Abstract
Objective
To evaluate the in vitro antifungal activity of Aegle marmelos leaf extracts and fractions on the clinical isolates of dermatophytic fungi like Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum canis, Microsporum gypseum and Epidermophyton floccosum.
Methods
The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of various extracts and fractions of the leaves of Aegle marmelos were measured using method of National Committee for Clinical Laboratory Standards (NCCLS).
Results
Aegle marmelos leaf extracts and fractions were found to have fungicidal activity against various clinical isolates of dermatophytic fungi. The MIC and MFC was found to be high in water and ethyl alcohol extracts and methanol fractions (200µg/mL) against dermatophytic fungi studied.
Conclusions
Aegle marmelos leaf extracts significantly inhibites the growth of all dermatophytic fungi studied. If this activity is confirmed by in vivo studies and if the compound is isolated and identified, it could be a remedy for dermatophytosis.
Keywords: Antifungal activity, Aegle marmelos, Dermatophytosis, Dermatophytes
1. Introduction
Mycotic infections are the most common cause of skin infection in tropical developing countries. The incidence of dermatophytosis raised dramatically in the past one decade. Humid weather, over population and poor hygiene are the ideal conditions for the growth of dermatophytes[1]. These dermatophytes invade skin, hair and nail and cause dermatophytosis. Though these dermatophytes respond to treatment with conventional antifungal agents, the disease had a tendency to reoccur in the same area or other ones[2].
Medicinal plants represent a rich source of antimicrobial agents[3]–[36]. Therapeutic efficacy of many indigenous plants for several disorders has been described by practitioners of traditional medicine[37]. Treatment based on Indian medicinal plants is becoming increasingly popular among patients with dermatophytes and physicians are also looking for alternative treatments because the present-day cures have side effects[38].
Aegle marmelos (L.) Correa (A. marmelos), a tree species belonging to the family Rutaceae, is commonly called Vilvam (in Tamil), and often cultivated in temples for its leaves are used for pujas. The leaves, stem, bark and fruits of this plant have long been used in traditional medicine for its medicinal value. The leaves are widely used to treat diarrhoea, dysentery, skin and eye diseases[39]–[43]. They contain terpenoids which act as an antifungal agent[44]. After these facts were known, the present work was done to investigate the antifungal activity of the leaves of A. marmelos against the clinical isolates of dermatophytic fungi from patients attending the Department of Dermatology of Bharath Heavy Electrical Limited (BHEL) Hospital, Tiruchirappalli, India and Annal Gandhi Memorial Government Hospital, Tiruchirappalli, India.
2. Materials and methods
The plant material used in this study was collected from Tiruchirappalli, Tamil Nadu, India. It was identified and authenticated by the Botanist of Department of Plant Sciences, Bharathidasan University, Tiruchirappalli. Fresh leaves were collected and shade dried. The dried leaves were ground to powder and stored in an airtight container until further use. Known quantity of A. marmelos leaf powder was subjected to cold extraction with water and 100% ethyl alcohol separately and the aqueous extracts were collected. The extracts were dried in a vacuum desiccator and were stored in a sterile container for further use[45].
Known quantity of A. marmelos coarse powder was also successively extracted with various organic solvents like hexane, benzene, chloroform, ethyl acetate, methanol and water. Different fractions collected were filtered and evaporated to dryness in a vacuum concentrator. Coding was given to various extracts and fractions and was stored till use. The dried extracts and fractions were weighed and dissolved in 5% dimethyl sulfoxide (DMSO) and were used for further analysis.
Trichophyton mentagrophytes (T. mentagrophytes), Trichophyton rubrum (T. rubrum), Microsporum canis (M. canis), Microsporum gypseum (M. gypseum) and Epidermophyton floccosum (E. floccosum) were the five different clinical isolates of dermatophytic fungi taken for this study.
The selected isolates were grown on sabouraud dextrose agar (SDA). Twenty-one-day old culture of dermatophytic fungi was scraped with a sterile scalpel and macerated with sterile distilled water. The suspension was adjusted spectrophotometrically to an absorbance of 0.600 at 450 nm. By this way the fungal inoculum was prepared. For further study known quantity of this inoculum was used.
