Abstract
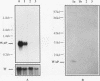
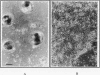
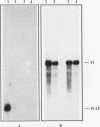
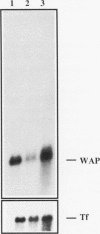
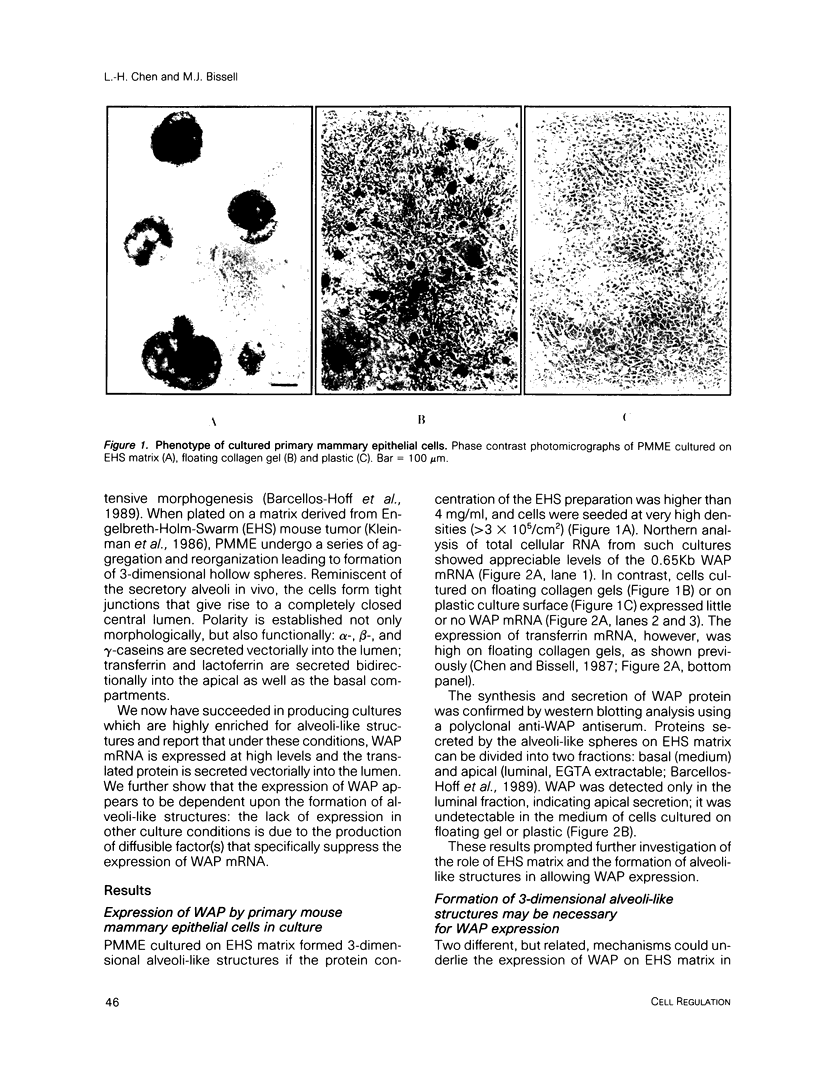
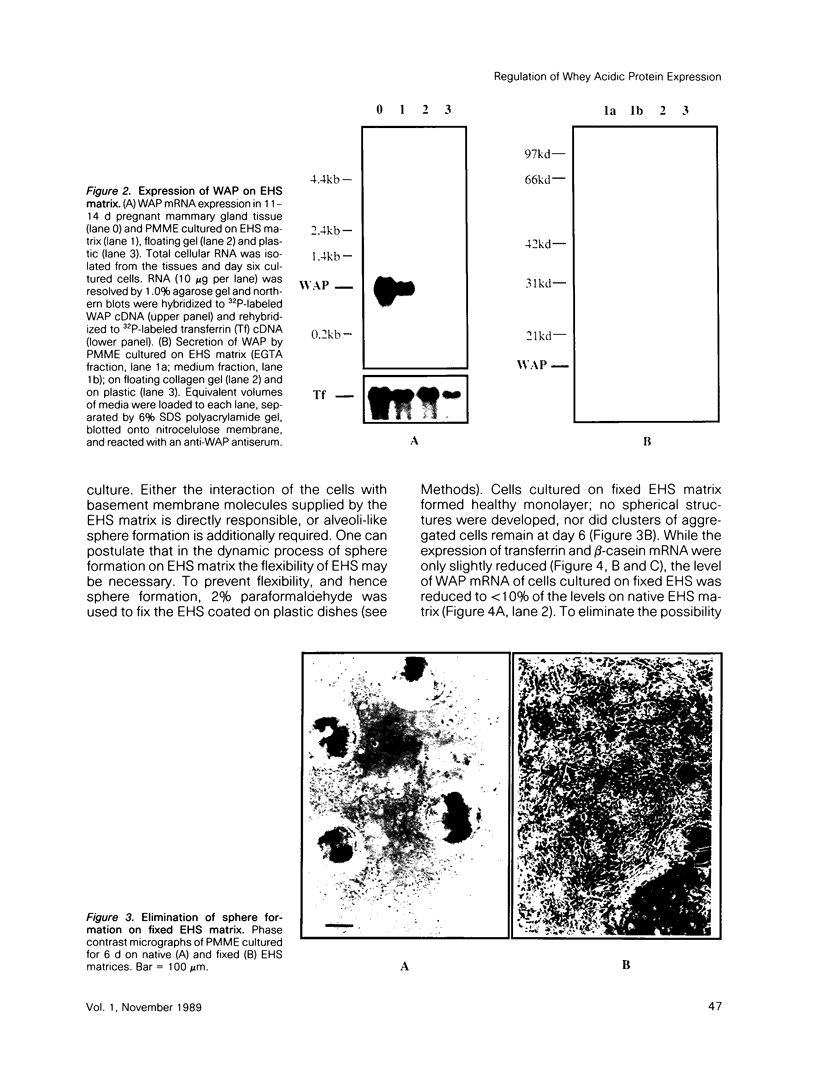
When primary mouse mammary epithelial cells (PMME) are cultured on a basement membrane type matrix, they undergo extensive morphogenesis leading to the formation of 3-dimensional alveoli-like spherical structures surrounding a closed lumen. We show for the first time that cells cultured on basement membrane-type matrix express high levels of whey acidic protein (WAP) mRNA and secrete the protein into the lumen. The expression of WAP appears to be dependent upon the formation of the alveoli-like spheres: prevention of sphere formation by fixation or drying of the matrix abolishes the expression of WAP. Co-culturing PMME on native and fixed basement membrane matrix indicates that the suppression of WAP expression is dominant, thereby revealing the existence of a diffusible inhibitor(s). The inhibitory activity is present in the conditioned medium of PMME cultured on plastic surface and floating collagen gels, substrata that do not form alveoli and do not allow WAP expression. These