Abstract
Background: The progressive accumulation of misfolded and aggregated proteins in neurons is an accepted mechanism in aging. Overproduction of reactive oxygen species (ROS), referred to as oxidative stress, is currently believed to play a pivotal role in this process. Lipofuscin as a histological index of aging results from cross-links between oxidized proteins and lipids. Therefore, to attenuate lipofuscin formation, it would be logical to use exogenous natural or synthetic antioxidants. Yakuchinone B (1-[4'-hydroxy-3'-methoxyphenyl]-7-phenylhept-1-en-3-one) is a component of Alpinia oxyphylla seeds with established antioxidant activity. Methods: To evaluate the neuroprotective roles of yakuchinone B (JC6) and its structural analogues (JC1-JC5), the free radical scavenging capabilities of yakuchinone B derivatives were studied in terms of cell viability, apoptosis, cells ROS content, catalase (CAT) and superoxide dismutase (SOD) activity and the intracellular lipofuscin content in SK-N-MC cells exposed to H2O2. The level of MDA (malondialdehyde), as an index of lipid peroxidation and acid phosphatase activity were also measured. Results: Our results indicated that derivatives especially JC4, JC5 and JC6 decreased the extent of apoptosis and ROS level, while they increased the activities of SOD and CAT in drug-pretreated cells as compared to H2O2-treated cells. A clear relationship between the structure and antioxidant activities of these compounds was established. In addition, JC4, JC5 and JC6 were capable of down-regulating the formation of MDA and lipofuscin. Conclusion: Our results indicated that free radicals play significant roles in lipofuscin formation and cellular aging which can be attenuated by yakuchinone B derivatives.
Key Words: Aging, Lipofuscin, Reactive oxygen species (ROS), Yakuchinone B
Introduction
Aging, characterized by progressive changes in cells and tissues of the body, is associated with attenuation of cellular function [1]. Over-production of reactive oxygen species (ROS) including free radicals, such as superoxide (O2•−), hydroxyl (•OH), peroxyl (RO2•−) as well as non-radical species such as hydrogen peroxide (H2O2) are accepted as the main modulators of aging [2]. Under oxidative stress condition, H2O2 diffuses into lysosomes (pH ~ 4-5) and reacts with iron metal ions released from metalloprotein degradation (Fenton chemistry). This leads to the formation of highly ROS such as hydroxyl radicals (•OH) which subsequently oxidize vital macromolecules, such as lipids, proteins and nucleic acids. The oxidized products would then react with each other leading to the formation of aggregates known as lipofuscin pigments or age pigments [3, 4].
Therefore, lipofuscin is an intracellular indigestible yellow-brown autofluorescent material mainly composed of oxidized proteins (30-58%) and lipids (19-51%). It is highly resistant to proteolytic degradation and accumulates mostly in post mitotic cells such as neurons [5-7]. The intracellular rate of lipofuscin formation is negatively correlated with the remaining life span of cells and increases with age [7, 8]. Regarding the elevated level of polyunsaturated fatty acids in brain, lipofuscin pigments are mostly accumulated within this tissue by age [9].
However, cells have several antioxidant defense mechanisms to prevent and/or to attenuate the destructive effects of ROS. These defense mechanisms include anti-oxidative enzymes, such as super-oxide dismutase (SOD), catalase (CAT), glutathione peroxidase and small molecules such as glutathione and vitamins C and E [10]. The efficiency of the antioxidant defense system is weakened under oxidative stress conditions. Therefore, to back up the system under these conditions, it might be beneficial to use exogenous natural or synthetic antioxidants.
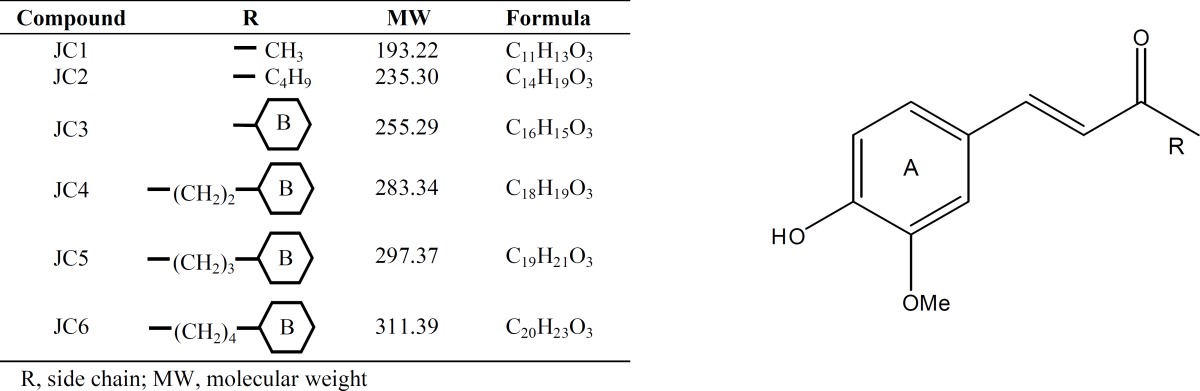
Recently, much attention has been devoted to the phenolic antioxidants of medicinal and dietary plants [11-14]. Yakuchinone B (1-[4'-hydroxy-3'-methoxy-phenyl]-7-phenylhept-1-en-3-one) has been charac-terized in Alpinia oxyphylla from Zingiberaceae family [15]. This conjugated 1,4-enones belongs to chalcone compounds with known diverse biological activities, such as anti-inflammatory [16], anti-proliferative [17], anti-viral [18] and anti-neurodegenerative [19]. To get a better understanding on the structural entities required for free radical scavenging, we evaluated the protective effects of yakuchinone B (JC6) and its derivatives (JC1-JC5, Table 1) against H2O2-induced damage on SK-N-MC cells in terms of cell viability, intracellular ROS content, malondialdehyde (MDA) and lipofuscin levels and also on the CAT and SOD activity. Our results indicated that derivatives especially JC4, JC5 and JC6 decreased the extent of apoptosis and ROS levels. However, they increased the activities of SOD and CAT as compared to H2O2-treated cells. The antioxidant activities of yakuchinone B and its derivatives seemed to be related to their molecular structure, the presence of a hydroxyl group on ring A (JC1-JC6), presence of an alkylated ring B (JC3, JC4, JC5 and JC6) and the conjugation. In addition, resonance effects on rings A and B seemed to be essential for JC activities.
