Abstract
A case of pulmonary coinfection by Strongyloides stercoralis and Pneumocystis jiroveci has been detected in an AIDS patient treated in the Respiratory Intensive Care Unit of the Muñiz Hospital. At diagnosis, the patient presented cough with mucopurulent expectoration, dyspnea, fever, bilateral pulmonary infiltrates on the chest X-ray, negative bacilloscopy for acid fast bacteria and a CD4+ T lymphocytes count of 52 cells/µL. The microbiological diagnosis was achieved by microscopic observation of the respiratory secretions obtained by bronchoalveolar lavage, while the wet mount examination revealed rhabditiform and filariform larvae of the nematode and foamy exudates, pathognomonic of the pulmonary pneumocystosis. It was the unique case of this association among about 3 000 samples performed in our laboratory in the last 10 years and diagnosed by microscopy. Other complementary stains (a rapid modification of Grocott, Kinyoun and Giemsa) were applied to the smears after the diagnosis of mycotic and parasitary infections achieved by fresh microscopy. Both physicians and microbiologists should take into account the possible coexistence of respiratory pathogens in immunocompromised patients, such as those with AIDS.
Keywords: Strongyloides stercoralis, Pneumocystis jiroveci, Pulmonary coinfection, AIDS, Bronchoalveolar lavage, Wet mout examination, Pulmonary pneumocystosis
1. Introduction
The severe immunosupression that affects AIDS patients makes them susceptible to numerous infections, called “opportunistic” due to the poor pathogenicity of their etiologic agents or the immunologic deterioration of the host. These infections may occur in joint form, appearing as generalized infections or just localized in certain organs. Pneumocystis jiroveci (P. jiroveci), recently incorporated in the Fungi Kingdom, is responsible for the pulmonary pneumocystosis (PCP) and considered as one of the most frequent opportunistic infections in AIDS patients. Its clinical picture becomes evident when the CD4+ T lymphocyte count is <200 cells/µL[1].
The nematode Strongyloides stercoralis (S. stercoralis) is observed less frequently than P. jiroveci in these patients and produces symptomatic infections disseminated out of the intestines, which can reach inusitated severity[2]. Although a considerable percentage (15%-30%) of the millions of infected individuals remains asymptomatic, S. stercoralis can produce hyperinfection and turn into a dangerous pathogen even mortal when accompanied by certain favorable events associated with the host immunity[2].
Despite the fact that the association of opportunistic infections in AIDS patients could be considered as a usual event, the pulmonary coexistence of P. jiroveci and other respiratory pathogens has been previously found in approximately 10% of the cases[3]. The diagnosis of a pulmonary coinfection of P. jiroveci and S. stercoralis in an AIDS patient assisted in the Respiratory Intensive Care Unit (RICU) of the Infectious Diseases “Francisco Muñiz Hospital” of Buenos Aires is communicated.
2. Case report
The 43-year-old male patient, HIV positive, at the moment of remission of the respiratory secretions to the parasitology laboratory, was hospitalized at the RICU. As relevant background he referred to a previous hospitalization because of intestinal and extra intestinal strongyloidiasis treated with ivermectin. The patient was suffering from diarrhea with severe dehydration, impregnation syndrome, anemia, a pneumopathy associated with dyspnea, mucopurulent expectoration, fever, and bilateral pulmonary infiltrates on the chest X-ray. At the moment of hospitalization in February, 2007, the CD4+ T lymphocyte count was 52 cells/µL and the result of bacilloscopy from the sputum sample was negative. A few days later, microscopy of CSF was conducted to discard the presence of S. stercoralis in that location, but it revealed the presence of capsulated yeasts. In spite of receiving the treatment with usual dosis of trimethoprim, sulfamethoxazole, ivermectin and amphotericin B, the general condition of the patient deteriorated with progresive respiratory insufficiency and died in March, 2007.
