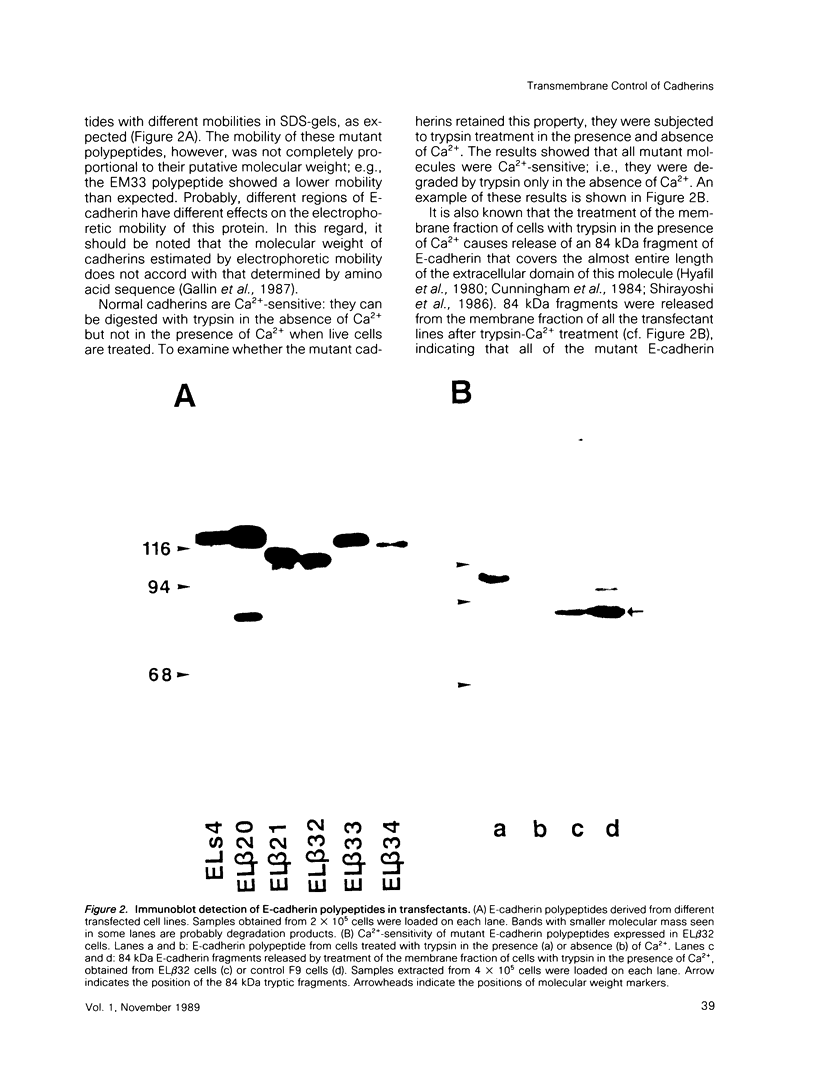
Abstract
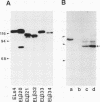
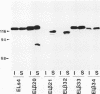
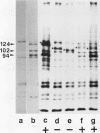
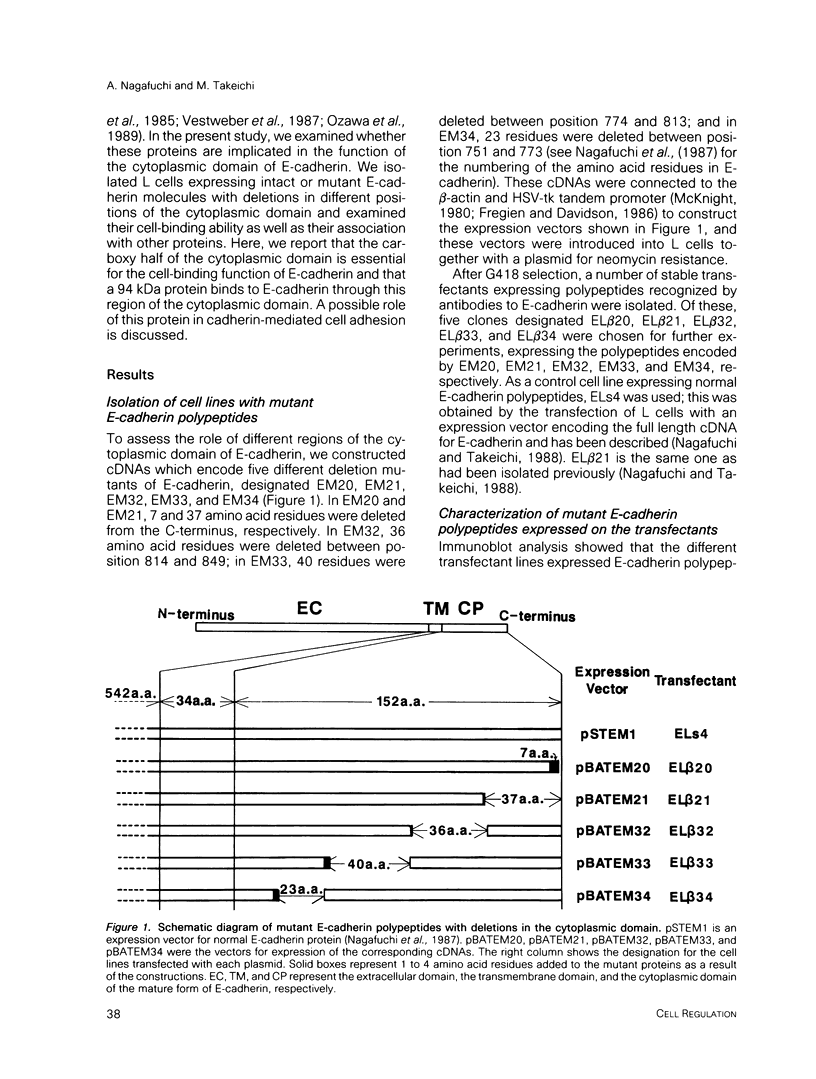
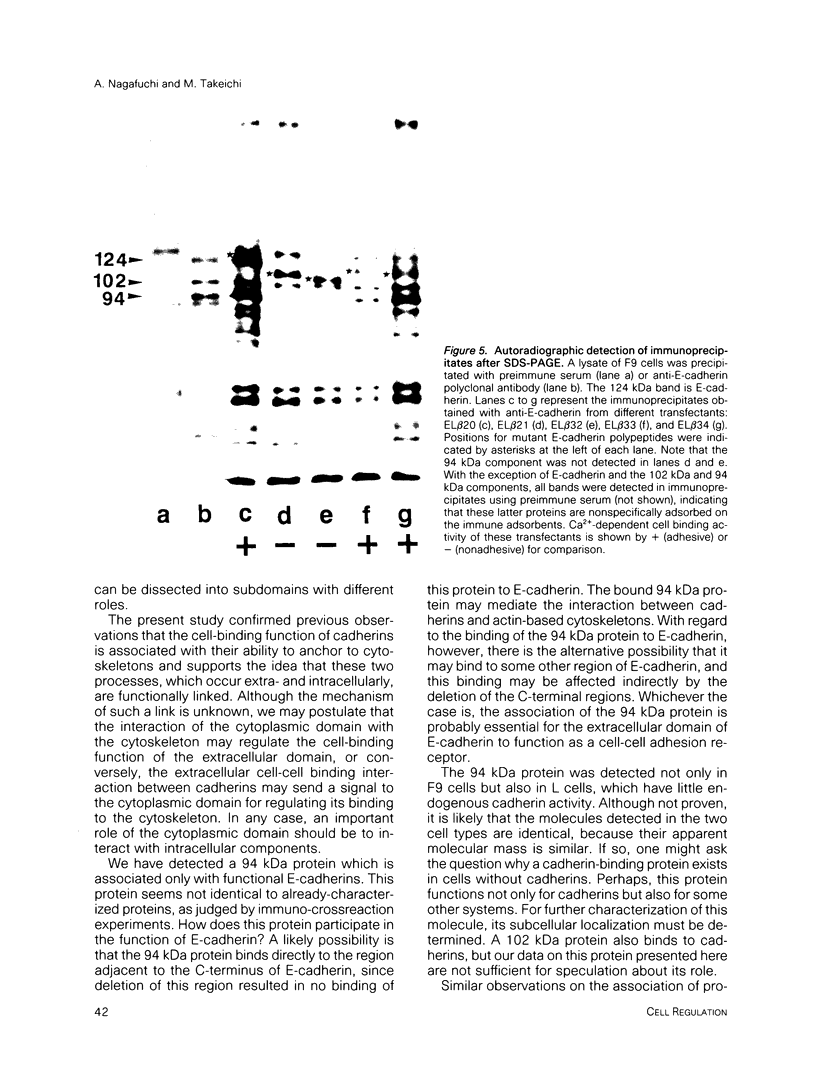
Cadherins are a family of transmembrane glycoproteins which play a key role in Ca(2+)-dependent cell-cell adhesion. Cytoplasmic domains of these molecules are anchored to the cell cytoskeleton and are required for cadherin function. To elucidate how the function of cadherins is controlled through their cytoplasmic domains, we deleted five different regions in the cytoplasmic domain of E-cadherin. After transfecting L cells with cDNA encoding the mutant polypeptides, we assayed aggregating activity of these transfectants; all these mutant proteins were shown to have an extracellular domain with normal Ca(2+)-sensitivity and molecular weight. Two mutant polypeptides with deletions in the carboxy half of the cytoplasmic domain, however, did not promote cell-cell adhesion and had also lost the ability to bind to the cytoskeleton, whereas the mutant molecules with deletions of other regions retained the ability to promote cell adhesion and to anchor to the cytoskeleton. Thus, the cytoplasmic domain contains a subdomain which was involved in the cell adhesion and cytoskeleton-binding functions. When E-cadherin in F9 cells or in L cells transfected with wild-type or functional mutant cadherin polypeptides was solubilized with nonionic detergents and immunoprecipitated, two additional 94 and 102 kDa components were coprecipitated. The 94 kDa component, however, was not detected in the immunoprecipitates from cells expressing the mutant cadherins which had lost the adhesive function. These results suggest that the interaction of the carboxy half of the cytoplasmic domain with the 