Table 1.
Substituted benzylideneacetophenone derivatives (JC1-JC6) used in the present study. For the synthetic methods, please refer to Oh et al. [19].
|
MATERIALS AND METHODS
Materials. Human SK-N-MC neuroblastoma cells were obtained from the Pasteur Institute of Iran (Tehran). The cell culture medium (RPMI 1640), penicillin-streptomycin and fetal bovine serum (FBS) were purchased from Gibco BRL (Life Technology, Paisley, Scotland). EDTA was obtained from Sigma-Aldrich (Germany). Ethidium bromide, acridine orange and Triton X-100 were purchased from Pharmacia LKB Biotechnology (Sweden). MTT [3-(4,5-dimethyl tiazol-2-yl)-2,5-diphenyl tetrazolium bromide] and PMSF were from Sigma Chemical Co. (Germany). Dimethyl sulfonate, nicotinamide adenine dinucleotide reduced, phenazine methosulfate, glutaraldehyde, H2O2 and 2-thio-barbituric acid were obtained from Merck (Germany). The 2′,7′-dichlorofluorescein diacetate (DCFH-DA) was obtained from Molecular Probe (Eugene, Oregon, USA). Benzylideneacetophenone derivatives (JC1-JC6) were a generous gift from Dr. Seikwan Oh (Ewha Woman’s University of Korea). JC1-JC6 were dissolved in a minimum amount of DMSO and then diluted with the culture medium to get the desired concentration. The concentration of DMSO in the culture medium has been kept lower than 0.1% and the control cells have been treated with the vehicle containing the same amount of DMSO.
Cell culture. The SK-N-MC cells were cultured at a density of 5 × 104/ml in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin (100 µg/ml) and incubated under humidified atmosphere of 5% CO2 at 37C.
Cytotoxicity evaluation of JC1-JC6 in SK-N-MC cells. Cell viability was estimated using the MTT assay. This method is dependent on the conversion of yellow tetrazolium bromide to its purple formazan derivative by mitochondrial succinate dehydrogenase of the viable cells [20]. The cells were seeded in 96-well plates at a concentration of 5 × 104 cells/ml for 24 h, and then treated with JC1-JC6 at different concentrations (10, 20, 40, 60 and 80 µM). After 24 h, MTT stock solution (10 µl, 5 mg/ml) was applied to each well. After 4 h of incubation, the plates were centrifuged at 900 ×g for 15 min and the supernatants were aspirated. The formazan crystals in each well were dissolved in 200 µl of DMSO, and the absorbance was measured via ELISA technique at a wavelength of 570 nm. Results were expressed in percentage of MTT reduction as compared to the control cell samples, presuming that the absorbance of the control cells was 100%.
Cytoprotective effect of JC1-JC6 in H 2 O 2 -treated SK-N-MC cells. We have previously reported that exposure of the cells to 300 µM of H2O2 has caused significant reduction in viability by almost 42% as compared to untreated cells [3]. Therefore, to induce oxidative stress, H2O2 was freshly prepared from 8.5 mM stock solution prior to each experiment. To evaluate the cytoprotective effects of JC1-JC6 against H2O2 damage, SK-N-MC cells were plated at a density of 5 × 104 cells/ml for 24 h. The cells were treated with 20 µM of JC1-JC6. Three hours later, 300 µM H2O2 was added to the plate followed by incubation for an additional 24 h and 48 h. Cell viability was estimated using the MTT assay and the result was expressed in percentage of survival as compared to the control cell samples.
Fluorescence microscopy and detection of apoptotic cells. The SK-N-MC cells were seeded in 24-well plates. After 24 h, the cells were pretreated with 20 µM of each drug and then treated with 300 μM H2O2 for a time course of 24 h. Apoptotic cells were characterized morphologically by staining the cells with acridine orange/ethidium bromide (AO/EtBr) followed by fluorescence microscopy inspection [21]. After treatment, the cells were washed twice with PBS and adjusted to a cell density of 1 × 106 cells/ml of PBS and stained with AO/EtBr solution (1:1 v/v) in a final concentration of 100 µg/ml. The nuclear morphology was evaluated by Axoscope 2 plus fluorescence microscope from Zeiss (Germany). The cells with condensed or fragmented nuclei were counted as apoptotic cells. All experiments were repeated three times. In each run, the total number of cells along with total number of apoptotic cells was determined in 10 different microscopic fields. The extent of apoptosis was then expressed as a percentage of the total cell count.
