Abstract
Objective
To examine the anti-bacterial activity of leaf extracts of Morus alba L. (Moraceae) and Piper betel L. (Piperaceae), and seed extracts of Bombax ceiba L. (Borabacaceae).
Methods
We have partially purified plant extracts by solvent extraction method, and evaluated the effect of individual fractions on bacterial growth using Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus) bacterial strains.
Results
Compared with Morus and Bombax fractions, Piper fractions showed significant growth inhibition on all the three types of bacteria studied. The EtOAc-hexane fractions of Piper leaves exhibited significant anti-bacterial activity with minimum inhibitory concentrations (MIC) of 50 µg/mL culture against both gram-positive and gram-negative bacteria. The EtOAc-fractions I, II, and IV inhibited bacterial colony formation on soft agar in addition to growth inhibition. A combination treatment of piper fractions with ampicillin resulted in significant growth inhibition in E. coli and P. aeruginosa, and combination with anticancer drug geldanamycin (2µg/mL) showed selective growth inhibition against P. aeruginosa and S. aureus. Three major compounds, i.e., eugenol, 3-hexene-ol and stigmasterol, were primarily identified from Piper betel leaf extractions. Among the individual compounds, eugenol treatment showed improved growth inhibition compared with stigmasterol and 3-hexene-ol.
Conclusions
We are reporting potential anti-bacterial compounds from Piper betel against both gram-positive and gram-negative bacteria either alone or in combination with drug treatment.
Keywords: Piper betel, Anti-microbial activity, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Morus alba, Bombax ceiba, Minimum inhibitory concentration, Growth inhibition
1. Introduction
Microbial disease risk is much greater than the risk posed by chemicals suspected of causing cancer in humans[1]. Several new anti-bacterial agents are currently being developed in response to the emergence of bacterial resistance to existing drugs. The majority of such anti-microbial drugs are aimed at targeting nucleic acid, protein, or peptidoglycan synthesis[2],[3]. Microbial disease is of particular relevance from both fundamental and public health viewpoint. The large number of microbial genome sequences currently available facilitates completely new methods of cross-genome analyses, which aid in target identification and prioritization for anti-microbial drug discovery[4],[5].
Medicinal plants also represent a rich source of anti-microbial agents. Thus interest has revived recently in the investigation of medicinal plants to identify novel active phytochemicals that might lead to new classes of microbial drug development. The entire plant source or different parts which include root, leaf, seed, stem, flower, fruit, twigs exudates and modified plant organs can be used to identify potential active principles for various ailments either as a whole plant, crude extract, aqueous, or organic extracts[6]–[8]. Anti-microbial properties have been described by previous science fair investigators from some natural extracts, and the majority of these studies focus on crude extracts[9]. Morus species have been a rich food source, and the leaf extracts of Morus Alba (M. alba) are used for anticancer and antioxidants properties[10],[11]. Bombax seeds are useful for treating gonorrhea and chronic cystitis, bark for improving skin color, flowers for making tea and silk for cotton production. Methanolic extracts of Bombax have been shown to contain potential antioxidant properties[12]. Piper species have been used for wound healing in diabetic rats[13] and gastric ulcers[14].
The objective of this study was to evaluate partially purified fractions from three south Indian plant varieties, M. alba, Bombax ceiba L. (B. ceiba), and Piper betel L. (P. betel) for growth inhibition properties against two gram-negative bacteria, Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa) and one gram-positive bacteria, Staphylococcus aureus (S. aureus). We showed that among the three plant varieties studied P. betel has signficant anti-bacterial activity. We tested effects of P. betel extracts in combination with known anti-bacterial and anticancer drugs. After confirming the specificity and selectivity of these combinations, we identified three major compounds with potential anti-microbial activity.
2. Materials and methods
2.1. Material and plant source
Acetone, acetonitryl, ethyl acetate, hexane, methanol, chloroform, silica gel, sulphuric acid, Luria Broth (LB), yeast extract, sodium chloride, peptone, thin layer chromatography plates and other general chemicals and materials were procured from either Sigma or Calbiochem (USA). Capillary tubes, glass column (30 cm), glass beakers, measuring cylinders, conical flasks, petri plates were purchased from Borosil. P. betel L., B. ceiba L., and M. alba L. (varieties S-26 and S-36) were grown in the laboratory campus for ornamental and research purposes.
