Abstract
Background: Adult stem cells (ASC) are undifferentiated cells found throughout the body. These cells are promising tools for cell replacement therapy in neurodegenerative disease. Adipose tissue is the most abundant and accessible source of ASC. This study was conducted to evaluate effect of selegiline on differentiation of adipose-derived stem cells (ADSC) into functional neuron-like cells (NLC), and also level of the neurotrophin expression in differentiated cells. Methods: ADSC were transdifferentiated into NLC using selegiline where CD90, CD49d, CD31, CD106 and CD45 were used as markers for ADSC identification. Lipogenic and osteogenic differentiation of ADSC were used to characterize the ADSC. ADSC were treated with selegiline at different concentrations (from 10-6 to 10-11 mM) and time points (3, 6, 12, 24 and 48 h). Percentage of viable cells, nestin and neurofilament 68 (NF-68) immunoreactive cells were used as markers for differentiation. The optimal dose for neurotrophin expressions in differentiating cells was evaluated using reverse transcriptase-PCR. NLC function was evaluated by loading and unloading with FM1-43 dye. Results: ADSC were immunoreactive to CD90 (95.67 ± 2.26), CD49d (71.52 ± 6.64) and CD31 (0.6 ± 0.86), but no immunoreactivity was detected for CD106 and CD45. The results of neural differentiation showed the highest percentage of nestin and NF-68 positive cells at 10-9 mM concentration of selegiline (exposed for 24 h). The differentiated cells expressed synapsin and neurotrophin genes except brain-derived neurotrophic factor. Conclusion: ADSC can be an alternative source in cell-based therapy for neurodegenerative diseases using selegiline to induce ADSC differentiation to neuronal lineage.
Key Words: Selegiline, Neurotrophin, Transdifferentiation
Introduction
Adult stem cells (ASC) are multi-potent undifferentiated cells that found in some tissues of the adult body. ASC are capable of self-renewal and differentiate into specialized cells. These cells are very promising tools for replacement cell therapy in neurodegenerative disorders. Adipose tissue is the most abundant and accessible source of adult stem cells. Because of minimal invasive collection procedure, ASC ability for differentiation into different cell lineages, as well as their safe autologous transplantation are the factors that could be considered them as an alternative source for bone marrow cells [1]. Adipose tissue is composed of mature adipocytes and heterogenous cell population including stromal vascular fraction (SVF) [2]. The SVF is consisted of various cell types, such as immune cells, fibroblasts, pericytes, endothelial cells, and adipogenic progenitor stromal cells, which attach together by collagen fibers [3]. In culture, adipose-derived stem cells (ADSC) express cell surface markers that are similar to those expressed by mesenchymal stem cells, including CD105, SH3, CD90, and CD44; however, they do not express the hematopoietic marker CD45 and the endothelial marker CD31 [4, 5].
Epigenesis has been reported to be involved in the cellular differentiation during development, which is controlled by different factors such as growth and environmental factors [6]. These factors are involved directly or indirectly in the transcription and expression of genes by genetic reprogramming process [7]. For example, ADSC could be induced into neural and glial phenotypes by transdifferentiation mechanism [8]. On the other hand, selegiline, which is a monoamine oxidase inhibitor [9], has been reported to neuroprotect the neurons and induce bone marrow stromal cells (BMSC) into neuron-like cells (NLC) [10]. Currently, it is used to treat Parkinson's disease [11] and induce neuronal differentiation in embryonic stem cells associated with neurotrophic factor expression [12].
Therefore, the main purpose of this study was to determine the expression profile of neurotrophins in differentiating ADSC using selegiline during the induction process.
MATERIALS AND METHODS
Adipose-derived stem cell isolation and culturing. In this study, all surgical procedures were performed in according to the guidelines of Ethical Committee of Tarbiat Modares University, Tehran. In 1964, the isolation of a population of progenitor cells from adipose tissue was first described in rodents by Rodbell [13]. The animals were anesthetized under sterile condition, and the adipose tissue was obtained from the pararenal region. The tissue was processed according to the established method with minor modifications [2]. Briefly, the samples were washed several times in PBS containing penicillin and streptomycin. Then, the tissue was minced and treated with equal volumes of 0.075% collagenase type1 (Sigma, Belgium) with continuous agitation at 37°C for 1 h. Enzyme activity was neutralized with DMEM (Sigma, Belgium) containing 10% fetal bovine serum (Gibco, UK) and then centrifuged at 1200 ×g for 10 min to obtain a high-density cell pellet. The supernatant was discarded and SVF pellet was mixed with 2,000 µl DMEM using pipetting aid device. The suspended cells were passed through 100 µm nylon filter mesh (Falcon Company, USA) and incubated at 37°C in 5% CO2 in DMEM containing 10% fetal bovine serum. The medium was replaced every 2 days.
Osteogenic differentiation. For differentiation of ADSC (at the fourth passage) toward osteogenic phenotype, the ADSC culture medium was changed to osteogenic maintenance medium containing 10 mM β-glycerophosphate, 0.2 mM ascorbic acid and 10-7 M dexamethasone for 21 days. The culture cells were fed every three days throughout the study. For confirming the osteogenic differentiation, we used Alizarin Red S staining. Briefly, osteogenic medium was removed and washed three times in PBS. The cells were fixed in 70% ethanol at 4oC for 1 h. After fixation, the cells were washed in deionized water and allowed to air dry. The fixed cells were stained with 2% Alizarin Red S (pH 7.2, Sigma, Belgium) at 37oC for 1 h, washed in deionized water and photographed with inverted microscope (Olympus, Japan).