Measurement of intracellular reactive oxygen species. The ROS generation was monitored using DCFH-DA which readily diffuses into cells [22]. Within the cells, this non-fluorescent dye reacts with intracellular ROS and is converted into dichloro-fluorescein which is a fluorophor. SK-N-MC cells were cultured in a 12-well plate with 1 ml of medium for 24 h. Then, the cells were pretreated with drugs and then exposed to 300 µM H2O2. After 24 h of incubation, the cells were rinsed with serum-free RPMI medium and then DCFH-DA (10 µM) was added to the cells followed by incubation for 1 h at 37C. After incubation, the cells were washed twice with PBS and the fluorescence intensity was monitored using a Varian-spectrofluorometer, model Cary Eclipse with excitation and emission wavelengths of 485 nm and 530 nm, respectively.
Catalase activity assay. The activity of CAT was measured by the method of Aebi [23]. The decomposition of H2O2 was followed by monitoring the decrease in absorbance at 240 nm for 60 s using a spectrophotometer. The rate of decomposition of H2O2 was expressed as k/(s mg protein), where k represents the rate constant of the first-order reaction of CAT. Protein content was determined based on Lowry’s method [24] using crystalline bovine serum albumin for calibration.
Superoxide dismutase activity assay. SOD activity was measured based on the extent inhibition of amino blue tetrazolium formazan formation in the mixture of nicotinamide adenine dinucleotide, phenazine-methosulfate and nitroblue tetrazolium according to the method of Kakkar et al. [25]. One unit of enzyme activity was defined as the amount of enzyme which caused 50% inhibition of nitroblue tetrazolium reduction/mg protein.
Determination of lipid peroxidation. Lipid peroxidation is commonly measured in term of thiobarbituric acid reactive substance (TBARS) [26]. This assay is based on the ability of antioxidants toward the inhibition of lipid peroxidation in cells in the presence of H2O2. The extent of lipid peroxidation in the presence and absence of JC1-JC6 was evaluated based on the extent of TBARS according to the published method [27]. Briefly, the cells were seeded in 12-well plates and pretreated with 20 µM of each of the derivatives. After 3 h, the cells were exposed to 300 µM H2O2 for 24 h. Then, the cells were mixed with 0.5 mL of 10% trichloroacetic acid and heated at 95°C for 15 min. After cooling at room temperature, the samples were centrifuged at 1500 ×g for 10 min and 0.5 ml of each sample supernatant was transferred into a test tube containing 0.25 mL of 0.67% thiobarbituric acid. After cooling, the absorption of the organic layer was determined at 532 nm. The protein concentration was determined by the Lowry’s method [24]. The total amount of TBARS to form chromophore absorbing at 532 nm was estimated using a molar absorption coefficient of 1.56 × 105 cm−1 M−1. The results were expressed as nmol TBARS per mg of protein [28].
Acid phosphatase assay. The sodium acetate buffer (0.1 M, pH 5, 100 μl) containing 0.1% (vol/vol) Triton X-100 and 10 mM p-nitrophenyl phosphate was added to each well. The plates were placed in the incubator at 37C for 3 h. The reaction was stopped by the addition of 10 μl of NaOH (100 mM, pH 10.5) to each well, and the wells were measured via ELISA technique at a wavelength of 405 nm [29].
Evaluation of intracellular lipofuscin pigments. Extraction of intracellular lipofuscin was achieved following lysis of each cell sample according to the Emig and the colleagues’ procedure [30] with slight modification. The cells (5 × 104 cells/well) were seeded in 24-well plates for 24 h prior to pretreatments. After pretreatment with 20 µM of each derivative for 3 h, each cell sample was treated with 300 µM H2O2 for 24 h, 48 h and 72 h. The attached cells in each well were trypsinized with Trypsin-EDTA solution followed by cell counting using a hemocytometer. Each plate was then centrifuged and the cell pellet was washed with PBS, and the cell content was lysed with lysis buffer containing 1% Triton X-100, 1 mM EDTA and 1 mM PMSF. Each cell lysate was harvested and its fluorescence intensity was monitored on a Varian-spectrofluorometer, model Cary Eclipse with excitation and emission wavelengths of 310 and 620 nm, respectively [31]. All experiments were repeated three times and the fluorescence intensities of the samples were then normalized to equal cell numbers.
Statistical analyses. Data are expressed as mean ± SD of three independent experiments and statistically analyzed using Student’s t-test.Values of P<0.05 were considered significant.