Susceptibility testing was performed by the reference broth micro dilution method[46]. MIC & MFC were determined after 21 days incubation at 35 °C. To know the phytoconstituents of A. marmelos, the extracts were subjected to the analysis of macromolecules and secondary metabolites by using thin layer chromatography and high performance thin layer chromatography.
3. Results
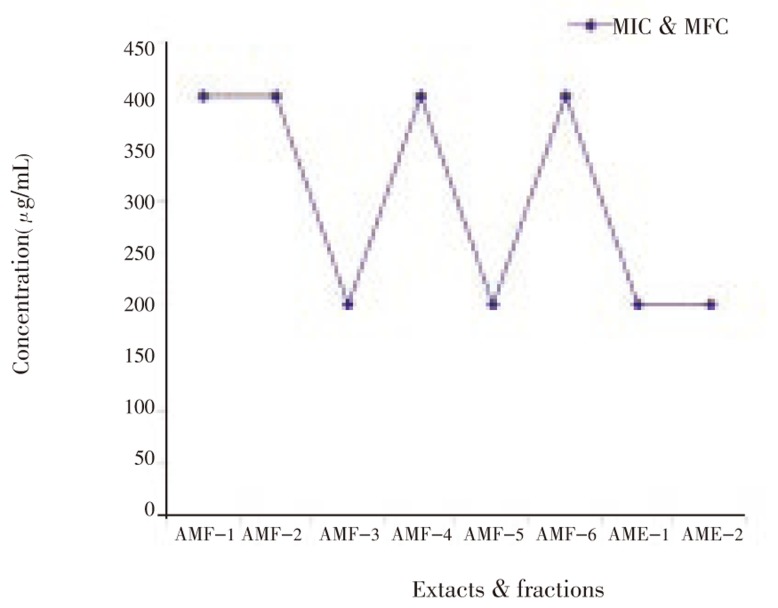
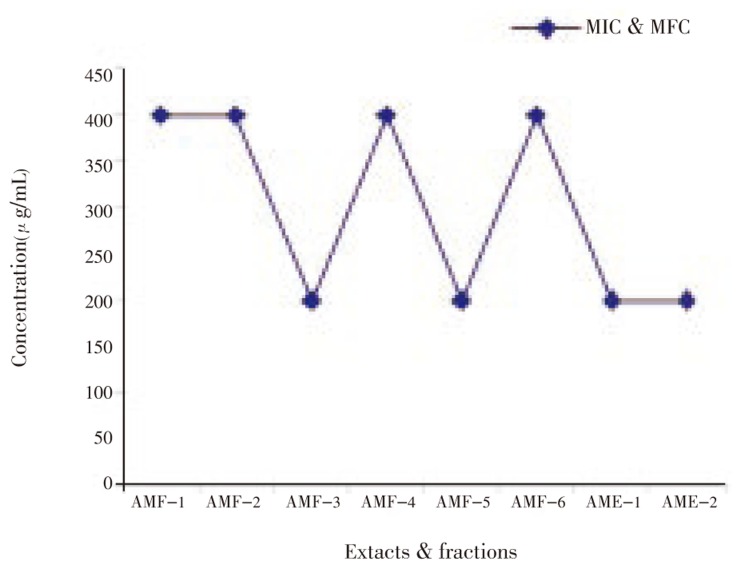
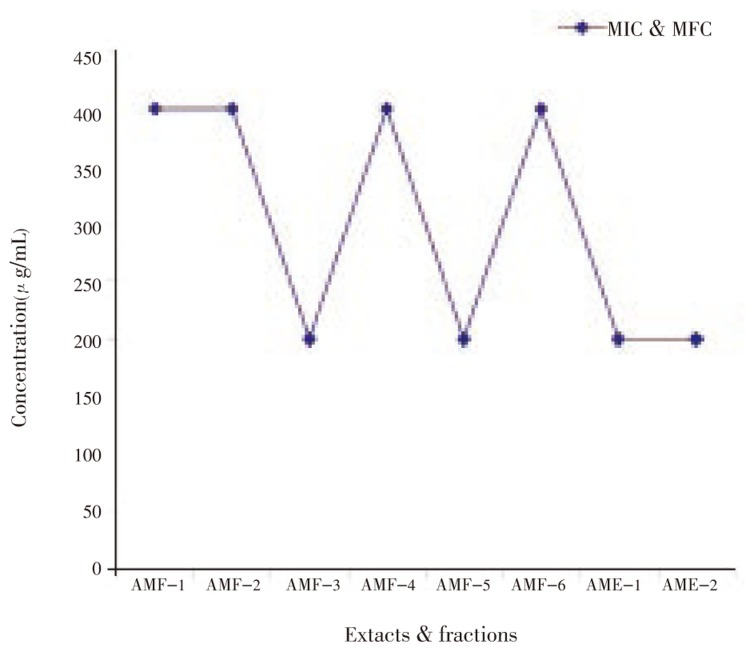
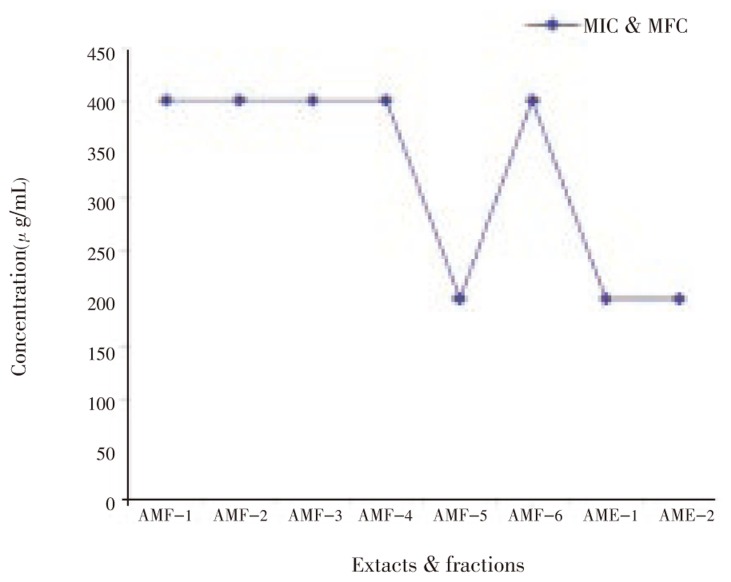
The results revealed that the extracts and fractions of A. marmelos leaves inhibited the growth of clinical isolates of dermatophytic fungi. All six fractions showed MIC and MFC at 400 µg/mL concentration against all the organisms tested. Methanol fraction, ethanol extract and water extract showed the MIC and MFC at 200 µg/mL against T. mentagrophytes, M. canis and E. floccosum (Figure 1–5). Steroids and alkaloids were totally absent in A. marmelos. Trace amounts of triterpenoids, phenolic compounds, tannins and flavonoids were seen in the extracts and fractions.
Figure 1. Effect of A. marmelos leaf extracts and fractions on T. rubrum.
AMF-1-Hexane fraction; AMF-2-Benzene fraction; AMF-3-Chloroform fraction; AMF-4-Ethyl acetate fraction; AMF-5-Methanol fraction; AMF-6-Water fraction; AME-1-Crude ethyl alcohol extract; AME-2-Crude water extract.
Figure 5. Effect of A. marmelos leaf extracts and fractions on E. floccosum.