findings are consistent with the model that the synthesis, or the action, of the WAP inhibitory factor is regulated by the tissue-like multicellular organization of mammary cells. When PMME do not have correct 3-dimensional structures, one (or more) inhibitor is secreted into the medium which suppresses WAP expression by an autocrine or paracrine mechanism. Nuclear run-on experiments suggest that the suppression of WAP expression is posttranscriptional. These results have obvious bearings on the understanding of the mechanisms by which cell-cell and cell-extracellular matrix interaction regulate tissue specific gene expressions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres A. C., Schönenberger C. A., Groner B., Hennighausen L., LeMeur M., Gerlinger P. Ha-ras oncogene expression directed by a milk protein gene promoter: tissue specificity, hormonal regulation, and tumor induction in transgenic mice. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1299–1303. doi: 10.1073/pnas.84.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
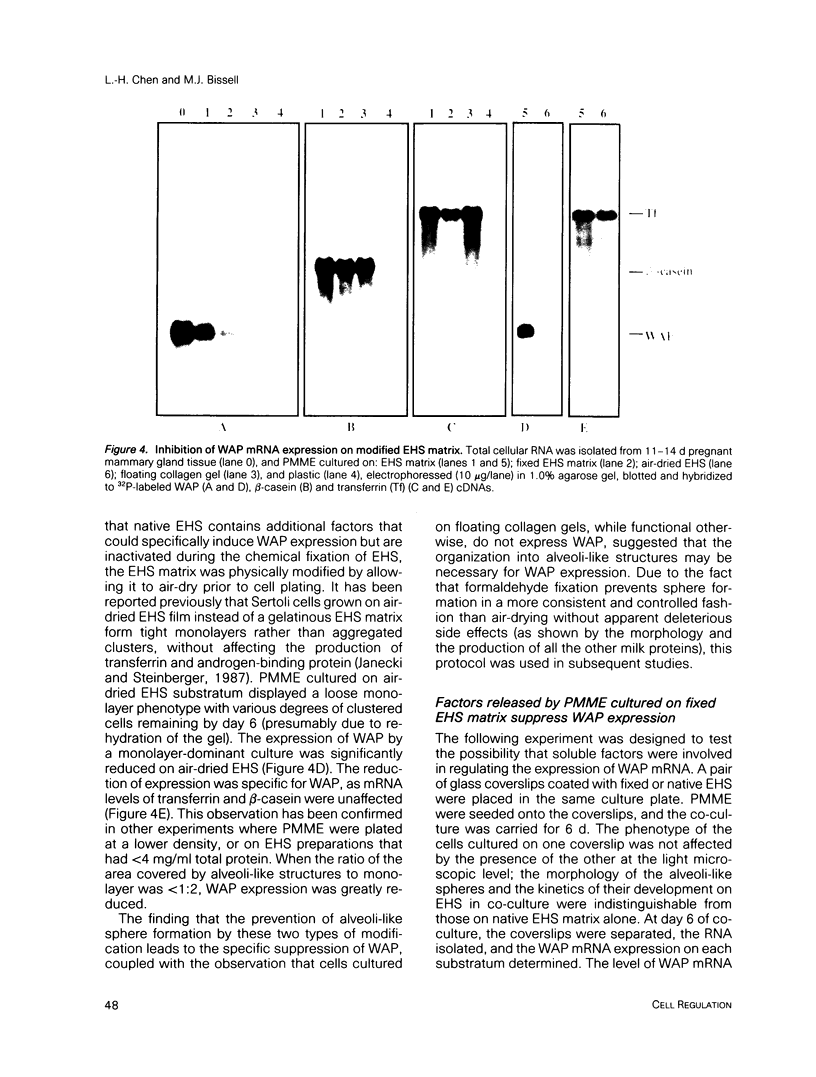