94 kDa component regulates the cell binding function of the extracellular domain of E-cadherin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller K., Vestweber D., Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985 Jan;100(1):327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Leutzinger Y., Gallin W. J., Sorkin B. C., Edelman G. M. Linear organization of the liver cell adhesion molecule L-CAM. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5787–5791. doi: 10.1073/pnas.81.18.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Sorkin B. C., Edelman G. M., Cunningham B. A. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Volk T., Volberg T. Molecular heterogeneity of adherens junctions. J Cell Biol. 1985 Oct;101(4):1523–1531. doi: 10.1083/jcb.101.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K., Nose A., Nagafuchi A., Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J Cell Biol. 1988 Mar;106(3):873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S., Nose A., Hatta K., Kawakami A., Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987 Dec;105(6 Pt 1):2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil F., Morello D., Babinet C., Jacob F. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell. 1980 Oct;21(3):927–934. doi: 10.1016/0092-8674(80)90456-0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acids Res. 1980 Dec 20;8(24):5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988 Sep 23;54(7):993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyriéras N., Louvard D., Jacob F. Characterization of antigens recognized by monoclonal and polyclonal antibodies directed against uvomorulin. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8067–8071. doi: 10.1073/pnas.82.23.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwald M., Schuh R., Vestweber D., Eistetter H., Lottspeich F., Engel J., Dölz R., Jähnig F., Epplen J., Mayer S. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J. 1987 Dec 1;6(12):3647–3653. doi: 10.1002/j.1460-2075.1987.tb02697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y., Hatta K., Hosoda M., Tsunasawa S., Sakiyama F., Takeichi M. Cadherin cell adhesion molecules with distinct binding specificities share a common structure. EMBO J. 1986 Oct;5(10):2485–2488. doi: 10.1002/j.1460-2075.1986.tb04525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S. Isolation of cell-to-cell adherens junctions from rat liver. J Cell Biol. 1989 Jan;108(1):31–41. doi: 10.1083/jcb.108.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D., Gossler A., Boller K., Kemler R. Expression and distribution of cell adhesion molecule uvomorulin in mouse preimplantation embryos. Dev Biol. 1987 Dec;124(2):451–456. doi: 10.1016/0012-1606(87)90498-2. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Kemler R. Some structural and functional aspects of the cell adhesion molecule uvomorulin. Cell Differ. 1984 Dec;15(2-4):269–273. doi: 10.1016/0045-6039(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Volk T., Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. I. Immunoelectron microscopic localization and biochemical studies. J Cell Biol. 1986 Oct;103(4):1441–1450. doi: 10.1083/jcb.103.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T., Geiger B. A-CAM: a 135-kD receptor of intercellular adherens junctions. II. Antibody-mediated modulation of junction formation. J Cell Biol. 1986 Oct;103(4):1451–1464. doi: 10.1083/jcb.103.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida C., Takeichi M. Teratocarcinoma cell adhesion: identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell. 1982 Feb;28(2):217–224. doi: 10.1016/0092-8674(82)90339-7. [DOI] [PubMed] [Google Scholar]