Adipogenic differentiation. The ADSC at fourth passage were incubated with adipogenic maintenance medium (50 μg/ml indomethacin, 50 μg/ml ascorbic acid and 100 nM dexamethasone, all purchased from Sigma, Belgium) for 21 days. The medium was changed every three days throughout the study [14]. The adipogenic differentiation was confirmed using of Oil Red O (Sigma, Belgium). Briefly, adipogenic medium was removed and washed three times in PBS. The cells were fixed in 10% formalin for 30-60 minutes at room temperature, washed in distillated water and treated with 2 mL isopropanol (60%) for 5 minutes. Then, they were removed, and stained with Oil Red O (2 mL to each well) at room temperature for 5 minutes. Finally, the cells were washed in tap water and photographed with an inverted microscope (Olympus, Japan).
Neuronal induction. Subconfluent cultures of rat ADSC at fourth passage were maintained in serum-free induction medium (DMEM containing selegiline). To determine optimal selegiline concentration and time of exposure, ADSC were treated with selegiline at 10-6, 10-7, 10-8, 10-9, 10-10, 10-11 and 10-12 mM concentrations at different time points (3, 6, 12, 24 and 48 h) and evaluated using the percentages of viable cells, nestin and neurofilament 68 (NF-68) immunoreactive cells. The viability was evaluated with trypan blue exclusion test and the percentage of viable cells was estimated, while the percentage of the cells with neural phenotype was evaluated by cresyl violet stain. To determine the time required to obtain the neural phenotype, ADSC was treated with optimal selegiline concentration and accordingly cultured medium was changed until the neural phenotype was noticed.
Immunofluoresence staining . The adherent ADSC (at fourth passage), cultured on a gelatin-coated glass coverslip, differentiated NLC using selegiline (24 h). The medium was discarded and the cells were washed three times in PBS, fixed with fresh 4% paraformaldehyde in PBS (pH 7.2) at room temperature for 60 min. For staining the intracellular antigens, the cells were permeabilized with 0.3% Triton X-100 (Sigma, Belgium) for 30 min. For blocking non-specific binding, the cells were rinsed with 10% BSA in PBS for 30 min, washed three times in PBS and incubated at 4°C overnight with the following primary antibodies: mouse anti-CD49d, monoclonal antibody (1:300), mouse anti-CD106, monoclonal antibody (1:300), mouse anti-CD31, monoclonal antibody (1:200), mouse anti-CD45, poly-clonal antibody (1:300), mouse anti-CD90, monoclonal antibody (1:300), mouse anti-nestin monoclonal antibody (1:100), mouse anti-NF-68 monoclonal antibody (1:200), mouse anti-NeuN monoclonal antibody (1:150) and mouse anti-synapsin monoclonal antibody (1:200), all from Millipore, Germany. Then, the primary antibodies were washed three times in PBS at room temperature and incubated with secondary antibody (rabbit anti-mouse IgG with conjugated FITC, 1:100, Millipore, Germany) for two h. Afterward, the cells were washed twice in PBS, counterstained with ethidium bromide (10 μg/mL in PBS) except NeuN (was not stained with ethidium bromide) for 15 seconds to demonstrate the nuclei and washed in PBS and examined with an inverted microscope (Olympus, Japan). Nuclear counting was performed for the untreated and induced ADSC and the percentage of the immunoreactive cells was calculated. The primary antibodies were omitted from negative controls.
Calculation of neuron-like cell percentage. Neural-like cells were evaluated based on presence of Nissl bodies, expression of nestin and NF-68. All experiments were repeated five times to obtain the NLC percentage. The mean and standard errors of the mean were carried out using SPSS software release 15.
Reverse transcriptase-polymerase chain reaction ( RT-PCR ). The expression of neurotrophins (nerve growth factor-β, brain-derived neurotrophic factor [BDNF], glial cell-derived neurotrophic factor, neurotrophin-3, neurotrophin-4, and ciliary neurotrophic factor) was evaluated by RT-PCR in NLC using 10-9 mM of selegiline incubated for 24 h. The spinal cords of newborn rats were used as positive controls. The primers used in the study have been presented in Table 1, The primers were designed using Generunner software (3.05) and prepared from the disributor (Genfanavaran Co., Iran). PurelinkTM RNA mini kit (Invitrogen, Germany) was used for extracting the total RNA [15] and the extracted RNA treated with DNaseI (Invitrogen, Germany) was checked using a spectroscope and agarose gel electrophoresis. The extracted RNA (1,000 ng) was used for synthesizing cDNA (Revert aid™: Fermentas, Germany) and the
Table 1.