- Barcellos-Hoff M. H., Aggeler J., Ram T. G., Bissell M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989 Feb;105(2):223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Blum J. L., Zeigler M. E., Wicha M. S. Regulation of rat mammary gene expression by extracellular matrix components. Exp Cell Res. 1987 Dec;173(2):322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Chen L. H., Bissell M. J. Transferrin mRNA level in the mouse mammary gland is regulated by pregnancy and extracellular matrix. J Biol Chem. 1987 Dec 25;262(36):17247–17250. [PubMed] [Google Scholar]
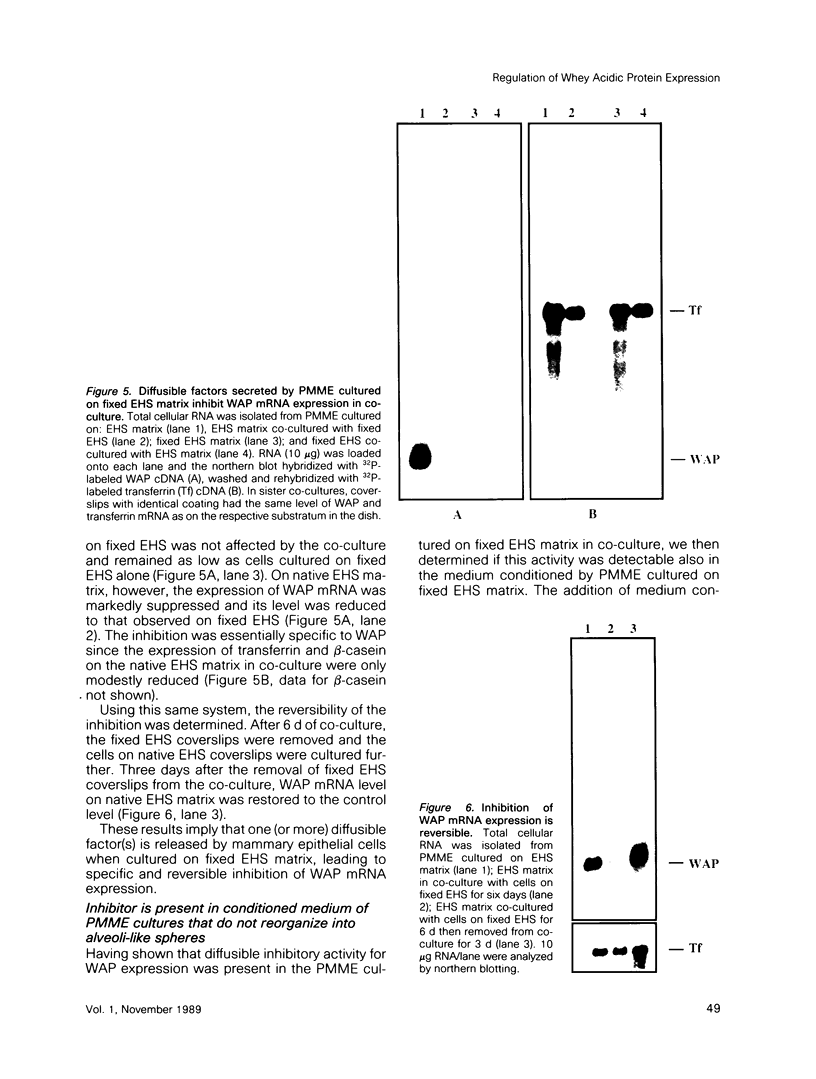
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar A. M., Robinson E. A., Appella E., Qasba P. K. Complete sequence analysis of cDNA clones encoding rat whey phosphoprotein: homology to a protease inhibitor. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3987–3991. doi: 10.1073/pnas.79.13.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C. W., Silberstein G. B., Van Horn K., Strickland P., Robinson S. TGF-beta 1-induced inhibition of mouse mammary ductal growth: developmental specificity and characterization. Dev Biol. 1989 Sep;135(1):20–30. doi: 10.1016/0012-1606(89)90154-1. [DOI] [PubMed] [Google Scholar]
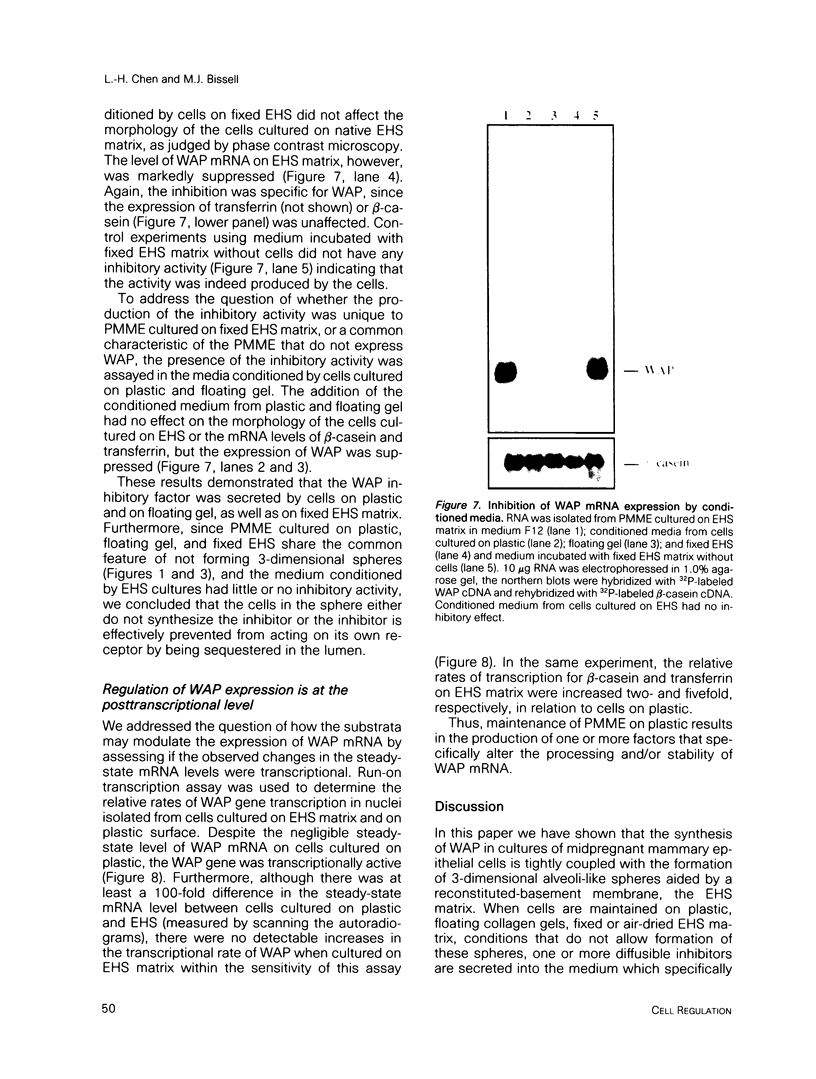