2.2. Extraction and purification
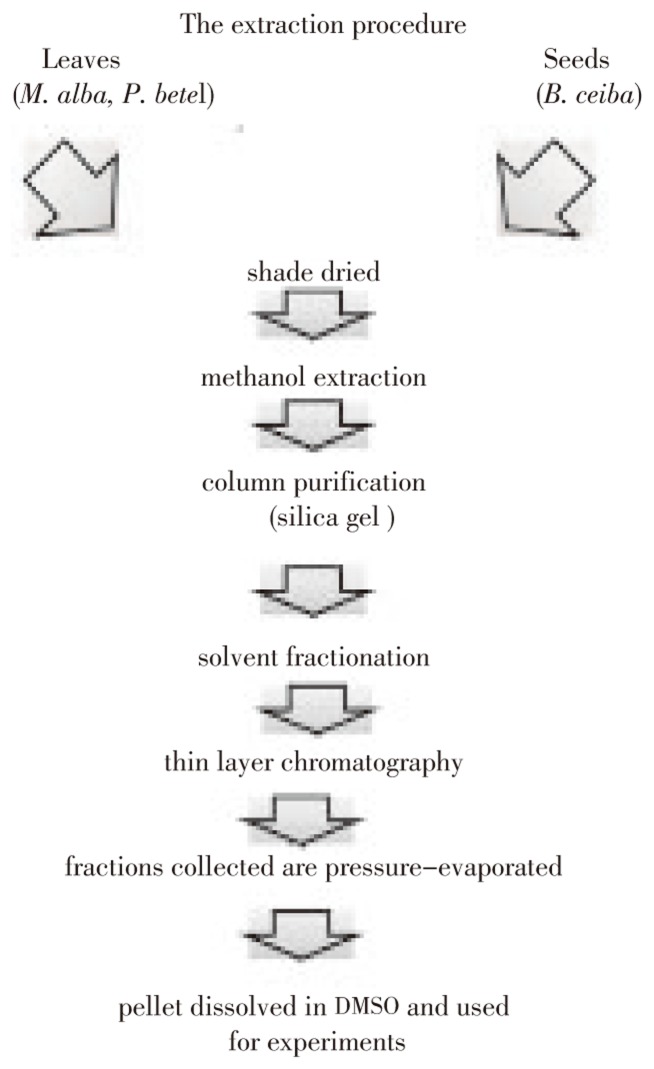
The plant materials (leaves or seeds) were collected and shade dried M. alba (2.0 kg dry leaf), B. ceiba (1.0 kg dry seed) and P. betel (1.0 kg dry leaf) were ground into powder and extracted with methanol (each 4 L) for 4 days at room temperature. The supernatant removing the debris that has been settled at the bottom of the flask was filtered 0.22 µ pore size PTFE filter (Millipore Co., Billericia, USA) and concentrated by vacuum-evaporation. The concentrate was then fractionated using silica gel column with appropriate solvent gradient (Figure 1 and Table 1). A stock solution of 10 mg/mL in DMSO (dimethylsulfoxide) was prepared and used for examining the anti-microbial activity.
Figure 1. Schematic representation of extractions procedure.
Table 1. Solvent fractionation of crude extracts.
| Fraction | P. betel (ethyl acetate -hexane) | B. ceiba (methanol-chloroform) | M. alba; S-36(methanol-chloroform) | M. alba; S-26(ethyl acetate - hexane) |
| I | 3% | 5% | 1% | 1% |
| II | 4% | 10% | 2% | 2% |
| III | 5% | 12% | 3% | 4% |
| IV | 6% | chloroform | 4% | 5% |
2.3. Identification and characterization of major compounds
The crude fractions were purified by column chromatography to get the pure compounds as shown in Figure 1. The structures of the compounds were elucidated using the spectral data (NMR and Mass) and by comparison with reported literature[18],[19].
2.4. Bacterial growth conditions and treatment
Bacteria were grown on either LB agar plates for maintenance or LB medium without any antibiotic in the growth medium. Briefly, in each experiment, a single bacterial colony was inoculated into 2 mL LB broth and grown over night at 37 °C, 200 rpm shaking. In the next day, fresh culture was inoculated from overnight culture (1:100 dilution) and allowed to grow for 3 h to obtain all cells in mid-log phase of growth. Partially purified natural compound fractions were initially tested for dose dependent cytotoxicity and a concentration of 200µg/mL was chosen as appropriate for all the fractions for subsequent treatment.
2.5. Colony forming ability versus growth inhibition studies
Two milliliter bacterial cultures at mid-log growth phase were treated with appropriate fractions for 5 h and cultures diluted to 1:100 in fresh LB medium and 50 µL cultures were spread on LB agar plate and incubated for further 16 h at 37 °C. The colony formation was monitored by counting number of bacterial colonies formed.
2.6. Analysis of bacterial growth by spectrometric measurements
The respective fractions were added to growing bacterial cultures in their mid-log phase and incubated for 5 h or overnight (18 h). Optical density was measured at 600 nm using the spectrophotometer (Shimadzu double beam spectrophotometer, Model 1 601). Control cells were maintained without treatment whereas solvent controls were maintained by treating cells only with DMSO. The optical density values obtained were converted to percent growth inhibition and plotted on a bar diagram. Each set of experiments was repeated at least three times and the values were represented as SEM±Mean. The significance was calculated using standard P value calculation by Sigma plot software and expressed as, *P<0.05, ** P<0.01, and *** P<0.001.
3. Results
3.1. Effect of natural compounds on bacterial growth
To understand the effect of natural compounds on bacteria, we adopted bacterial turbidity measurements to determine growth inhibition by the amount of light scattered by suspension of cells. We measured optical density (OD) of bacterial cultures after overnight treatment (18 h) with various partially purified compound fractions.