Results
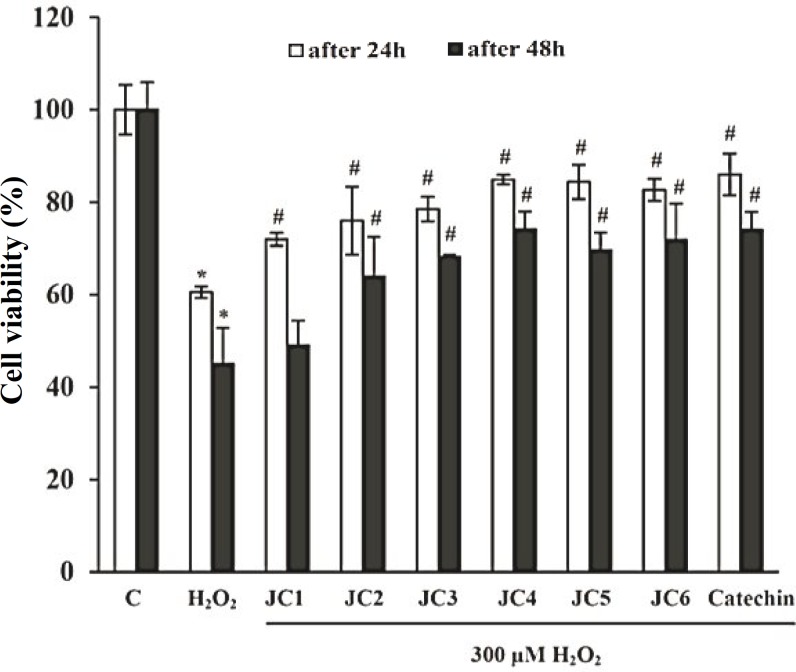
Effect(s) on cell viability. The toxicity of each of the compounds (JC1-JC6) was evaluated based on the viability of SK-N-MC cells exposed to variable concentrations of the compounds using MTT assay. Table 2 indicates that benzylideneacetophenone analogues (JC1-JC6) have slight cytotoxic effects on the cells following a 24 h evaluation time. Based on Table 2, the entire investigations with the derivatives were achieved at doses less than 20 µM to avoid the cytotoxic effects of the drugs. We evaluated the cytoprotective effect of JC1-JC6 against damage induced in cells by H2O2. For this reason, we pretreated SK-N-MC cells with 20 µM of JC1-JC6 for 3 h. Then, the pretreated cells were exposed to 300 µM H2O2 for 24 h and 48 h. As shown in Figure 1, treatment withH2O2 reduced cell viability by almost 40 and 55% as compared to H2O2-untreated cells after 24 and 48 h, respectively. However, pretreatment of cells with 20 µM of JC1-JC6 reduced the damaging effects of H2O2 by about 11, 15, 18, 24, 24 and 22% after 24 h and by about 4, 19, 23, 29, 25 and 27% after 48 h, respectively. Regarding these observations, it can be concluded that JC4, JC5 and JC6 at 20 µM concentration neutralized ROS to the same extent as catechin at the same concentration.
Table 2.
Effects of 20-80 µM benzylideneacetophenone analogues (JC1–JC6) on SK-N-MC cells viability.
| Compound (µM) |
Cell viability (%control)
|
|||||
|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 40 | 60 | 80 | |
| JC1 | 100 ± 3 | 104 ± 12 | 109 ± 5 | 102 ±1 | 95 ± 2 | 88 ± 1 |
| JC2 | 100 ± 3 | 99 ± 5 | 105 ± 2 | 98 ± 4 | 95 ± 3 | 90 ± 1 |
| JC3 | 100 ± 3 | 106 ± 1 | 94 ± 3 | 88 ±3 | 82 ± 3 | 77 ± 5 |
| JC4 | 100 ± 3 | 100 ± 1 | 110 ± 11 | 111 ± 6 | 104 ± 6 | 94 ± 3 |
| JC5 | 100 ± 3 | 105 ± 10 | 123 ± 1 | 113 ± 5 | 111 ± 2 | 90 ± 3 |
| JC6 | 100 ± 3 | 99 ± 8 | 101 ± 4 | 100 ± 1 | 100 ± 5 | 93 ± 4 |
| catechina | 100 ± 3 | 105 ± 3 | 102 ± 6 | 101 ± 2 | 99 ± 5 | 93 ± 7 |
aAs the standard antioxidant; Results are means ± SD of three measurements and all are significant from the control cells (P<0.05).
Fig. 1.
Protective effect of benzylideneacetophenone derivatives (JC1-JC6) on H2O2-induced cytotoxicity among SK-N-MC cells. SK-N-MC cells were pretreated with 20 µM of JC1-JC6 for 3 h, and then exposed to 300 µM H2O2 for further 24 h and 48 h. Cell viability was evaluated by MTT assay. Catechin was used as the positive control. Data were expressed as percent of values of untreated control cells. Each value represents the mean ± SD (n = 3). *Significantly different from control cells (P<0.05); #Significantly different from H2O2-treated cells (P<0.05).
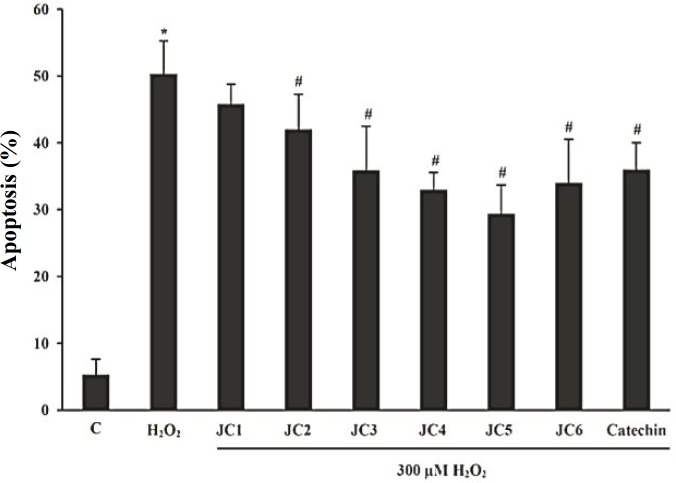
Inhibitory effects on apoptosis. To evaluate the inhibitory effects of benzylideneacetophenone analogues (JC1-JC6) against H2O2-induced toxicity, we studied the extent of cell death using AO/EtBr double staining technique [21]. In this approach, the non-apoptotic control cells appeared uniformly green and the apoptotic cells showed orange dots in their nuclei corresponding to nuclear DNA fragmentation. The extent of apoptotic cell death for untreated cells was lower than 5%. The number of apoptotic cells increased by 45% upon exposure to H2O2 for 24 h. However, treatment with H2O2 in the presence of JC1-JC6 decreased the extent of apoptotic cells by 4.5, 8.3, 14.5, 17.4, 21.0 and 16.3%, respectively (Fig. 2).
Fig. 2.
