–48. doi: 10.1007/s11671-006-9028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starowicz M, Stypuła B, Banaś J. Electrochemical synthesis of silver nanoparticles. Electrochem Commun. 2006;8(2):227–230. [Google Scholar]
- 9.Esau SR, Roberto SB, Ocotlan-Flores J, Saniger JM. Synthesis of AgNPs by sonochemical induced reduction application in SERS. J Nanopart Res. 2010;9:77. [Google Scholar]
- 10.Sreeram KJ, Nidin M, Nair BU. Microwave assisted template synthesis of silver nanoparticles. Bull Mater Sci. 2008;31(7):937–942. [Google Scholar]
- 11.Begum NA, Mondal S, Basu S, Laskar RA, Mandal D. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of black tea leaf extracts. Colloids Surf B Biointerfaces. 2009;71(1):113–118. doi: 10.1016/j.colsurfb.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya D, Gupta RK. Nanotechnology and potential of microoganisms. Crit Rev Biotechnol. 2005;25:199–204. doi: 10.1080/07388550500361994. [DOI] [PubMed] [Google Scholar]
- 13.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2007;10:507–517. [Google Scholar]
- 14.Vilchis-Nestor AR, Sanchez MV, Camacho-Lopez MA, Gomez-Espinoza RM. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett. 2008;62:3103–3105. [Google Scholar]
- 15.Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Abu Bakar NHH, Ismail J, Abu Bakar M. Synthesis and characterization of silver nanoparticles in natural rubber. Mater Chem Phys. 2007;104:276–283. [Google Scholar]
- 17.Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22:577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 18.Jaleel CA, Gopi R, Paneerselvam R. Alterations in non-enzymatic antioxidant components of Catharanthus roseus exposed to paclobutrazol, gibberellic acid and Pseudomonas fluorescens. Plant Omics J. 2009;2:30–40. [Google Scholar]
- 19.Jayanthi M, Sowbala N, Rajalakshmi G, Kanagavalli, Sivakumar V. Study of antihyerglycemic effect of Catharanthus roseus in alloxan induced diabetic rats. Int J Pharm & Pharm Sci. 2010;2(4):114–116. [Google Scholar]
- 20.Nayak BS, Pinto Pereira LM. Catharanthus roseus flower extract has wound-healing activity in Sprague Dawley rats. BMC Complement Altern Med. 2006;6:41. doi: 10.1186/1472-6882-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez C, Pauli M, Bazerque P. Antibiotic assay by agar well diffusion method. Acta Biol Med Exp. 1990;15:113–115. [Google Scholar]
- 22.Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N. Synthesis of silver hanoparticles using Acalypha indica leaf extracts and its antibacterial activity water borne pathogens. Colloids Surf B Biointerfaces. 2010;76:50–56. doi: 10.1016/j.colsurfb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Noginov MA, Zhu G, Bahoura M, Adegoke J, Small C, Ritzo BA, et al. The effect of gain and absorption on surface plasmon in metal nanoparticles. Appl Phys B. 2006;86:455–460. [Google Scholar]
- 24.Shrivastava S, Dash D. Agrifood nanotechnology: a tiny revolution in food and agriculture. J Nano Res. 2009;6:1–14. [Google Scholar]
- 25.Xu H, Kall M. Surface-plasmon-enhanced optical forces in silver nanoaggregates. Phys Rev Lett. 2002;89:246802. doi: 10.1103/PhysRevLett.89.246802. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, et al. Biosynthesis of silver andgold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18:105104–105114. [Google Scholar]
- 27.Gulbrason SH, Hud JA, Hansen RC. Argyria following the use of dietary supplements containing collied protein. Cutis. 2000;66:373–374. [PubMed] [Google Scholar]
- 28.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Yeo SY, Jeong SH. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mater Sci. 2003;38:2199–2204. [Google Scholar]
- 30.Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D. Characterization of enhanced antibacterial effect of novel silver nanopartides. Nanotechnology. 2007;18:225103. doi: 10.1088/0957-4484/18/22/225103. [DOI] [PubMed] [Google Scholar]