Figure 2. Effect of A. marmelos leaf extracts & fractions on T. mentagrophytes.
Figure 3. Effect of A. marmelos leaf extracts & fractions on M. canis.
Figure 4. Effect of A. marmelos leaf extracts and fractions on M. gypseum.
4. Discussion
Occurrence of fungal diseases is a serious problem of the present world. This is because of the development of antifungal drug resistance of the pathogens and side effects exhibited by the drugs used for fungal diseases. Hence there is a great demand for safer, alternative and effective chemotherapeutic agents. Use of medicinal herbs in the treatment of skin diseases including mycotic infection is an age old practice in many parts of the world[47]. Plants contain a spectrum of secondary metabolites such as triterpenoids, phenols, flavanoids, quinines, tannins and their glycosides, alkaloids and their essential oils. The importance of these substances as antimicrobial agents against pathogens has been emphasized by several workers[1].
A wide spectrum of antifungal activity of ethanol and water extracts and methanol and chloroform fractions of A. marmelos leaves against 5 different dermatophytic fungi was observed in this study. In an earlier study Rana et al[44] verified that the essential oil of this plant inhibited the growth of dermatophytes and Fusarium species at a concentration of 500 µg/mL. Souza et al[48] showed that a crude extract of Hyptis ovalifolia had activity against the same organisms at a concentration that ranges from 500 µg/mL to 1 000 µg/mL.