Measurement of the extent of cell apoptosis under the influence of the drugs (JC1-JC6). Cells exposed to 20 μM of each derivative for 3 h followed by exposure to 300 μM H2O2 for 24 h. Data were expressed as percentage of the apoptotic cells randomly counted in 10 microscopic fields. Catechin was used as the positive control. Each value represents the mean ± SD (n = 3). *Significantly different from control cells (P<0.05); #Significantly different from H2O2-treated cells (P<0.05).
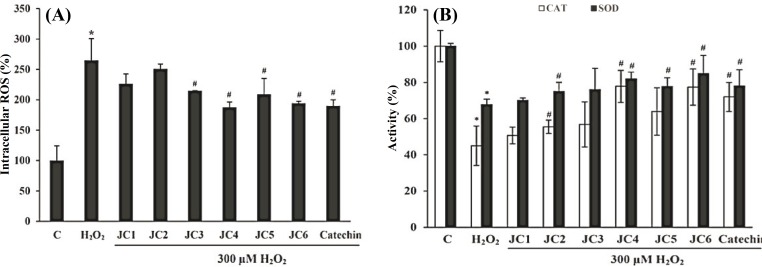
Deactivation of reactive oxygen species. Exposure of the cells to 300 μM H2O2 caused 165% increase in ROS content as compared to H2O2-untreated control cells. Pretreatment of the cells with JC1-JC6 at 20 μM diminished the levels of ROS by 38, 14, 50, 77, 56 and 71%, respectively compared to cells exposed only to H2O2 (Fig. 3A). The intracellular ROS level did not varied among the cells treated solely with each of the drugs (JC1-JC6, 20 μM, data not shown). Based on our data, JC3, JC4, JC5 and JC6 can certainly be classified as free-radical scavengers.
Fig. 3.
Effects of JC1-JC6 on the extent of intracellular ROS and the activities of CAT and SOD. The SK-N-MC cells were incubated without or with JC1-JC6 (20 μM) for 3 h before addition of H2O2. Intracellular ROS was measured using DCFH-DA (A). Restoring effect of JC1-JC6 on SOD and CAT activities in H2O2-treated SK-N-MC cells (B). Catechin was used as the positive control. Results are means ± SD of three measurements. *Significantly different from control cells (P<0.05); #Significantly different from H2O2-treated cells (P<0.05).
Effects on endogenous antioxidant enzymes . The H2O2-treated SK-N-MC cells showed significantly lower levels of the CAT and SOD activity as compared to the untreated control cells by 55 and 32%, respectively (Fig. 3B). However, pre-treatment of the cells with each of derivatives at a concentration of 20 µM increased the CAT activity by 5.3, 10.5, 11.8, 32.6, 18.9 and 32.4% and SOD activity by 2.3, 7.2, 8.2, 14.2, 10.0 and 17.1%, respectively as compared to H2O2-treated cells.
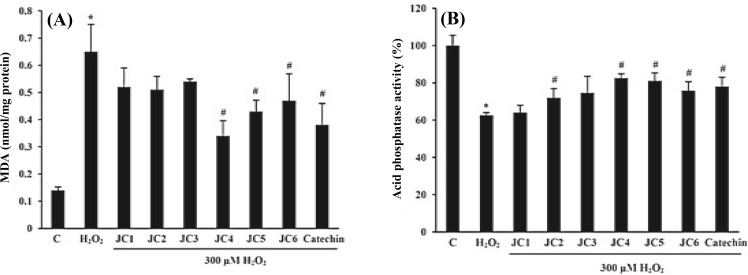
Inhibition of lipid peroxidation. Biological membranes and intracellular components, which are rich in polyunsaturated fatty acids, can be easily affected by free radicals through peroxidation. The extent of lipid peroxidation was significantly increased among the cells exposed to 300 µM H2O2 for 24 h as measured in terms of MDA (Fig. 4A). The level of MDA in SK-N-MC cells treated with H2O2 for 24 h was 0.65 nmol/mg protein which was about 4.3 foldshigher than that of untreated cells (0.15 nmol/mg protein). As shown in Figure 4A, JC4, JC5 and JC6 effectively inhibited the formation of MDA. The MDA levels in cells pretreated with JC1-JC6 were 0.52, 0.50, 0.54, 0.34, 0.43 and 0.47 nmol/mg protein, respectively. In other words, JC1-JC6 have quenched the formation of MDA by 20.4, 22.7, 16.8, 47.9, 34.2 and 28.1% as compared to H2O2-treated cells.
Fig. 4.
Inhibitory effects of derivatives (JC1-JC6) on the H2O2-induced lipid peroxidation after 24 h (A) and restoring effects on acid phosphatase activity in H2O2-treated SK-N-MC cells (B). Cells were pretreated with 20 μM of JC1-JC6 for 3 h before addition of H2O2. Catechin was used as the positive control. Results are means ± SD of three measurements. *Significantly different from control cells (P<0.05); #Significantly different from H2O2-treated cells (P<0.05).
Effects on acid phosphatase activity. Acid phosphatase is a stable lysosomal enzyme. Accumulation of lipofuscin in lysosome acts as a trap for acid phosphatase leading to its inactivation. Our results showed a significant decrease (by 38%) in acid phosphatase activity among the H2O2-treated cells. However, pretreatment of the cells with each of the drugs (JC1-JC6) increased the acid phosphatase activity by 1, 8, 12, 20, 19 and 13%, respectively as compared to H2O2-trested cells (Fig. 4B).