Primers sequences, size of the fragment amplified and GenBank accession numbers of BDNF, GDNF, NGF, NT-3, NT-4, CNTF and GAPDH genes.
| Gene | Accession no. | Sense 5→ 3 | Antisense 5→ 3 | Size (bp) |
|---|---|---|---|---|
| BDNF | NW_001084813 | TGTATCCGACCCTCTCTG | CCTGGTGGAACTTTACG | 165 |
| GDNF | NM_019139 | CTGACCAGTGACTCCAATATGC | GCCTCTGCGACCTTTCCC | 192 |
| NGF | XM_227525 | ACCTCTTCGGACACTCTG | GTGGCTGTGGTCTTATCTC | 164 |
| NT-3 | NM_031073 | CTTCTGCCACGATCTTAC | AACATCTACCATCTGCTTG | 197 |
| NT-4 | NC_005100.2 | CTAATGTGTGACTCTGCTAAC | GATACGGTGCTCAGGATAG | 180 |
| CNTF | NC_005100 | TCGTTGGAGTGAGATGAC | AGTATGTATTGCCTGATGG | 149 |
| GAPDH | NM_002046 | CAAGGTCATCCATGACAACTTTG | GTCCACCACCCTGTTGCTGTAG | 496 |
BDNF, brain-derived neurotrophic factor; GDNF, glial cell-derived neurotrophic factor; NGF, nerve growth factor; NT-3, neurotrophin 3; NT-4, neurotrophin 4; CNTF, ciliary neurotrophic factor; and GAPDH, glyceraldehyde-3-phosphate dehydrogenase (internal control).
cDNA (500 ng) was subjected to PCR Master Mix 2X (Fermentas, Germany) using a thermocycler (Bio-Rad, USA) for 35 cycles. The negative control was without cDNA. The product size of PCR was checked on 2% agarose gel electrophoresis and the ladder used in this study was 100 bp (Fermentas, Germany).
Functionality assay. In this study, the NLC were stained by stimulating the exocytosis with a high [K+] solution in the presence of 10 mM FM1-43 dye for 2 min. Then, NLC was incubated in a low [K+] solution in the presence of 10 mM FM1-43 for an additional 15 min. Afterward, FM1-43 dye was removed by washing. For detection of vesicles, several images were taken (one photograph each minute) from the same field using an inverted fluorescence microscope (Olympus IX71, Olympus, Japan) for ten minutes. The standard low [K+] solution was consisted of the following components: 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, 10 mL Hepes, and 8 mM CaCl2 mM. The high [K+] solution had the same composition and extra 100 mM KCl and 45 mM NaCl [16].
Statistics . Statistical analysis was performed using SPSS software release 15. All data are presented as means ± standard error of the means (SEM). To compare multiple means in groups, one-way ANOVA using Tukey's post hoc comparison was used. Values of P<0.05 were considered statistically significant.
RESULTS
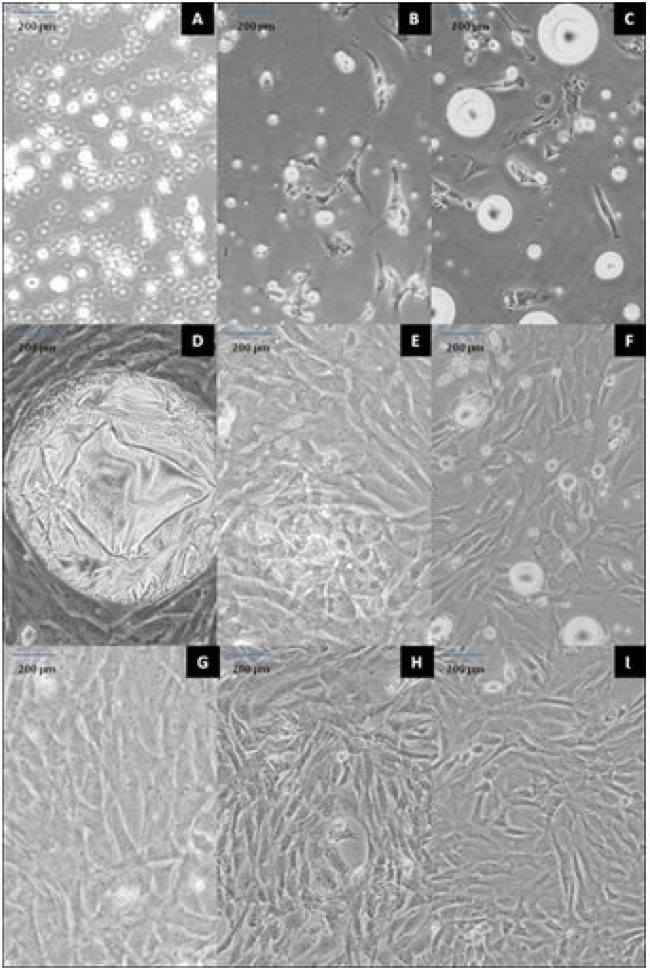
Adipose-derived stem cell culture. The cultured ADSC showed that ADSC adherent to plastic flask is similar to mesenchymal stem cells which were characterized by a rapid proliferation. At earlier hours, the cells were floating and theirs nucleus was not visible (Fig.1A).
Fig. 1.
ADSC preparation. Floating cells at 12 h (A); attached fibroblast-like cells at (B) 24 and (C) 48 h; cultured cells with large fat droplet at day 5 (D); first (E), second (G) and third (I) passages at days 7, 11 and 13, respectively; subculture cells at days 10 (F) and 12 (H). Scale bar = 200 µm.