- Eisenstein R. S., Rosen J. M. Both cell substratum regulation and hormonal regulation of milk protein gene expression are exerted primarily at the posttranscriptional level. Mol Cell Biol. 1988 Aug;8(8):3183–3190. doi: 10.1128/mcb.8.8.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman J. T., Bartley J. C., Bissell M. J. Glucose metabolite patterns as markers of functional differentiation in freshly isolated and cultured mouse mammary epithelial cells. Exp Cell Res. 1981 Jul;134(1):241–250. doi: 10.1016/0014-4827(81)90481-x. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Burwen S. J., Pitelka D. R. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell. 1979;11(1):109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Enami J., Pitelka D. R., Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
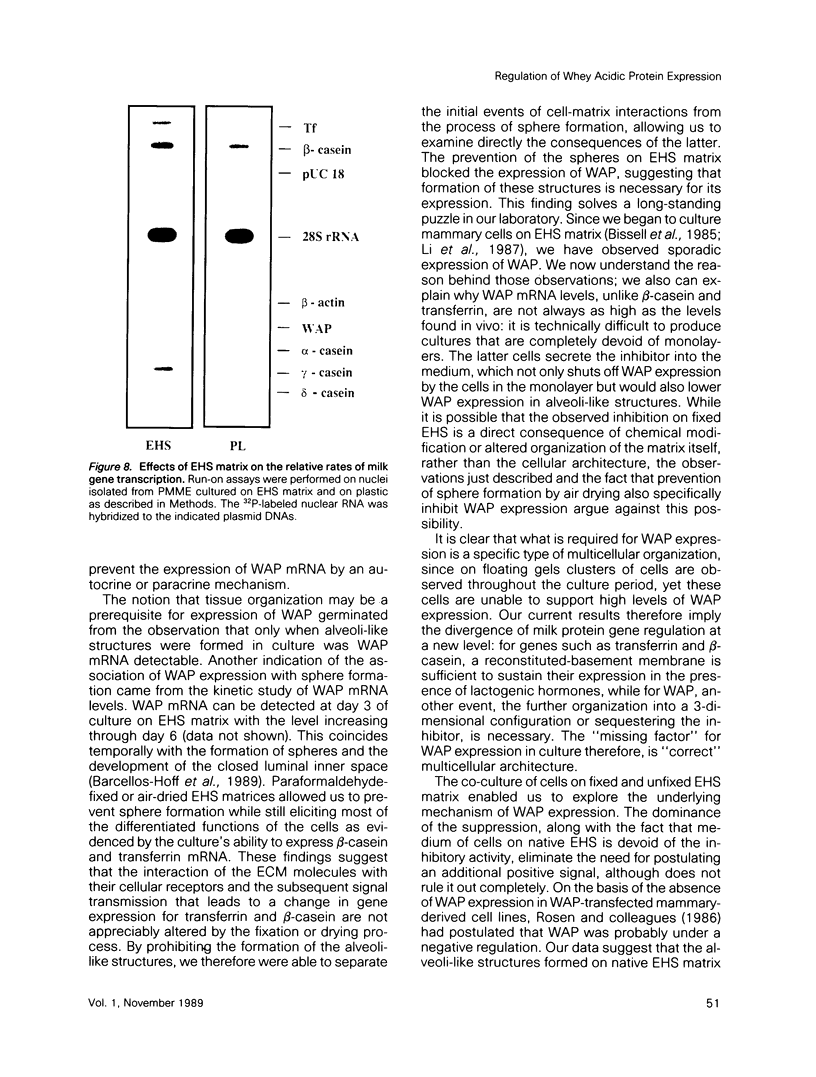
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Haeuptle M. T., Suard Y. L., Bogenmann E., Reggio H., Racine L., Kraehenbuhl J. P. Effect of cell shape change on the function and differentiation of rabbit mammary cells in culture. J Cell Biol. 1983 May;96(5):1425–1434. doi: 10.1083/jcb.96.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E. Characterization and cloning of the mRNAs specific for the lactating mouse mammary gland. Eur J Biochem. 1982 Jun 15;125(1):131–141. doi: 10.1111/j.1432-1033.1982.