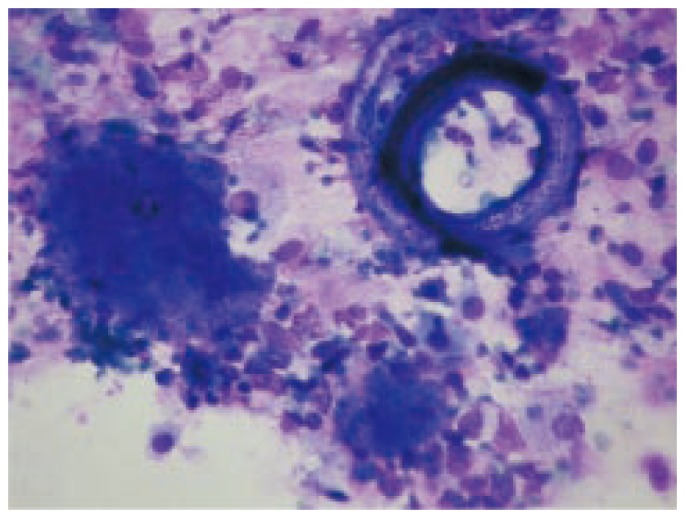
The respiratory secretions from the patient were obtained by bronchoalveolar lavage (BAL) and sent to the parasitology laboratory in an approximate volume of 10 mL, in a sterile, plastic tube with a screw cap. The sample was centrifuged (1 500 rpm/15′), and fresh microscopy, smears stained with Kinyoun[4], Giemsa (Figure 1) and a rapid modification of the Grocott technique[5] were performed. Also, in our center the number of pulmonary cryptococcosis diagnosed in AIDS patients is significantly lower than expected as compared with the cases of meningoencephalitis which is produced by the infection of Cryptococcus neoformans (C. neoformans) through inhalation of the propagules[6]. Part of the respiratory secretions was processed in the microbacteriology, mycology and bacteriology services of the Muñiz Hospital and the result was negative. The wet smear revealed numerous leukocytes, filariform and rhabditiform larvae of S. stercoralis and foamy exudates[6]. A quick modification of the Grocott technique to the smears showed P. jiroveci cysts, while the Kinyoun stain was negative for acid resistant bacteria and protozoa.
Figure 1. Rhabditiform larva of S. stercoralis (left) and foamy exudate (honey coombs exudate), patognomonic of pulmonary pneumocystosis (right), observed in a smear stained with Giemsa (1 000×).
3. Discussion
Even though the presence of strongyloidiasis or pulmonary pneumocystosis is frequent in AIDS patients, the coexistence of both of them at pulmonary level, especially between the AIDS patients assisted in the Muñiz Hospital, is exceptional. It was the unique case of this association among about 3 000 processed samples in the past 10 years and diagnosed by microscopy.
The severe immunologic deterioration of the patient evidenced indirectly for the low CD4+ T count and fostered the simultaneous coexistence not only of P. jiroveci and S. stercoralis but also of C. neoformans which produced a meningoencephalitis.
Neither the microscopy nor the culture, as it can be noticed from the clinical history of the patient, revealed the presence of C. neoformans in the respiratory secretions. Regarding the respiratory coinfection in patients with P. jiroveci (PCP), the respiratory secretions sent during 2007 to the parasitology laboratory for PCP were analyzed in a previous study[7]. Only 12% of the 52 evaluated samples revealed the coexistence of P. jiroveci with other respiratory pathogens.
Each analyzed sample corresponded to respiratory secretions obtained, as in the present case, by BAL, and they belonged to AIDS patients hospitalized in the RICU with PCP as presumptive diagnosis. The coinfection occurred with Mycobacterium tuberculosis (n=2) and with potentially pathogenic bacteria (Haemophilus influenzae, Streptococcus pneumoniae and Pseudomonas aeruginosa) in 4 cases, but no fungi or protozoa was detected. The presence of filariform larvae in the respiratory secretions obeys the endogenous reinfection process in this case, which may occur in severely immunocompromised patients, in response to hyperinfection. In these cases, rhabditiform larvae mature inside the bowels and break through the mucous membrane or through the perianal skin in order to get to the respiratory tract via blood, and finally return the intestinal location. Exceptionally, eggs of S. stercoralis were observed by fresh microscopy in a respiratory secretion obtained by BAL belonging to an AIDS patient with presumptive diagnosis of pulmonary pneumocystosis[8].