After 24 h, the floated cells were attached to culture dish to form fibroblast-like colonies. During 24 to 48 h, ADSC cells became spindle in shape (fibroblast like) and were loaded with several lipid granules within the cells (Fig. 1B and 1C). The lipid granules were fused together and formed large droplets then released into the cells culture medium (Fig. 1D). The first passage was made in 7 days when the cells reached 80% confluency (Fig. 1E). After the first passage, the cells exhibited extensive proliferative capacity. The second passage was performed in the following 4 day (Fig. 1F and Fig. 1G). After the four passages were performed in 13 days, the cells were used for differentiation experiments (Fig. 1H and Fig. 1I). In this study, we found that ADSC were very resistant and spontaneous differentiations were rarely happened.
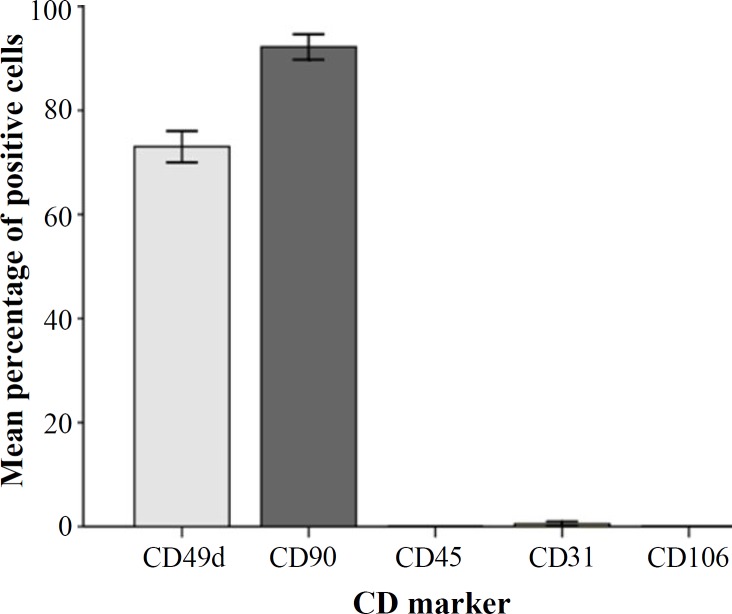
Immunofluoresence reactivity of CD markers. The percentages of the immunoreactive cells have been presented in Figure 2. The results were as follows:
Fig. 2.
The mean percentages of immunoreactive cells to different CD markers.
CD49d = 71.52 ± 6.64; CD90 = 95.67 ± 2.26 and CD31 = 0.60 ± 0.86. The results also showed that ADSC were negative for immunofluorescence staining with CD106 (specific BMSC CD marker) and CD45 (specific hematopoietic cells CD marker) (Fig. 3).
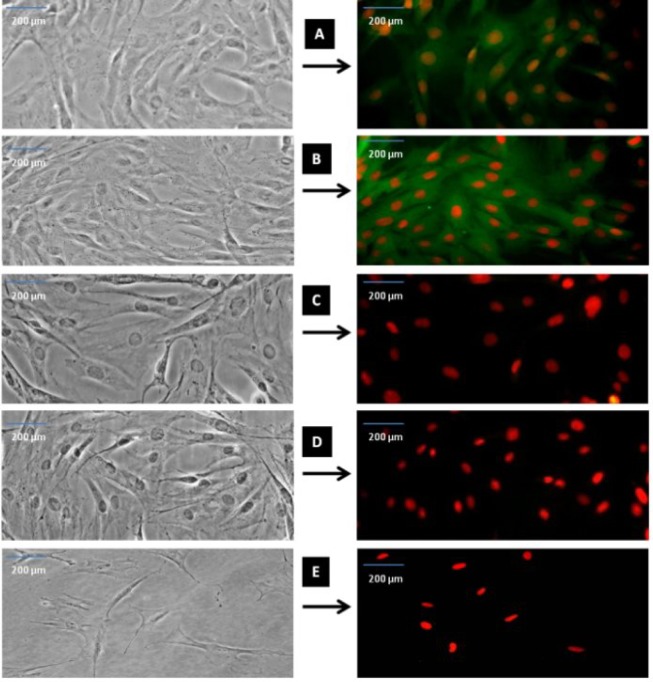
Fig. 3.
Immunostaining of CD marker immunoreactivity after fourth passage. As representative photomicrographs shows, CD49d is specific for ADSC (A), CD90 is a specific mesenchymal stem cells (B), CD45 is a specific for hematopoietic cells (C), CD31 for endothelial cells (D) and CD106 for BMSC (E). The left sides of the fluorescent photomicrographs represent the phase contrast images from the same field of the immunofluorescence images. The cells were immunostained with relevant primary antibodies and labeled with FITC-conjugated secondary antibody (green color shows positive cells) and the red colors are ethidium bromides for counterstaining of the nuclei. Scale bar = 200 µm.
Osteogenic and adipogenic differentiation. The in vitro differentiation of ADSC into osteogenic and adipogenic phenotypes using induction cocktail medium has been shown in Figure 4. The results showed that osteoblast-like cells were capable of mineralizing extracellular matrix and staining with Alizarin Red S dye. Also, the adipogenic differentiation had cell morphology with lipid accumulation in the form of small vacuoles or droplets, which were stained with Oil Red O. During the differentiation process, the cells had various sizes due to the different amounts of lipid.