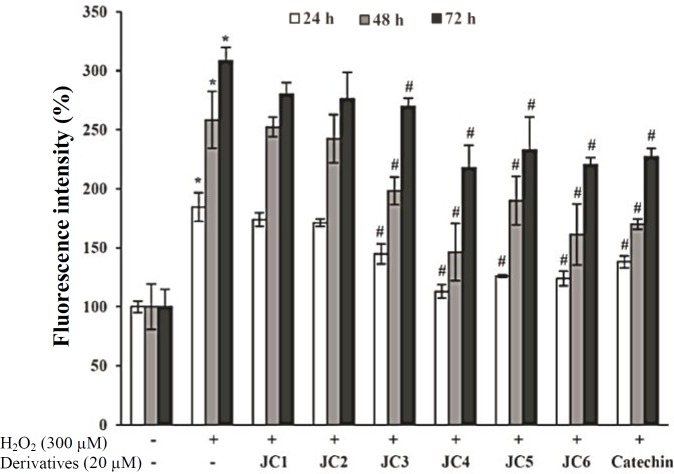
Influence on lipofuscin formation. Exposure of the cells to 300 μM H2O2 for 24 h, 48 h and 72 h caused 85, 158 and 208% increase in the intracellular level of lipofuscin pigments as compared to H2O2-untreated control cells, respectively. Pretreatment of the cells with each of the derivatives (20 μM) attenuated the formation of lipofuscin pigments by 11, 13, 40, 72, 58 and 61% after 24 h of exposure (Fig. 5). Regarding these data, JC drugs can certainly reduce lipofuscin formation due to their free-radical scavenging capabilities as one of their route of action.
Fig. 5.
Inhibitory effects of JC1-JC6 on H2O2-induced lipofuscin formation. Cells exposed to 20 μM of JC1-JC6 for 3 h followed by exposure to 300 μM H2O2 for 24-72 h. Then, the extent of lipofuscin in each cell lysate was evaluated using a varian spectrofluorometer; model Cary Eclipse with excitation and emission wavelengths of 310 nm and 620 nm, respectively. Catechin was used as the positive control. Each value represents the mean ± SD (n=3). *Significantly different from control cells (P<0.05); #Significantly different from H2O2-treated cells (P<0.05).
Discussion
It is by now well accepted that free radicals play vital roles in initiation and progression of age-related complications [32]. H2O2, as a pro-oxidant, is capable of penetration into lysosomes, where it interacts with redox-sensitive transition metals via Fenton reaction and produces hydroxyl radicals [33]. Hydroxyl radicals, in turn, initiate lipid peroxidation through MDA production, which will guide the cross-linking of macromolecules, such as proteins and the lipid peroxidation products leading to the formation of lipofuscin aggregates [4]. Thus, lipofuscin accumulation is linked to oxidative stress and/or inefficient antioxidant defense system. In addition, liposomal, iron overload and mitochondrial dysfunction are considered to play roles in lipofuscin accumulation within the cells [34]. Based on these facts, the antioxidant-based therapeutical approaches are expected to attenuate lipofuscin accumulation via perturbation of free radical chain reactions [3, 35].
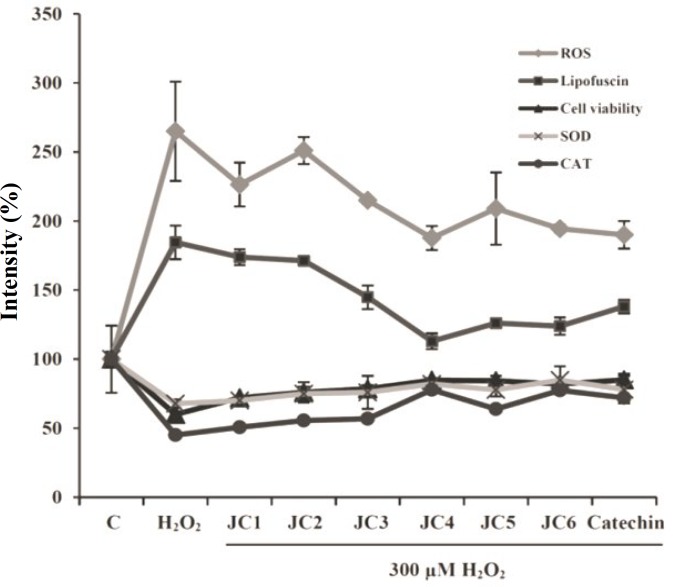
In the present investigation, we attempted to correlate the antioxidant property of yakuchinone B (JC6) and some of its derivatives (JC1-JC5) to the intracellular build up of lipofuscin aggregates. Our cumulative results indicated that the damaging effects of H2O2 on cell viability, morphology and apoptosis were all reversed by pretreating the cells with each of the analogues. For example, the viabilities of the JC-pretreated cells were increased by 11, 15, 18, 24, 24 and 22% after 24 h and by about 4, 19, 23, 29, 24 and 27% after 48 h, respectively as compared to H2O2-treated cells (Fig. 1). The extent of apoptosis among the JC-pretreated cells was decreased by 4.5, 8.3, 14.5, 17.4, 21.0 and 16.3% as compared to H2O2-treated cells (Fig. 2). While the H2O2-treated cells have heterogeneity in shape and size and mostly detached from the culture plate surface, the drugs pre-treated cells showed resistance to damaging effects of H2O2 and this was evident through their almost uniform shapes and sizes besides being mostly attached to the plate surface. Based on Figure 3A, H2O2 enhanced the intracellular level of ROS and this was accompanied with significant depression of CAT and SOD activities as shown in Figure 3B. However, pre-treatments of the cells with a single dose of each of the drugs (JC1-JC6) decreased the intracellular level of ROS and restored the majority of cellular CAT and SOD activities, as it is evident from Figure 3A and 3B, respectively. SOD catalyzes the dismutation of superoxide anion into oxygen and H2O2 and CAT catalyzes the conversion of H2O2 to H2O and molecular oxygen. Repression of SOD and CAT activities by H2O2 (Fig. 3B) was accompanied with enhanced intracellular level of lipofuscin as indicated in Figure 5. Therefore, our data clearly indicated that there is a link between ROS content, antioxidant enzyme activities as well as the lipofuscin content (Fig. 6). As mentioned before, lysosome is engaged in lipofuscin ogenesis. Accumulation of age pigments within the lysosome might interfere with the activities of lysosomal enzymes [36]. For instance, our results indicated that the acid phosphatase activity upon exposure to H2O2 was significantly declined. However, pre-treatment of the cells with JC3, JC4, JC5 and JC6, followed by exposure to H2O2, restored the acid phosphatase activity (Fig. 4B).