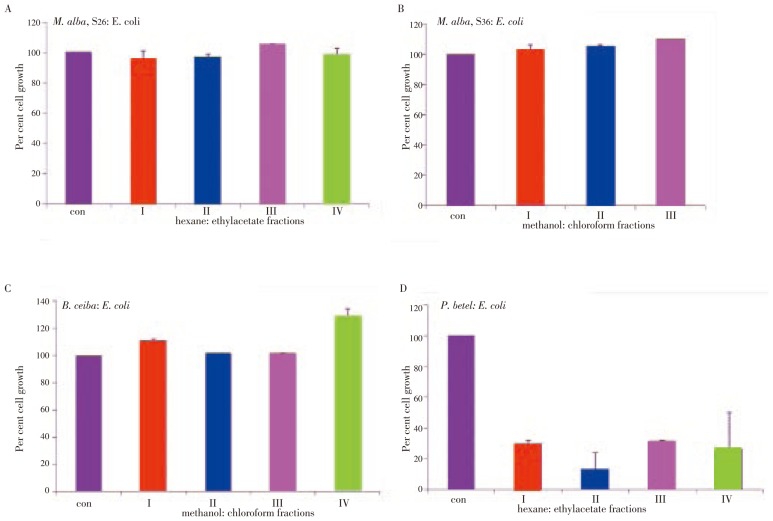
E. coli: Between S26 and S36 mulberry varieties, S26 variety showed growth inhibitory property against bacterial growth. Among the S26 fractions, except fraction III all other three factions, I, II, IV showed a small but significant growth inhibition (Figure 2A). The S36 treatment, instead of showing growth inhibition, showed growth stimulation. The stimulatory effect increased in fractions I and III (Figure 2B). The B. ceiba extracts also showed growth stimulatory effect. The fractions I and IV showed maximum proliferation, i.e., 11 and 29 percent, and fractions II and III showed negligible stimulation (Figure 2C). P. betel extracts showed a significant growth inhibition compared with M. alba or B. ceiba extracts. While fractions II and IV showed 80 and 75 percent growth inhibition, fractions I and III showed 70 percent growth inhibition (Figure 2D).
Figure 2. Plant extracts induced growth inhibition in E. coli.
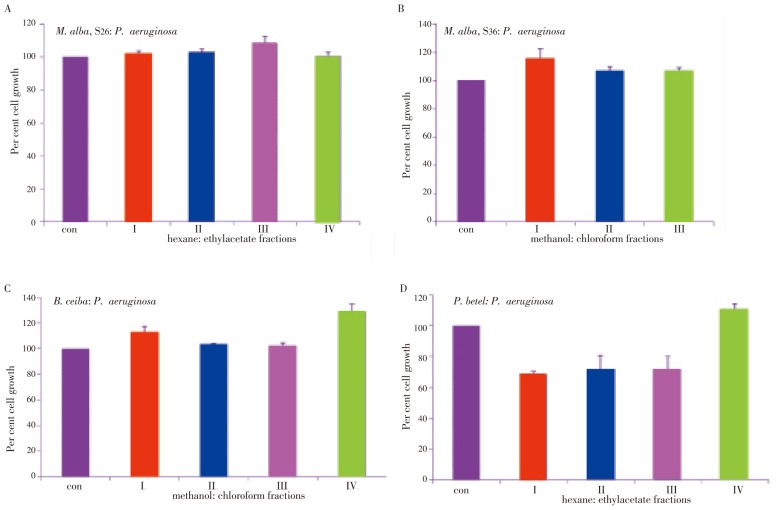
P. aeruginosa: Both S26 and S36 fractions showed a significant growth stimulatory effect. Compared with S26 fractions (I-IV), which showed 10% growth stimulation, S36 fractions (I-IV) showed 20% growth stimulation (Figure 3A and 3B). Similar to M. alba fractions, B. ceiba fractions showed growth stimulatory effect on P. aeruginosa. While fractions I, II, and III showed (12%-15%) growth increase, fraction IV showed 50% increase (Figure 3C). Interestingly and in agreement with E. coli growth inhibition, all the fractions from P. betel induced significant growth inhibition. The growth inhibition found for fractions I, II, III and IV were 70%, 40%, 30% and 20%, respectively (Figure 3D).
Figure 3. Plant extracts induced growth inhibition in P. aeruginosa.