The ethanol extract of Azadiracta indica leaves showed MIC and MFC at 250 µg/mL concentration against T. rubrum and Microsporum nanum[2]. Crude methanol extract of the plant Piper solmsianum exhibited antifungal activity against M. canis, M. gypseum, T. mentagrophytes, E. floccosum and T. rubrum[49].
Bagy et al[50] demonstrated an in vitro antifungal activity of some natural compounds like onion oil, aloe sap and garlic extracts. All of these showed less antifungal activity against T. rubrum. Cassia alata leaf extract showed antimicrobial activity against dermatophytic fungi T. mentagrophytes, T. rubrum, M. canis and M. gypseum. It also showed susceptibility towards the dermatophytic fungi than the non dermatophytic fungi like Fusarium solani, Aspergillus niger, Cladosporium werneckii and Penicillium sp.[51].
Rai and Acharya[52] reported that the essential oil of both the species of the genus, Tagetes erecta and Tagetes patula can be utilized topically on dermatophytic infections. It showed maximum inhibition against T. mentagrophytes and Fusarium oxysporum. Monica Bedi et al[53], reported that Allium sativum (garlic) and Scontains ajoene, showed antifungal activity. They have also reported that tea tree oil has been widely used topically for the treatment of bacterial and fungal infections. They showed in vitro antimicrobial activity against Propionibacterium acnes, Staphylococcus aureus, Escherichia coli, Candida albicans, T. mentagrophytes and T. rubrum. Angela Malheivos et al[54], reported that Drimys brasiliensis could be of use for developing new antifungal agents for treating dermatomycosis produced by E. floccosum.
From the present study it was concluded that the crude extracts made of water and ethanol and methanol fractions of the plant A. marmelos has potent antifungal activity against the clinical isolates of dermatophytes. Leaves of A. marmelos yield an essential oil, 4 alkaloids besides aegelenine and aegeline; also condensed tannins, phlobotannins, flavan-3-oil, leucoanthocyanins anthocyanins, flavonoid glycosides, skimmianine,β-sitosterol, rutin and marmesinin. These compounds stated either alone or in combination may be the reason for its antifungal activity. Thin layer and high performance thin layer chromatography also confirmed the presence of these compounds. It is evident that the antidermatophytic activity of these plants may be due to secondary metabolites present in it. Further work on this study may help to design a new drug against dermatophytosis.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Vaijayantimala J, Rajendra Prasad N, Pugalendi KV. Antifungal activity of oils. Indian J Microbiol. 2001;41:325–328. [Google Scholar]
- 2.Natarajan V, Venegopal PV, Menon T. Effect of Azadiracta indica (neem) on the growth pattern of dermatophytes. Indian J Med Microbiol. 2003;21(2):98–101. [PubMed] [Google Scholar]
- 3.Anpin Raja RD, Prakash JW, Jeeva S. Antibacterial activity of some medicinal plants used by Kani tribe, southern Western Ghats, Tamilnadu, India. In: Trivedi PC, editor. Ethnic tribes and medicinal plans. Jaipur: Pointer Publishers; 2010. pp. 28–45. [Google Scholar]
- 4.Bhattacharjee I, Chatterjee SK, Chandra G. Isolation and identification of antibacterial components in seed extracts of Argemone mexicana L. (Papaveraceae) Asian Pac J Trop Med. 2010;3(7):547–551. doi: 10.1016/S1995-7645(11)60028-X. [DOI] [PubMed] [Google Scholar]