Fig. 6.
Effects of JC1-JC6 on the cell viability, ROS, CAT and SOD activity and lipofuscin formation. The SK-N-MC cells were incubated without or with JC1-JC6 (20 μM) for 3 h before addition of 300 μM H2O2. Catechin was used as the positive control. Results are means ± SD of three measurements (P<0.05).
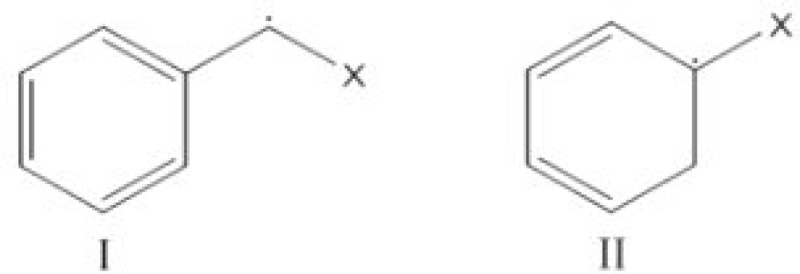
Our cumulative data clearly documented the yakuchinone B derivatives as free radical scavengers with the following order of activity: JC4> JC6> JC5≥ JC3> JC2> JC1. Previous studies have shown that the phenolic hydroxyl group on the vanillin ring (ring A, Table 1) is required for the antioxidant activity of each of the derivatives [3, 37, 38]. The stronger free radical scavenging activity of JC4, JC5 and JC6, as compared to JC1, JC2 and JC3, might be attributed to two structural features: the presence of another aromatic ring (ring B, Table 1) and the presence of at least one methylene group between the carbonyl group and the aromatic ring B. These structural elements would add up to higher free radical scavenging activity due to the formation of the following intermediates (I and II) from JC4, JC5 and JC6 as additional routes for neutralization of free radicals such as •OH. The other analogues (JC1, JC2 and JC3) cannot form either of these intermediates and thus, they will be capable of neutralizing the free radicals only through ring A. This is in contrast to compounds JC4, JC5 and JC6 which would neutralize the free radicals through both rings A and B. Therefore, they have higher antioxidant activities [39].
In summary, the results of this investigation, besides the reconfirming the free-radical hypothesis of aging, clearly indicate that the molecular complications of aging such as intra-molecular accumulation of lipofuscin pigments could be attenuated by balancing the intracellular levels of free radicals to their normal physiological levels. This might be achieved through delicate administration of appropriate exogenous antioxidants.
ACKNOWLEDGMENTS
The authors appreciate the financial support of this investigation by the Research Council of University of Tehran (Iran).
References
- 1.Balcombe, N.R , Sinclair, A. Ageing: definitions, mechanisms and the magnitude of the problem. Best. Pract. Res. Clin. Gastroenterol. 2001;15:835–849. doi: 10.1053/bega.2001.0244. [DOI] [PubMed] [Google Scholar]
- 2.Lenaz, G. Role of mitochondria in oxidative stress and ageing. Biochim. Biophys. Acta. 1998;1366:53–67. doi: 10.1016/s0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 3.Bayati, S , Yazdanparast, R , Majd, S.S , Oh, S Protective effects of 1,3-diaryl-2-propen-1-one derivatives against H2O2-induced damage in SK-N-MC cells. J. Appl. Toxicol. 2011;31:545–553. doi: 10.1002/jat.1594. [DOI] [PubMed] [Google Scholar]
- 4.Brunk, U.T , Terman, A Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 5.Terman, A , Gustafsson, B , Brunk, U.T Autophagy, organelles and ageing. J. Pathol. 2007;211:134–143. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- 6.Kurz, T , Terman, A , Gustafsson, B , Brunk U.T Lysosomes and oxidative stress in aging and apoptosis. Biochim. Biophys. Acta. 2008;1780:1291–1303. doi: 10.1016/j.bbagen.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Jung, T , Bader, N , Grune, T Lipofuscin: formation, distribution, and metabolic consequences. Ann. N.Y. Acad. Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- 8.Szweda, P.A , Camouse, M , Lundberg, K.C , Oberley, T.D , Szweda, L.I Aging, lipofuscin formation, and free radical-mediated inhibition of cellular proteolytic systems. Aging Res. Rev. 2003;2:383–405. doi: 10.1016/s1568-1637(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 9.Yin, D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic. Biol. Med. 1996;21:871–888. doi: 10.1016/0891-5849(96)00175-x. [DOI] [PubMed] [Google Scholar]
- 10.Ardestani, A , Yazdanparast, R Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104:21–29. [Google Scholar]
- 11.Rice-Evans, C.A , Miller, N.J , Paganga, G Antioxidants properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 12.Nijveldt, R.J , van Nood, E , van Hoorn, D.E , Boelens, P.G , van Norren, K , van Leeuwen, P.A Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 13.Silva, B.A , Ferreres, F , Malva, J.O , Dias, A.C.P Phytochemical and antioxidant characterization of Hypericumperforatum alcoholic extracts. Food Chem. 2005;90:157–167. [Google Scholar]