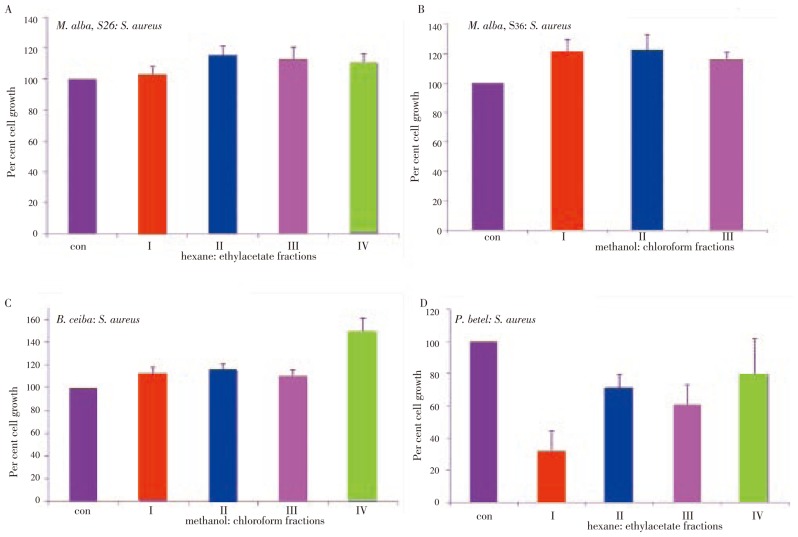
S. aureus: All S26 fractions showed growth stimulation (Figure 4A). While S36 fraction I showed 15% growth stimulation, fractions II and III showed 6% and 7% growth stimulation respectively (Figure 4B). Collectively M. alba fractions showed a growth stimulatory effect on S. aureus. In accordance with previous results of E. coli and P. aeruginosa, the B. ceiba fractions I and IV showed maximum growth stimulation, 12% and 29% respectively (Figure 4C). Since fraction IV found to enhance proliferation rates in all the three bacteria studied, this fraction appears to contain compounds that have growth stimulatory property. Except the fraction IV, fractions I, II and III showed 30% growth inhibition (Figure 4D).
Figure 4. Plant extracts induced growth inhibition in S. aureus.
3.2. Inhibition of bacterial growth in ampicillin combination treatment
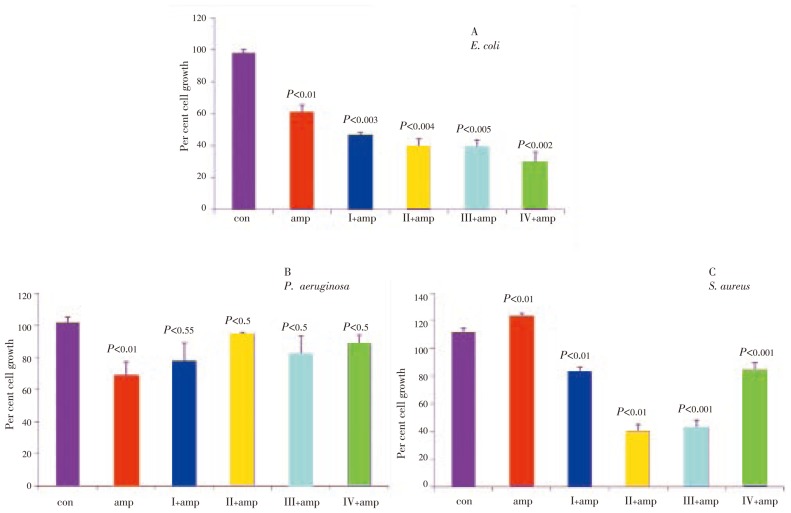
Ampicillin is an antibiotic belonging to the group of beta-lactam antibiotics and found to be effective against both gram-negative and gram-positive bacteria with differential sensitivity. Ampicillin when used in combination with other growth inhibitor drugs has been shown to enhance growth inhibition and potentiate the cytotoxic effects[15]. Therefore, we examined effect of ampicillin in combination with P. betel fractions against S. aureus, P. aeruginosa and E. coli in the present study.
Bacterial cells were treated with 10 µg of ampicillin either alone or in combination with P. betel fractions in their mid-log phase of growth, and the growth inhibition was monitored after 18 h. While E. coli showed 40% growth inhibition with ampicillin alone (P<0.05), a combination of P. betel fractions (I-IV, each 200 µg) potentiated ampicillin effect on growth inhibition, which was 64%, 60%, 60% and 70% respectively for fractions I, II, III and IV (Figure 5A; P<0.05). The ampicillin either alone or in combination with P. betel failed to show any inhibitory effect on P. aeruginosa (Figure 5B; P<0.05). However, ampicillin in combination with P. betel induced 17%, 60%, 57% and 26% growth inhibition respectively for fractions I, II, III and IV on S. aureus (Figure 5C; P<0.05).
Figure 5. Growth inhibition of P. betel extracts in combination with ampicillin.