The patient also had positive results for S. stercoralis in the feces and gastric content analysis, as it was expected to consider the usual location of the nematode[2]. Taking into account the disseminated character of strongyloidiasis in immunocompromised patients, parasitological studies were made to search the etiologic agent in urine and CSF, and both results were negative.
In the described case, the coexistence of many S. stercoralis larvae and foamy exudates, typical of PCP, improved the microscopic diagnosis. The low CD4+ count is also associated with the presence of great amounts of microorganisms in the clinical samples, just as it was seen in our patient with P. jiroveci and S. stercoralis. BAL is the gold standard for the PCP diagnosis and is most frequently used in the collection of sputum samples which were sent to our laboratory. The casuistics reveals a very low sensitivity of the expectorated sputum as clinical sample and induced sputum is very difficult to obtain because of the lack of biosafety cabinets and the high number of bacilliferous patients with multiresistant tuberculosis[10].
The diagnostic methodology is the same as the one usually used to process respiratory secretions samples in the parasitology laboratory of the Muñiz Hospital. Other most sensitive techniques for identification of P. jiroveci, such as direct immunofluorescense with monoclonal antibodies, are not employed in our laboratory. When it was applied at BAL samples belonging AIDS patients suspected for PCP, the obtained results were not significantly different with those produced by wet mount microscopy[11]. The modified Grocott technique allows the visualization of fungal structures in general, particularly in the P. jiroveci cysts, and the confirmation of the wet smear findings. Apart from that, the Giemsa stain is used because of the possibility of finding intracellular yeasts of Histoplasma capsulatum or other fungi or parasites, in spite of its low sensitivity for the PCP diagnosis. Finally, the Kinyoun technique is used in these materials to investigate acid resistant elements: filamentous bacteria from the Nocardia genus and oocysts of Cryptosporidium spp. and Cyclospora cayetanensis[4].
The wet mount microscopy raises the possibility of arriving at a micrological or parasitological diagnosis given by the mentioned stains, revealing helminthic larvae and foamy exudates that are of the same diagnostic importance as other fungal structures[6]. Strongyloidiasis, a disease recognized as a potentially fatal parasitic infection in immunocompromised patients, is becoming a major concern in many endemic regions of Latin America such as in the northern tropical area of Argentina. Development or exacerbation of pulmonary symptoms is seen, and the detection of increased numbers of larvae in sputum and/or BAL is the hallmark of hyperinfection. Larvae in nondisseminated hyperinfection are increased in numbers but confined to the organs normally involved in the pulmonary autoinfective cycle (i.e., gastrointestinal tract, peritoneum, lungs). Sputum or BAL may demonstrate filariform or rhabditiform larvae and even, occasionally, eggs. These findings suggest that filariform larvae develop into adults in the lungs and a new generation of rhabditiform larvae are then produced locally. This hypothesis is supported by reports of adult parasites being expectorated post-treatment and autopsy studies showing adult worms in lung tissue.
Parasitologic diagnosis of S. stercoralis hyperinfection is relatively straightforward, given the high numbers of larvae that exist in stool and, usually, sputum. Although AIDS has been associated with hyperinfection, the number of reported cases is much less than the overlapping endemicities of these conditions. Therefore, any individual with risk factors for acquiring S. stercoralis infection who is diagnosed with HIV-1 should be screened. Because of the longevity of the parasitic infection, even remote histories of travel or residence in places where the disease was considered endemic decades ago, that is, northeastern Argentina, should prompt screening. HIV-positive individuals coinfected with S. stercoralis should be treated with conventional doses of anthelmintics to prevent hyperinfection [11]–[15].
The coexistence of different infections in the pulmonary tissue is an important event to be considered in the severely immunocompromised patients, such as those who suffer from AIDS, and leads to the exhaustive study of the respiratory secretions. Finding a pathogen in a clinical sample should not discourage the microbiologist in the search of other pathogens that might be present in some occasions in small amounts. The laboratorist must be qualified to recognise every kind of pathogens, possibly present in the samples. The physician has to take into account the possibility of respiratory coinfections at the time of setting the appropriate treatment.