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Janecki A., Steinberger A. Vectorial secretion of transferrin and androgen binding protein in Sertoli cell cultures: effect of extracellular matrix, peritubular myoid cells and medium composition. Mol Cell Endocrinol. 1987 Jul;52(1-2):125–135. doi: 10.1016/0303-7207(87)90105-5. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H., Kaetzel C. S., Parry G., Bissell M. J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Parry G., Bissell M. J. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J Cell Biol. 1984 Jan;98(1):146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubon H., Hennighausen L. Nuclear proteins from lactating mammary glands bind to the promoter of a milk protein gene. Nucleic Acids Res. 1987 Mar 11;15(5):2103–2121. doi: 10.1093/nar/15.5.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D., Li M. L., Oborn C. J., Bissell M. J. Casein gene expression in mouse mammary epithelial cell lines: dependence upon extracellular matrix and cell type. Exp Cell Res. 1987 Sep;172(1):192–203. doi: 10.1016/0014-4827(87)90105-4. [DOI] [PubMed] [Google Scholar]
- Piletz J. E., Heinlen M., Ganschow R. E. Biochemical characterization of a novel whey protein from murine milk. J Biol Chem. 1981 Nov 25;256(22):11509–11516. [PubMed] [Google Scholar]
- Rocha V., Ringo D. L., Read D. B. Casein production during differentiation of mammary epithelial cells in collagen gel culture. Exp Cell Res. 1985 Jul;159(1):201–210. doi: 10.1016/s0014-4827(85)80049-5. [DOI] [PubMed] [Google Scholar]
- Rosen J. M., Rodgers J. R., Couch C. H., Bisbee C. A., David-Inouye Y., Campbell S. M., Yu-Lee L. Y. Multihormonal regulation of milk protein gene expression. Ann N Y Acad Sci. 1986;478:63–76. doi: 10.1111/j.1749-6632.1986.tb15521.x. [DOI] [PubMed] [Google Scholar]
- Schoenenberger C. A., Andres A. C., Groner B., van der Valk M., LeMeur M., Gerlinger P. Targeted c-myc gene expression in mammary glands of transgenic mice induces mammary tumours with constitutive milk protein gene transcription. EMBO J. 1988 Jan;7(1):169–175. doi: 10.1002/j.1460-2075.1988.tb02797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. J., Hasan H. R., Mayer R. J. Comparison of collagen gels and mammary extracellular matrix as substrata for study of terminal differentiation in rabbit mammary epithelial cells. Exp Cell Res. 1984 Apr;151(2):519–532. doi: 10.1016/0014-4827(84)90400-2. [DOI] [PubMed] [Google Scholar]