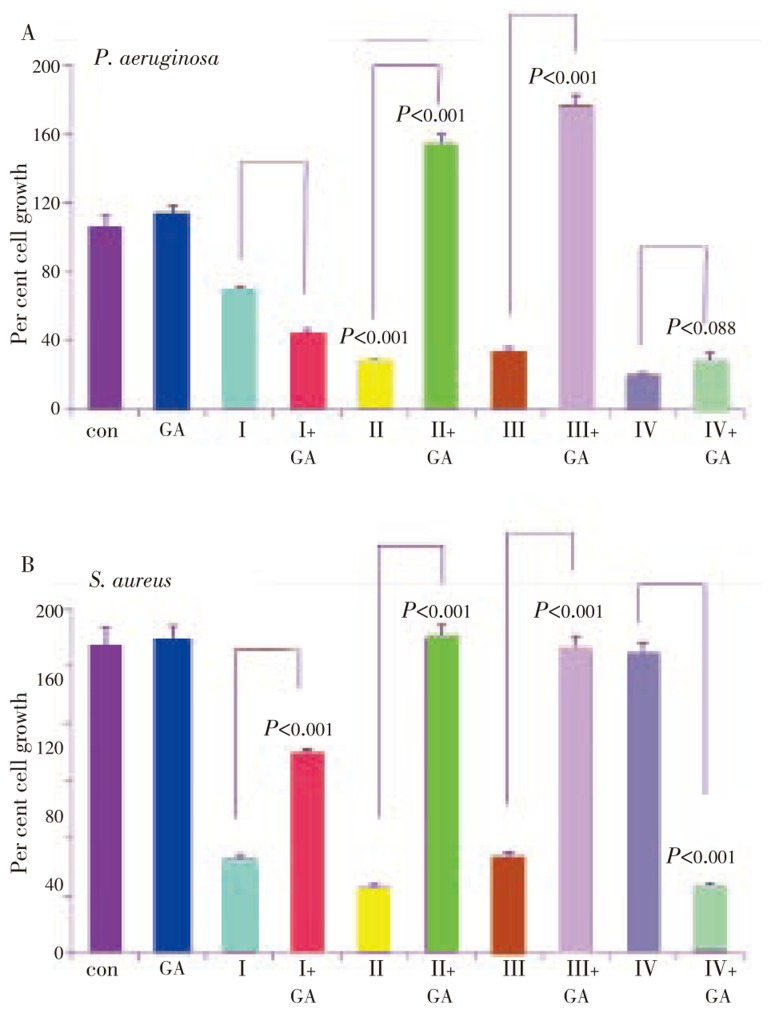
3.3. Inhibition of bacterial growth in geldanamycin combination treatment
Geldanamycin and its derivatives are selective inhibitors of heat shock protein-90, a pharmacological target to combat mammalian cancer[16]. While mammalian Hsp90 has been shown to be involved in induced cell proliferation, its bacterial homologue, HtpG has not been reported to have limited functional role in bacteria[17]. We examined geldanamycin effect in combination with P. betel fractions. A pre-treatment of bacterial cells with geldanamycin for 1 h followed by the addition of P. betel fraction was evaluated after 18 h of co-treatment in P. aeruginosa and S. aureus. Since E. coli did not respond to geldanamycin, data pertaining to E. coli were not included. Geldanamycin treatment alone was not effective but its combination with P. betel fractions I and IV significantly inhibited bacterial growth in both P. aeruginosa and S. aureus. However, fractions II and III in combination with geldanamycin were not effective (Figure 6A and B).
Figure 6. Growth inhibition of P. betel extracts in combination with geldanamycin.
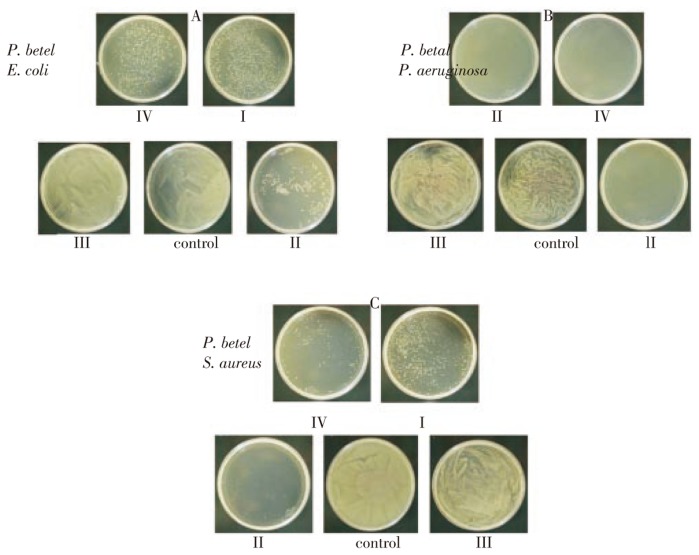
3.4. Inhibition of bacterial colony formation by P. betel extracts
Cells with differential drug uptake differently respond to growth stimulation, which provides non-conclusive information that interferes with the data interpretation. Since drug evaluation is crucial and assigning appropriate drug concentration with IC50 (growth inhibition concentration) is essential, we adapted colony formation assay to evaluate growth inhibition of natural compounds that were isolated and used in our cytostatic studies.
E. coli: While control plating showing lawn of bacterial growth, a significant inhibition of colony formation in fraction II was observed, showing 88% colony inhibition. Fractions I and IV showed 75% and 70% inhibition but fraction III showed no colony inhibition (Figure 7A; P<0.01).
Figure 7. Structural analysis of lead compounds isolated from P. betel extracts.