Footnotes
Foundation Project: Supported by Scientific Research Fund for Education Department of the the School of Medicine-Buenos Aires University (No: J500798759)
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Wazir JF, Ansari NA. Pneumocystis carinii infection: update and review. Arch Pathol Lab Med. 2004;128:1023–1027. doi: 10.5858/2004-128-1023-PCI. [DOI] [PubMed] [Google Scholar]
- 2.Keiser PB, Nutran TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mootsikapun P, Chetchotisakd P, Intarapoka B. Pulmonary infections in HIV infected patients. J Med Assoc Thai. 1996;79:477–485. [PubMed] [Google Scholar]
- 4.Bava AJ, Bellegarde JE. La coloracion de Kinyoun aplicada a muestras fecales de pacientes con SIDA. Rev Argent Infectol. 2002;15:19–21. [Google Scholar]
- 5.Bava AJ. Coloración rápida para la identificación de quistes de Pneumocystis carinii en materiales respiratorios. Acta Bioquím Clín Latinoam. 2003;37:189–192. [Google Scholar]
- 6.Bava AJ, Cattaneo S, Bellegarde E. Diagnosis of pulmonary pneumocystosis by microscopy on wet mount preparations. Rev Inst Med Trop Sao Paulo. 2002;44:279–282. doi: 10.1590/s0036-46652002000500009. [DOI] [PubMed] [Google Scholar]
- 7.Longworth DL, Weller PF. Hyperinfection syndrome with strongyloidiasis. In: Remington JS, Swartz MN, editors. Current clinical topics in infection diseases. 7th ed. New York: Mc Graw Hill; 1986. pp. 1–26. [Google Scholar]
- 8.Procop GW. The role of the clinical microbiology laboratory in the diagnosis and therapy of infectious diseases. In: Cockerill FR III, editor. Gastrointestinal infections. Philadelphia: WB Saounders Co; 2001. pp. 1073–1108. [Google Scholar]
- 9.Kroe DM, Kirsch CM, Jensen WA. Diagnostic strategies for Pneumocystis carinii peumonia. Semin Respir Infect. 1997;12:70–78. [PubMed] [Google Scholar]
- 10.Lehmann E, De Vedia L, Prieto R, Bava AJ. Pneumocystis jiroveci en secreciones respiratorias de pacientes con SIDA internados en cuidados intensivos respiratorios. El Muñiz Hoy. 2006;4:75–78. [Google Scholar]
- 11.Bava AJ, Troncoso AR. Strongyloides stercoralis hyperinfection in a patient with the AIDS. J Int Assoc Physicians AIDS Care (Chic) 2009;8:235–238. doi: 10.1177/1545109709336882. [DOI] [PubMed] [Google Scholar]
- 12.Baughman RP, Dohn MN, Frame PT. The continuing utility of bronchoalveolar lavage to diagnose opportunistic infection in AIDS patients. Am J Med. 1994;97:515–522. doi: 10.1016/0002-9343(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez PR, Guzman AP, Guillen SM, Adell RI, Estruch AM, Gonzalo IN, et al. Endemic strongyloidiasis on the Spanish Mediterranean coast. QJM. 2001;94:357–363. doi: 10.1093/qjmed/94.7.357. [DOI] [PubMed] [Google Scholar]
- 14.Cirioni O, Giacometti A, Burzacchini F, Balducci M, Scalise G. Strongyloides stercoralis first-stage larvae in the lungs of a patient with AIDS: primary localization or a noninvasive form of dissemination? Clin Infect Dis. 1996;22(4):737. doi: 10.1093/clinids/22.4.737. [DOI] [PubMed] [Google Scholar]
- 15.Maayan S, Wormser GP, Widerhorn J, Sy ER, Kim YH, Ernst JA. Strongyloides stercoralis hyperinfection in a patient with the acquired immune deficiency syndrome. Am J Med. 1987;83:945–948. doi: 10.1016/0002-9343(87)90656-5. [DOI] [PubMed] [Google Scholar]