- 5.Bhimba BV, Meenupriya J, Joel EL, Naveena DE, Kumar S, Thangaraj M. Antibacterial activity and characterization of secondary metabolites isolated from mangrove plant Avicennia officinalis. Asian Pac J Trop Med. 2010;3(7):544–546. [Google Scholar]
- 6.Darabpour E, Motamedi H, Nejad SMS. Antimicrobial properties of Teucrium polium against some clinical pathogens. Asian Pac J Trop Med. 2010;3(2):124–127. [Google Scholar]
- 7.Irudayaraj V, Janaky M, Johnson M, Selvan N. Preliminary phytochemical and antimicrobial studies on a spike-moss Selaginella inaequalifolia (hook. & grev.) Spring. Asian Pac J Trop Med. 2010;3(12):957–960. [Google Scholar]
- 8.Jarrar N, Abu-Hijleh A, Adwan K. Antibacterial activity of Rosmarinus officinalis L. alone and in combination with cefuroxime against methicillin-resistant Staphylococcus aureus. Asian Pac J Trop Med. 2010;3(2):121–123. [Google Scholar]
- 9.Jeeshna MV, Manorama S, Paulsamy S. Antimicrobial property of the medicinal shrub, Glycosmis pentaphylla. J Basic Appl Biol. 2009;3(1&2):25–27. [Google Scholar]
- 10.Johnson M, Wesely EG, Zahir Hussain MI, Selvan N. In vivo and in vitro phytochemical and antibacterial efficacy of Baliospermum montanum(Wïlld.) Muell. Arg. Asian Pac J Trop Med. 2010;3(11):894–897. [Google Scholar]
- 11.Kannan RRR, Arumugam R, Anantharaman P. Antibacterial potential of three seagrasses against human pathogens. Asian Pac J Trop Med. 2010;3(11):890–893. [Google Scholar]
- 12.Kingston C. Medicinal plants used in the endemic art of Travancore. J Basic Appl Biol. 2007;1(1):38–39. [Google Scholar]
- 13.Koochak H, Nejad SMS, Motamedi H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran) Asian Pac J Trop Med. 2010;3(3):180–184. [Google Scholar]
- 14.Laila Banu NR, Sreeja S, Pinky VR, Prakash JW, Jeenath Jasmine A. Medicinal plants used by the rural people of Kattathurai, Kanyakumari district, Tamilnadu. J Basic Appl Biol. 2007;1(1):18–22. [Google Scholar]
- 15.Mansour A, Enayat K, Neda MS, Behzad A. Antibacterial effect and physicochemical properties of essential oil of Zataria multiflora Boiss. Asian Pac J Trop Med. 2010;3(6):439–442. [Google Scholar]
- 16.Moghadam MS, Maleki S, Darabpour E, Motamedi H, Nejad SMS. Antibacterial activity of eight Iranian plant extracts against methicillin and cefixime restistant Staphylococcous aureus strains. Asian Pac J Trop Med. 2010;3(4):262–265. [Google Scholar]
- 17.Naik MI, Fomda BA, Jaykumar E, Bhat JA. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac J Trop Med. 2010;3(7):535–538. [Google Scholar]
- 18.Nejad SMS, Koochak H, Darabpour E, Motamedi H. A survey on Hibiscus rosa-sinensis, Alcea rosea L. and Malva neglecta Wallr as antibacterial agents. Asian Pac J Trop Med. 2010;3(5):351–355. [Google Scholar]
- 19.Okoye TC, Akah PA, Okoli CO, Ezike AC, Mbaoji FN. Antimicrobial and antispasmodic activity of leaf extract and fractions of Stachytarpheta cayennensis. Asian Pac J Trop Med. 2010;3(3):189–192. [Google Scholar]
- 20.Pugazharasi G, Meenakshi SA, Ramesh KN, Bastin CM, Natarajan E. Screening of antimicrobial activity of Phyllanthus maderaspatensis L. J Basic Appl Biol. 2009;3(3&4):43–49. [Google Scholar]
- 21.Rajan S, Jeevagangai TJ. Studies on the antibacterial activity of Aegle marmelos-fruit pulp and its preliminary phytochemistry. J Basic Appl Biol. 2009;3(1&2):76–81. [Google Scholar]
- 22.Sadheeshna KS, Huxley AJ, Sasikala In vitro propagation of medicinally important plant Mimosa invisa. J Basic Appl Biol. 2009;3(3&4):27–32. [Google Scholar]