Fig. 4.
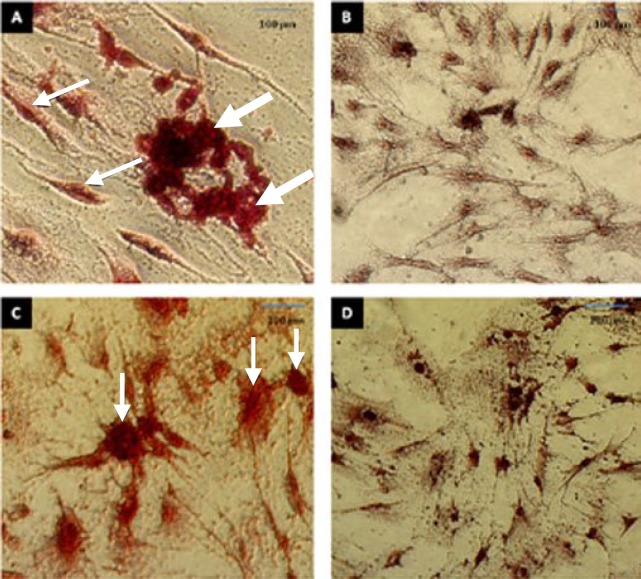
The in vitro osteogenesis and adipogenic differentiation. (A) ADSC after incubation for 21 days in osteogenic differentiation medium. The cells were visualized with Alizarin Red S staining. The thin arrows indicate osteoblasts and thick arrows indicate the deposition of a mineralized extracellular matrix. (B) Alizarin Red S staining of ADSC before osteogenic differentiation; (C) ADSC after incubation for 21 days in adipogenic differentiation medium. The cells were visualized with Oil Red O staining. The arrows indicate adipocytes and accumulation of fat droplets; (D) Oil Red O staining of ADSC before adipogenic differentiation. Scale bar = 100 µm.
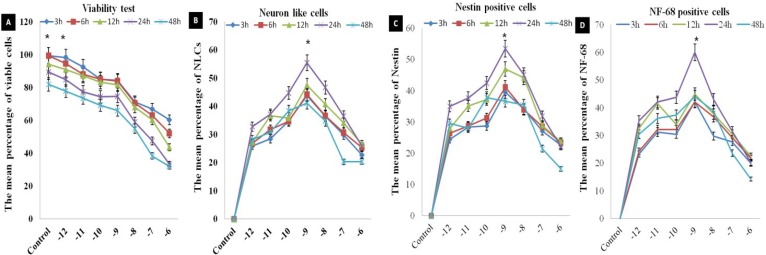
Neural induction. The cells were cultured in low concentrations of selegiline for a short time, resulting in a significant increase in the cell viability (Fig. 5A). The immunostaining results showed that there were significant increases in the percent of NLC nestin and NF-68 immunoreactive cells (incubated at 10-9 mM concentration for 24 h) as compared with other concentrations (Fig. 5B, 5C and 5D, 6A and 6B and Table 2). In the time course study of neural phenotype induction, incubation with selegiline resulted in retraction of cell body and processes and these changes
Fig. 5.
The viability study in dose-response and time course evaluation of selegiline on the neuronal differentiation of the ADSC. (A) the viability of the cells exposed to various doses of selegiline and various time points and the percentage of viable cells is determined; (B) the percentage of NLC using cresyl violet staining; (C) the percentages of nestin and (D) NF-68 immunoreactive cells. These barographs show that there is a significant difference in 10-9 mM (incubated at 24 h) compared to the other time points and concentrations of selegiline (*P<0.05). The data presented as the mean ± SEM of five repeated experiments.
Fig. 6.
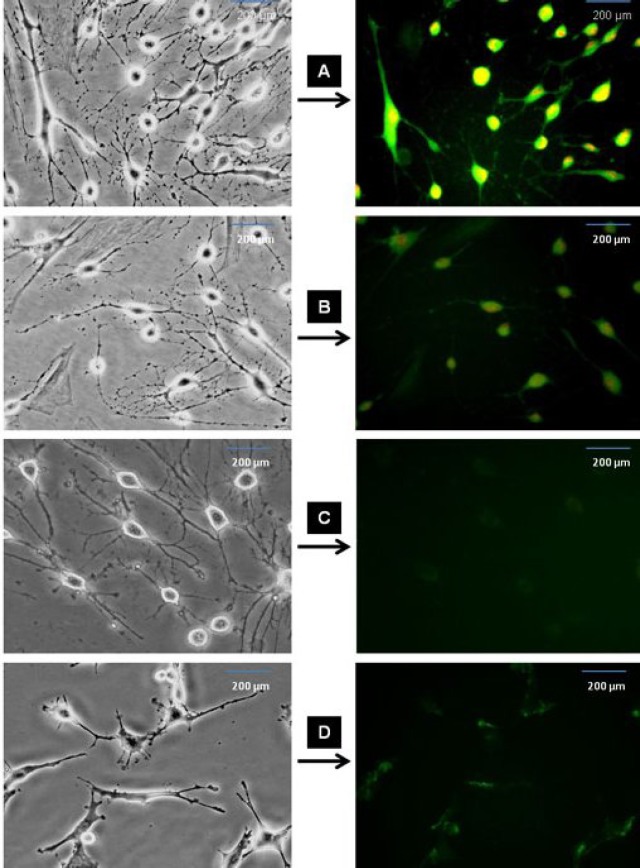
Immunofluorescence staining of differentiated ADSC into NLC using selegiline at 10-9 mM, incubated for 24 h. The cells were immunostained (left side) for the primary antibodies against nestin, NF-68, NeuN and synapsin (A, B, C and D, respectively), labeled with FITC-conjugated secondary antibody and counterstained with ethidium bromide. Right side images represent the phase contrast of the same field in the left side panel. Scale bar = 200 µm.