P. aeruginosa: Surprisingly, fractions I, II, and IV showed 100 percent inhibition on colony formation and bacterial growth, and fraction III treated cells appeared to be normal comparable with that of control untreated cells (Figure 7B; P<0.01).
S. aureus: Only cells treated with fraction II showed 100 percent inhibition, whereas cells treated with fractions I, III, and IV showed 25, 2 and 90 percent inhibition respectively. The results were comparable with other cells, showing that the Piper betel fraction II appears to contain significant growth inhibitory components (Figure 7C; P<0.01).
3.5. Bacteriostatic effect of lead compounds on gram-positive and gram-negative bacteria
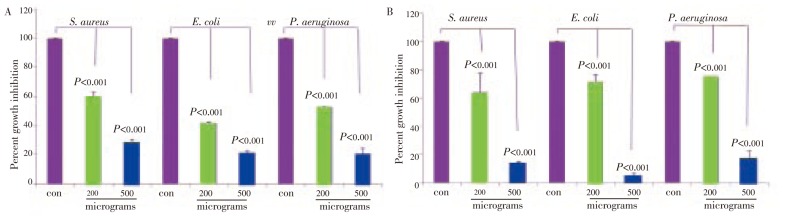
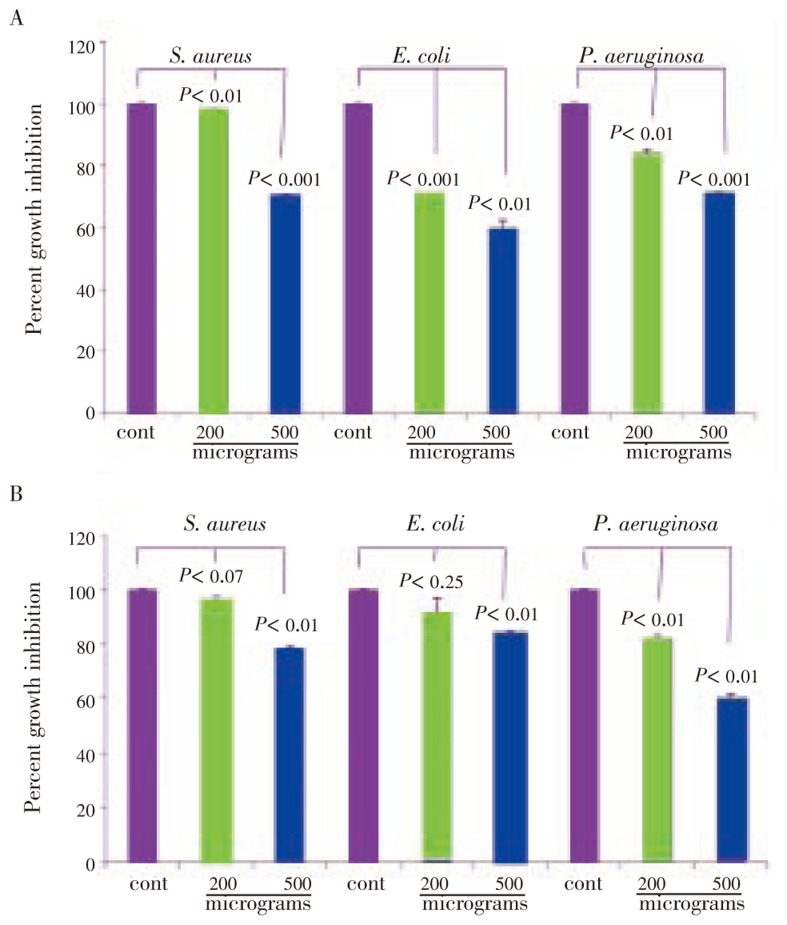
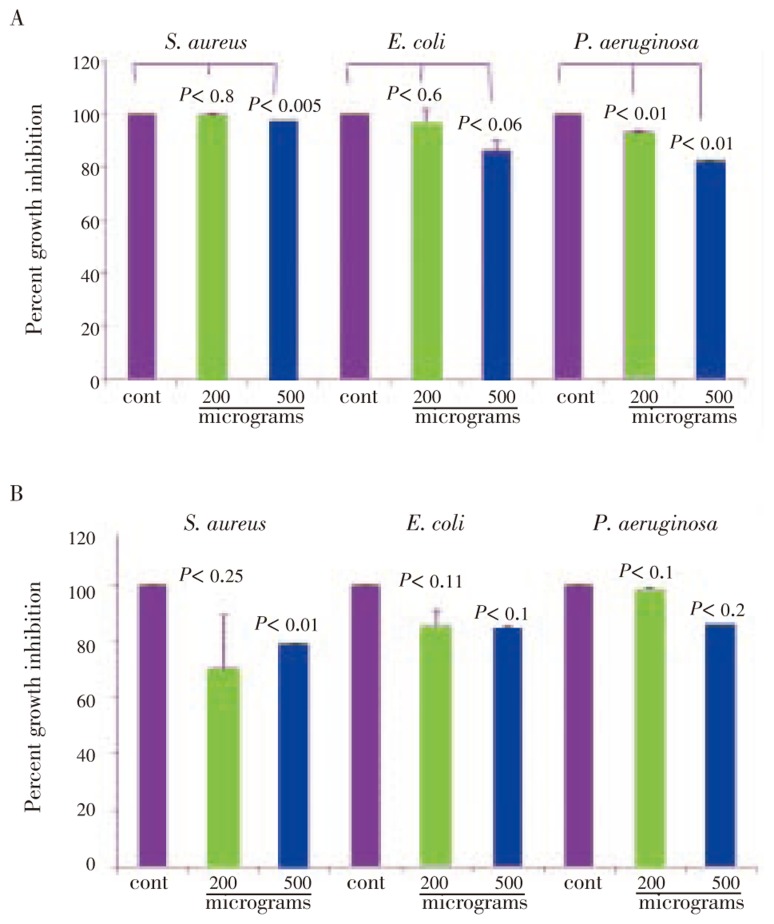
On further evaluation of partially purified fractions, we identified three lead compounds, i.e., eugenol, stigmasterol and 3-hexene-ol. The isolation, purification, structural analysis and stability of these compounds were assayed as explained earlier[18],[19]. Eugenol showed a dose dependent, significant and enhanced anti-bacterial activity by 5 h treatment(P<0.001) on all the three bacteria, E. coli, S. aureus and, P. aeruginosa. On prolonged incubation(24 h) with the drug at 200 microgram concentration, there was a slow recovery(Figure 8A). However at 500 microgram concentration, pronounced growth inhibition was observed (Figure 8B). Stigmasterol also showed a dose dependent growth inhibition. When compared with eugenol it was 42% less by 5 h treatment, but did not show any further increase on prolonged treatment of 24 h and 48 h(Figure 9A and 9B). The 3-hexene-ol showed insignficant growth inhibition at 24 h and 48 h (Figure 10A and 10B).
Figure 8. Growth inhibition of eugenol on S. aureus, E. coli, P. aeruginosa.
Figure 9. Growth inhibition of stigmasterol on S. aureus, E. coli, P. aeruginosa.
Figure 10. Growth inhibition of 3-hexene-ol on S. aureus, E. coli, P. aeruginosa.
4. Discussion