- 23.Suresh SN, Nagarajan N. Preliminary phytochemical and antimicrobial activity analysis of Begonia malabarica Lam. J Basic & Appl Biol. 2009;3(1&2):59–61. [Google Scholar]
- 24.Suresh KP. Anti-fungal activity of Leptadenia reticulata in rat animal model in vivo. J Basic & Appl Biol. 2008;2(1):9–13. [Google Scholar]
- 25.Emamuzo ED, Miniakiri SI, Tedwin EJO, Ufouma O, Lucky M. Analgesic and anti-inflammatory activities of the ethanol extract of the leaves of Helianthus Annus in Wistar rats. Asian Pac J Trop Med. 2010;3(5):341–347. [Google Scholar]
- 26.Georgewill OA, Georgewill UO, Nwankwoala RNP. Anti-inflammatory effects of Morninga oleifera lam extract in rats. Asian Pac J Trop Med. 2010;3(2):133–135. [Google Scholar]
- 27.Saha S, Goswami G. Study of anti ulcer activity of Ficus religiosa L. on experimentally induced gastric ulcers in rats. Asian Pac J Trop Med. 2010;3(10):791–793. [Google Scholar]
- 28.Shenoy S, Shwetha K, Prabhu K, Maradi R, Bairy KL, Shanbhag T. Evaluation of antiinflammatory activity of Tephrosia purpurea in rats. Asian Pac J Trop Med. 2010;3(3):193–195. [Google Scholar]
- 29.Patience OO, Edwin OO, Philip FU, Ernest KD, Damian Chiedozie Obiorah. Seasonal variation for the antidiabetic activity of Loranthus micranthus methanol extract. Asian Pac J Trop Med. 2010;3(3):196–199. [Google Scholar]
- 30.Bagepalli SAK, Kuruba L, Jayaveera KN, Devangam SS, Avalakondarayappa AK, Bachappa M. Antioxidant and antipyretic properties of methanolic extract of Amaranthus spinosus leaves. Asian Pac J Trop Med. 2010;3(9):702–706. [Google Scholar]
- 31.Krishanti P Melinda, Xavier R, Kasi Marimuthu, Ayyalu Diwakar, Surash Ramanathan, Sadasivam Kathiresan, et al. A comparative study on the antioxidant activity of methanolic leaf extracts of Ficus religiosa L, Chromolaena odorata (L.) King & Rabinson, Cynodon dactylon (L.) Pers. and Tridax procumbens L. Asian Pac J Trop Med. 2010;3(5):348–350. [Google Scholar]
- 32.Kannan Rengasamy Ragupathi Raja, Arumugam Rajasekaran, Anantharaman Perumal. In vitro antioxidant activities of ethanol extract from Enhalus acoroides (L.F.) Royle. Asian Pac J Trop Med. 2010;3(11):898–901. [Google Scholar]
- 33.Haripriya D, Selvan N, Jeyakumar N, Periasamy RS, Johnson M, Irudayaraj V. The effect of extracts of Selaginella involvens and Selaginella inaequalifolia leaves on poultry pathogens. Asian Pac J Trop Med. 2010;3(9):678–681. [Google Scholar]
- 34.Selvamaleeswaran P, Wesely EG, Johnson M, Velusamy S, Jeyakumar N. The effect of leaves extracts of Clitoria ternatea Linn against the fish pathogens. Asian Pac J Trop Med. 2010;3(9):723–726. [Google Scholar]
- 35.Jeeva S, Jasmine T Sawian, Febreena G Lyndem, Laloo RC, Venugopal N. National Seminar on Past, Present and Future Scenario in Medicinal Plants and Phytochemistry. Tamil Nadu: Department of Plant Science, Bharathidasan University, Thiruchirappalli; 2007. Medicinal plants in Northeast India: past, present and future scenario. [Google Scholar]
- 36.Hasan MF, Khan A, Rahman M, Sikdar B. Determination of antibacterial and antifungal activities of Polygonum hydropepper L. root extract. J Basic Appl Biol. 2009;3(1&2):6–10. [Google Scholar]
- 37.Venugopal Pankajalakshmi V, Venugopal Taralakshmi V. Antidermatophytic activity of neem (Azadiracta indica) leaves in-vitro. Indian J Pharmacol. 1994;26:141–143. [Google Scholar]
- 38.Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. Part I. J American Academy Dermatol. 1994;30(5):677–698. [PubMed] [Google Scholar]