Table 2.
The mean percentages of the NLC (stained with cresyl violet), nestin and NF-68 immunoreactive cells of differentiated ADSC using 10-9 mM concentration of selegiline at different time points.
| Time (h) | NLC | Nestin | NF-68 |
|---|---|---|---|
| 3 | 43.84 ± 2.52 | 39.48 ± 1.60 | 42.22 ± 2.61 |
| 6 | 44.13 ± 3.04 | 41.16 ± 3.22 | 42.11 ± 2.54 |
| 12 | 47.50 ± 3.14 | 46.94 ± 2.72 | 44.93 ± 3.44 |
| 24 | 55.49 ± 2.75 | 53.45 ± 2.22 | 60.02 ± 2.44 |
| 48 | 41.00 ± 1.90 | 36.61 ± 1.07 | 44.07 ± 2.42 |
were noticed during 90 min (Fig. 7). Also, Nissl bodies were demonstrated with cresyl violet and the result showed that there was an increase in the percentage of NLC incubated with selegiline (10-9 mM) for 24 h (Fig. 8). NLC were immunostained NeuN and synapsin and the result showed that the differentiated cells were able to express synapsin, but not NeuN (Fig. 6C and 6D).
Fig. 8.
The Nissl body staining using cresyl violet. Dark blue particles in the cytoplasm show Nissl bodies and the arrows show synaptic contact regions. Scale bar = 100 µm.
Fig. 7.
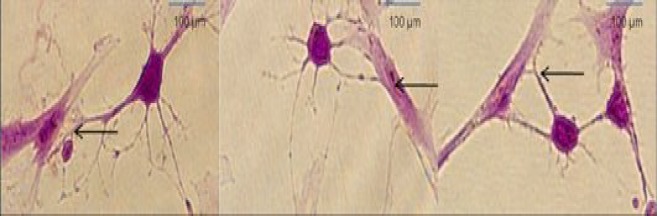
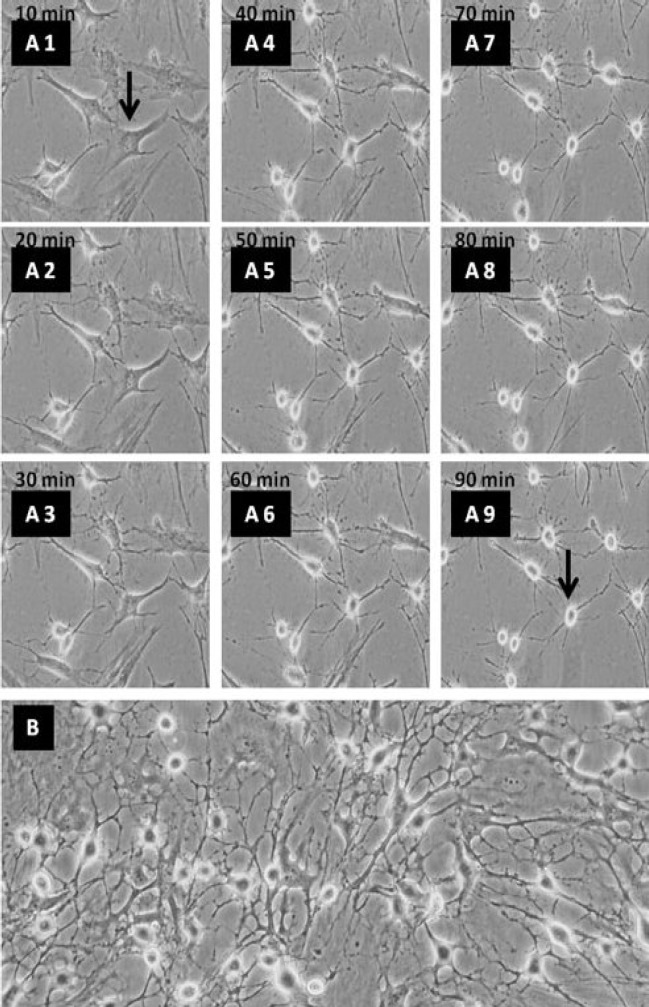
Phase contrast images of the NLC differentiation of ADSC using selegiline. (A1-A9) time course of ADSC transdifferentiated into NLC using selegiline, incubated for 90 min. Retraction of cell body and process formation are evident at the higher time points; (B) ADSC treated with selegiline (10-9 mM, incubated for 24 h), resulted in NLC. The arrows indicate single cell prior to differentiation and the same cell after differentiation at 90 min (magnification 20×).