Natural compounds have been the major source of drug discovery and drug designing. Our study examined the anti-bacterial properties of natural compounds isolated from three remedial south Indian plant varieties. The reason to use these plant sources are: (1) they are readily available, (2) easy to grow and maintain at tropical temperatures and (3) undefined but possessing known medicinal value. Three different strains of bacteria were chosen because (1) they have potential pathogenecity in the human host on infection, (2) being the common types of infectious bacteria and (3) from both gram-negative and gram-positive members. Interestingly, M. alba and B. ceiba extracts did not show any significant bacterial growth inhibition, compared with P. betel which showed marked growth inhibition against three different bacteria. The aqueous extracts of P. betel have been reported to have antioxidant activity but not have anti-bacterial activity[20]. Interestingly, Nalina and Rahim[21] reported that the aqueous extracts have anti-bacterial activity only against mutant bacteria but not against the wild type. Using organic extractions of P. betel we demonstrated their anti-bacterial activity.
The combined treatment of drug with natural compounds demonstrated[22],[23] that the treatment potentiates drug toxicity, prevents the emergence of resistant strains and takes advantage of antibiotic synergism. Our combination treatment of drug with ampicillin and geldanamycin further demonstrated the potential anti-bacterial components present in P. betel fractions. It can be argued that the active principles of crude extracts lose their activity when used in isolation of active principles because the activity assigned to the crude extract is always assumed to be a synergistic effect of mixture of compounds. However, the three major compounds isolated from P. betel also showed significant anti-bacterial activity, suggesting that there may be mixture of active principles present in the partially purified P. betel extract. Antibiotic penicillin targets gram-positive bacteria, whereas streptomycin targets gram-negative bacteria. However, there is no universal antibiotic drug that can target both gram-positive and gram-negative bacteria. We demonstrated that P. betel fractions target both gram-negative(E. coli and P. aeruginosa) and gram-positive(S. aureus) bacteria. Primarily we could isolate three major compounds but there may be several other compounds present in P. betel leaves, which needs further study.