- 39.Jeeva S, Kingston C, Kiruba S, Kannan D. Sacred forests-treasure trove of medicinal plants: a case study from south Travancore. In: Trivedi PC, editor. Indigenous medicinal plants. Jaipur: Pointer Publishers; 2007. pp. 262–74. [Google Scholar]
- 40.Prakash JW, Leena Suman L, Vidhya Devi MS, Berin Premila A, Asbin Anderson N, Veni P, et al. The medicinal plant diversity of Scott Christian College (Autonomous) campus, Nagercoil, South Tamil Nadu, India. J Nat Conservat. 2006;18(1):81–89. [Google Scholar]
- 41.Kingston C, Jeeva S, Jeeva GM, Kiruba S, Mishra BP, Kannan D. Indigenous knowledge of using medicinal plants in treating skin diseases in Kanyakumari District, Southern India. Indian J Traditional Knowledge. 2009;8(2):196–200. [Google Scholar]
- 42.Jeeva GM, Jeeva S, Kingston C. Traditional treatment of skin diseases in South Travancore, southern peninsular India. Indian J Traditional Knowledge. 2007;6(3):498–501. [Google Scholar]
- 43.Kingston C, Nisha BS, Kiruba S, Jeeva S. Ethnomedicinal plants used by indigenous community in traditional healthcare system. Ethnobot Leaflets. 2007;11:32–37. [Google Scholar]
- 44.Rana BK, Singh UP, Taneja Antifungal activity and kinetics of inhibition by essential oil isolated from leaves of Aegle marmelos. J Ethnopharmacol. 1997;57:29–34. doi: 10.1016/s0378-8741(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 45.Kelmanson JE, Jager AK, Staden JV. Medicinal plants with antibacterial activity. J Pharmacol. 2000;69:241–246. doi: 10.1016/s0378-8741(99)00147-6. [DOI] [PubMed] [Google Scholar]
- 46.National Committee for Clinical Laboratory Standards . Reference methods for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi: proposed standard M38-A. Wayne: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- 47.Irobi ON, Darambolo SO. Antifungal activity of crude extracts of Mitracarpus villosus (Rubiaceae) J Ethnopharmacol. 1993;40:137–140. doi: 10.1016/0378-8741(93)90059-e. [DOI] [PubMed] [Google Scholar]
- 48.Souza LKH, Oliveiva CMA, Ferri PH, Santos SC, Oliveira JG, junior, Miranda ATB, et al. Antifungal properties of Brazilian cerrado plants. Brazil J Microbiol. 2002;33:247–249. [Google Scholar]
- 49.DE Campos Marina Pereira, Filho Valsir Cechniel, DA Silva Rosi Zanoni, Yunes Rosendo Augusto, Zacchino Susana, Rosana Sabina Juarez, Cruz CE Bella, et al. Evaluation of antifungal activity of Piper solmsianum C. DC Var. solmsianum (Piperaceae) Biol Pharm Bull. 2005;28(8):1527–1530. doi: 10.1248/bpb.28.1527. [DOI] [PubMed] [Google Scholar]
- 50.Bagy MMK, Abdel-Mallek AY, EL-Sahanawany AA, Gamal A Morsi. Studies on fungi associated with laboratory Animal Golden Hamster and antibiotic effects of Aloe sap, garlic extract and onion oil. J Islamic Acad Sci. 1997;10(1):62–65. [Google Scholar]
- 51.Ibrahim Darah, Osman Halim. Antimicrobial activity of Cassia alata from Malaysia. J Ethnopharmacol. 1995;45:151–156. doi: 10.1016/0378-8741(94)01200-j. [DOI] [PubMed] [Google Scholar]
- 52.Rai MK, Acharya D. Search for fungitoxic potential in essential oils of Asteraceous plants. Composite Newsletter. 2000;35:18–23. [Google Scholar]
- 53.Bedi Monica K, Shenefelt Philip D. Herbal therapy in dermatology. Arch Dermatol. 2002;138:232–241. doi: 10.1001/archderm.138.2.232. [DOI] [PubMed] [Google Scholar]
- 54.Malheiros Angela, Filho Valdir Cechinel, Schmitt Clarisse B, Yunes Rosendo A, Escalante Andrea, Svetaz Laura, et al. Antifungal activity of Drimane sesquiferpenes from Drimys brasiliensis using bioassay guided fractionation. J Pharm Sci. 2005;8(2):335–339. [PubMed] [Google Scholar]