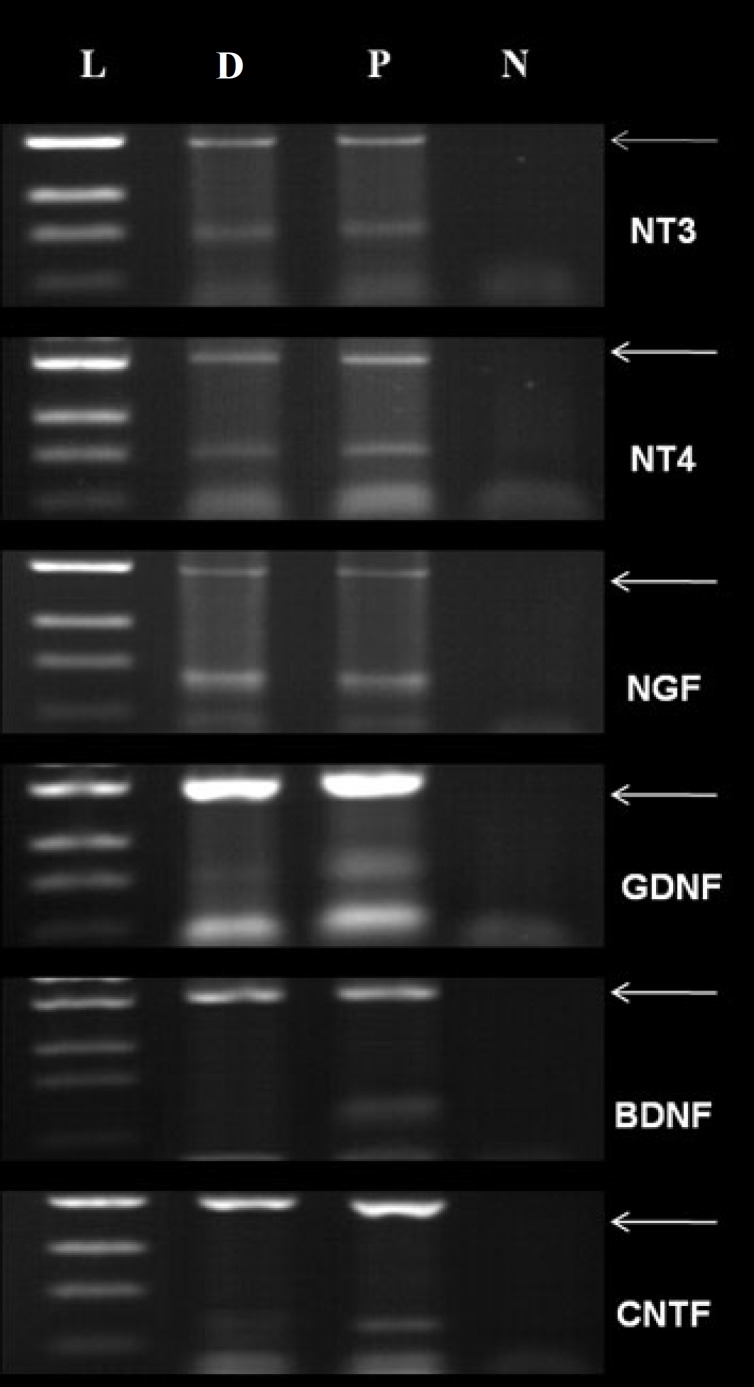
Neurotrophins expression . NLC and the newborn spinal cords (positive control) were evaluated for the expressions of neurotrophins with primers specific for (nerve growth factor-β, BDNF, glial cell-derived neurotrophic factor, neurotrophin-3, neurotrophin-4, and ciliary neurotrophic factor) using RT-PCR. The results of the neurotrophin expression and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, internal control) using 35 cycles have been presented in Figure 9. NLC expressed all neurotrophins genes except BDNF.
Fig. 9.
The expression of neurotrophins and GAPDH genes (internal control). L, D, P and N indicate ladder, ADSC differentiated cells into NLC, the newborn spinal cord (positive control) and negative control (without cDNA), respectively. The arrows indicate GAPDH.
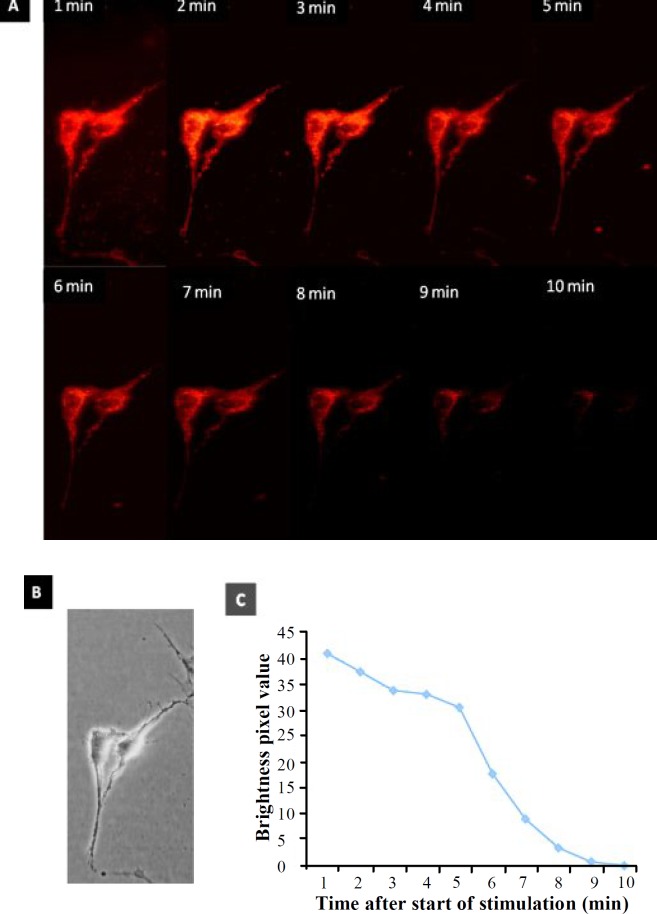
Functionality assay. NLC showed synaptic terminals, which were labeled with 10 µm FM1-43 dye using stimulating solution for 120 seconds (Fig. 10). The labeled cells showed several puncta, which were noticed on the surface of perikarya and neurites. The images from FM1-43 destaining were acquired every 60 second for 10 min. FM1-43 K+ induced destaining in NLC showed a reduction in synaptic vesicles (1- 10 min) (Fig. 11).