Footnotes
Foundation Project: Supported by Council of Scientific and Industrial Research (CSIR), Government of India.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Adamowicz W, Dupont D, Krupnick A, Zhang J. Resources for the future. Washington: REF Press; 2007. Valuation of cancer and microbial disease risk reductions in municipal drinking water: An analysis of risk context using multiple valuation methods; pp. 1–30. [Google Scholar]
- 2.Chopra I. The increasing use of silver-based products as anti-microbial agents: a useful development or a cause for concern? J Antimicrob Chemother. 2007;59(4):587–590. doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- 3.Xing B, Rao J, Liu R. Novel beta-lactam antibiotics derivatives: their new applications as gene reporters, antitumor prodrugs and enzyme inhibitors. Mini Rev Med Chem. 2008;8(5):455–471. doi: 10.2174/138955708784223558. [DOI] [PubMed] [Google Scholar]
- 4.Das Gupta B, Konwar KM, Mandoiu II, Shvartsman AA. DNA-BAR: distinguisher selection for DNA barcoding. Bioinformatics. 2005;15(16):3424–3426. doi: 10.1093/bioinformatics/bti547. [DOI] [PubMed] [Google Scholar]
- 5.Lindeberg M, Biehl BS, Glasner JD, Perna NT, Collmer A, Collmer CW. Gene ontology annotation highlights shared and divergent pathogenic strategies of type III effector proteins deployed by the plant pathogen Pseudomonas syringae pv tomato DC3000 and animal pathogenic Escherichia coli strains. BMC Microbiol. 2009;19(9) Suppl. 1:S4. doi: 10.1186/1471-2180-9-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dev S. Impact of natural products in modern drug development. Indian J Exp Biol. 2010;48(3):191–198. [PubMed] [Google Scholar]
- 7.Pavithra PS, Janani VS, Charumathi KH, Indumathy R, Sirisha P, Verma RS. Anti-bacterial activity of plants used in Indian herbal medicine. Int J Green Pharmacol. 2010;4(1):22–28. [Google Scholar]
- 8.Newman DJ, Crag GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 9.Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15(8):639–652. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev. 2010;6(4):247–254. doi: 10.2174/157339910791658826. [DOI] [PubMed] [Google Scholar]
- 11.Naowaratwattana W, De-Eknamkul W, De Mejia EG. Phenolic-containing organic extracts of mulberry (Morus alba L.) leaves inhibit HepG2 hepatoma cells through G2/M phase arrest, induction of apoptosis, and inhibition of topoisomerase IIα activity. J Med Food. 2010;13(5):1045–1056. doi: 10.1089/jmf.2010.1021. [DOI] [PubMed] [Google Scholar]
- 12.Vieira TO, Said A, Aboutabl E, Azzam M, Creczynski-Pasa TB. Antioxidant activity of methanolic extract of Bombax ceiba. Redox Rep. 2009;14(1):41–46. doi: 10.1179/135100009X392485. [DOI] [PubMed] [Google Scholar]
- 13.Keat EC, Razak SS, Fadil NM, Yusof FM, Chan LH, Chyi FK, et al. The effect of Piper betel extract on the wound healing process in experimentally induced diabetic rats. Clin Ter. 2010;161(2):117–120. [PubMed] [Google Scholar]
- 14.Bhattacharya S, Chaudhuri SR, Chattopadhyay S, Bandyopadhyay SK. Healing properties of some Indian medicinal plants against indomethacin-induced gastric ulceration of rats. J Clin Biochem Nutr. 2007;41(2):106–114. doi: 10.3164/jcbn.2007015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eumkeb G, Sakdarat S, Siriwong S. Reversing β-lactam antibiotic resistance of Staphylococcus aureus with galangin from Alpinia officinarum Hance and synergism with ceftazidime. Phytomedicine. 2010;18(1):40–45. doi: 10.1016/j.phymed.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Taldone T, Gozman A, Maharaj R, Chiosis G. Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol. 2008;8(4):370–374. doi: 10.1016/j.coph.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchner J. Bacterial Hsp90-desperately seeking clients. Mol Microbiol. 2010;76(3):540–544. doi: 10.1111/j.1365-2958.2010.07140.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Shyamveer S, Richa K, Mishra SK, Varshney SC, Gupta KC. Volatile constituents of essential oil of betel leaf ( Piper betel Linn) Cv. Meetha. Indian Perfumer. 2007;51(4):55–57. [Google Scholar]
- 19.Yin Y, Huang X, Wang J, Dai J, Liang H, Dai Y. Studies on the chemical constituents of the stems of Piper betel. Zhongyaocai. 2009;32:887–890. [PubMed] [Google Scholar]
- 20.Rathee JS, Patro BS, Mula S, Gamre S, Chattopadhyay S. Antioxidant activity of Piper betel leaf extract and its constituents. J Agric Food Chem. 2006;54(24):9046–9054. doi: 10.1021/jf061679e. [DOI] [PubMed] [Google Scholar]
- 21.Nalina J, Rahim ZHA. The crude aqueous extract of Piper betel L. and its anti-bacterial effect towards Streptococcus mutans. Am J Biotech Biochem. 2007;3(1):10–15. [Google Scholar]
- 22.Hemaiswarya S, Kruthiventi AK, Doble M. Antioxidant activity of Piper betel leaf extract and its constituents. J Agric Food Chem. 2006;54(24):9046–9054. doi: 10.1021/jf061679e. [DOI] [PubMed] [Google Scholar]
- 23.Jyothi D, Vanathi P, Mangala GP, Rama SRV, Madhusudana RJ, Sreedhar AS. Diferuloylmethane augments the cytotoxic effects of piplartine isolated from Piper chaba. Toxicol In Vitro. 2009;23(6):1085–1091. doi: 10.1016/j.tiv.2009.05.023. [DOI] [PubMed] [Google Scholar]