- 14.Seyoum, A , Asres, K , El-Fiky, F.K Structure-radical scavenging activity relationships of flavonoids. Phytochemistry . 2006;67:2058–2070. doi: 10.1016/j.phytochem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki, R , Hatano, H , Aiyama, R , Matsuzaki, T , Hashimoto, S , Yokokura, T Diaryl-heptanoids suppress expression of leukocyte adhesion molecules on human vascular endothelial cells. Eur. J. Pharmacol. 2000;404:375–385. doi: 10.1016/s0014-2999(00)00620-8. [DOI] [PubMed] [Google Scholar]
- 16.Srimal, R.C , Dhawan, B.N Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973;25:447–452. doi: 10.1111/j.2042-7158.1973.tb09131.x. [DOI] [PubMed] [Google Scholar]
- 17.Samaha, H.S , Kelloff, G.J , Steele, V , Rao, C.V , Reddy, B.S Modulation of apoptosis by sulindae, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylnexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997;57:1301–1305. [PubMed] [Google Scholar]
- 18.Ninomiya, Y , Shimma, N , Ishitsuka, H Change of processing and nucleocytoplasmic transport of mRNA in HSV-1-infected cells. Antiviral. Res. 1990;13:61–74. doi: 10.1016/0168-1702(89)90087-7. [DOI] [PubMed] [Google Scholar]
- 19.Oh, S , Jang, S , Kim, D , Han, I.O , Jung, J.C Synthesis and evaluation of biological properties of yakuchinone B and its analogues. Arch. Pharm. Res. 2006;29:469–475. doi: 10.1007/BF02969418. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Cohen, J.J. Apoptosis. Immunol. Today. 1993;14:126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 22.LeBel, C.P , Ischiropoulos, H , Bondy, S.C Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 23.Aebi, H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Lowry, O.H , Rosebrough, N.J , Farr, A.L , Randall, R.J Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Kakkar, P , Das, B , Viswanathan, P.N A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 26.Wei, T , Zhao, X , Hou, J , Ogata, K , Sakaue, T , Mori, A , Xin, W The antioxidant ESeroS-GS inhibits NO production and prevents oxidative stress in astrocytes. Biochem. Pharmacol. 2003;66:83–91. doi: 10.1016/s0006-2952(03)00231-4. [DOI] [PubMed] [Google Scholar]
- 27.Draper, H.H , Hadley, M Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 28.Ardestani, A , Yazdanparast, R , Nejad, A.S 2-Deoxy-D-ribose-induced oxidative stress causes apoptosis in human monocytic cells: prevention by pyridoxal-5'-phosphate. Toxicol. invitro. 2008;22:968–979. doi: 10.1016/j.tiv.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Shamsi, F.A , Boulton, M Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Invest. Ophthalmol. Vis. Sci. 2001;42:3041–3046. [PubMed] [Google Scholar]
- 30.Emig, S , Schmalz, D , Shakibaei, M , Buchner, K The nuclear pore complex protein p62 is one of several sialic acid-containing proteins of the nuclear envelope. J. Biol. Chem. 1995;270:13787–13793. doi: 10.1074/jbc.270.23.13787. [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki, Y , Park, M.K , Mori, T , Kawashima, S The difference in autofluorescence features of lipofuscin between brain and adrenal. Zoolog. Sci. 1995;12:283–288. doi: 10.2108/zsj.12.283. [DOI] [PubMed] [Google Scholar]
- 32.Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 33.Cai, Z.Y Pathway and mechanism of oxidative stress in Alzheimer's disease. J. Med. Coll. PLA. 2007;22:320–324. [Google Scholar]
- 34.Riga, D , Riga, S , Halalau, F , Schneider, F Brain lipopigment accumulation in normal and pathological aging. Ann. Y. Acad. Sci. 2006;1067:158–163. doi: 10.1196/annals.1354.019. [DOI] [PubMed] [Google Scholar]
- 35.Castro, M , Encarnação, P , Tully, O The effect of dietary antioxidants on lipofuscin accumulation in the crustacean brain. J. Exp. Mar. Bio. Eco. 2002;269:53–64. [Google Scholar]
- 36.Wassell, J , Davies, S , Bardsley, W , Boulton, M Thephotoreactivity of the retinal age pigment lipofuscin. J. Biol. Chem. 1999;274:23828–23832. doi: 10.1074/jbc.274.34.23828. [DOI] [PubMed] [Google Scholar]
- 37.Bayati, S , Yazdanparast, R Attenuation of lipid and protein oxidation by chalcone derivatives in neuroblastoma cells against H2O2-induced oxidative stress. Pharmacologyonline . 2011;2:479–489. [Google Scholar]
- 38.Patil, C.B , Mahajan, S.K , Katti, S.A Chalcone: A Versatile Molecule. J. Pharm. Sci. Res. 2009;1:11–22. [Google Scholar]
- 39.Huang, M , Wang, Z , Hao, L , Zhang, W Theoretical investigation on the mechanism and kinetics of OH radical with ethylbenzene. Int. J. Quantum. Chem. 2010;111:3125–3134. [Google Scholar]