Fig. 10.
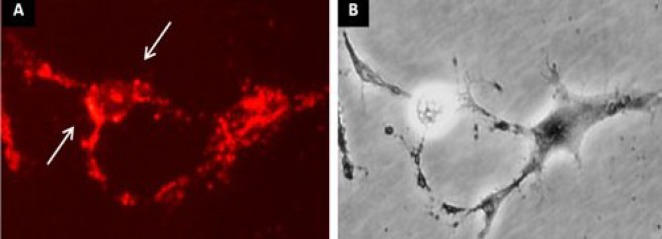
FM 1-43 staining of ADSC differentiated into NLC. (A) The ADSC incubated with selegiline (10-9 mM) at 24 h and transdifferentiated into NLC. The arrows represent the region of fluorescent sites (synaptic vesicles) after 120 s exposure to FM1-43; (B) the phase contrast image of the same field. Scale bar = 100 µm.
Fig. 11.
Staining and destaining of ADSC differentiated into NLC labeled with FM1-43. (A) The fluorescence images of the FM 1-43 staining (synaptic vesicle) using 120 s exposure to FM1-43 in 10 minute after the start of the stimulation; (B) the phase contrast image of the same field; (C) total number of the pixels at the time course (1-10 min) used in the study, where the FM1-43 fluorochrome stained NLC then the cells were destained. There was a reduction in the synaptic vesicle recycling following a long time delay (magnifications 40×).
Discussion
The adult CNS was documented to be incapable of repair following neuronal loss and injury [17]. The interest in the cell replacement strategies for the treatment of neurodegenerative disorders dates back to the late 1970s [18]. For the time being, although our best stem cell source is not completely defined, somatic stem cells have been considered as an accessible source [19]. These cells are populations with fibroblast-like morphology, self-renewal capacity, long-term viability, and multi-lineage differentiation potential [20]. BMSC are good candidates for stem cell therapy, but the clinical use was limited due to the following reasons: 1) highly invasive and painful collection procedure for patients [21] and 2) low yield, short life span and decrease in differentiation potential increase with patients’ age [22]. In comparison to BMSC, ADSC collection can be obtained in relatively large quantities with minimal risk for patients [22].
In this study, we found that the growth of ADSC is higher than BMSC, which is in agreement with other investigation [23]. Moreover, we could demonstrate that ADSC are able to differentiate into both mesenchymal (osteogenic and lipogenic cells) and ectodermal lineages (NLC). Although the osteogenic and lipogenic differentiation was consistent with the finding of Tapp et al. [24], the expression of cell-surface antigens were in agreement with other investigation [1]. The property of ADSC was confirmed by CD49d (ADSC specific marker) and CD90 (specific mesenchymal stem cell marker) expression [25]; however, they did not express CD45 (specific hematopoietic stem cell marker) and CD106 (specific BMSC marker) [26].
Selegiline was synthesized in Hungary in 1960s [27], and used for treatment of Parkinson's disease [11]. Esmaeili et al. [12] have reported that selegiline could induce embryonic stem cell differentiation into NLC, and neurotrophins expression. Ghorbanian et al. [28] has also shown that selegiline was an efficient and a potent inducer for BMSC into neuronal phenotype. Our results demonstrate that selegiline is an inducer for expression of nestin, NF-68 and neurotrophins (except BDNF). Nagatsu and Sawada [29] have reported that selegiline is able to increase the expression of neurotrophins.
The type of neural inducer is one of the important factors in cellular differentiation. For example, retinoic acid alone, retinoic acid combined with BDNF, β- mercaptoethanol, DMSO and others were used as inducers of neuronal differentiation [30, 31]. In this investigation, we used selegiline. The results of the study indicated that this agent in comparison with other inducers could increase the viability and the percentage of nestin immunoreactivity and also it had low toxicity, which was consistent with the previous results [12, 32]. In dose-response evaluation, our results showed in 10-9 mM concentration of selegiline at 24-h incubation, the percent of nestin and NF-68 immunoreactive cells were significantly increased when it was compared with other concentrations and times. Also, FM1-43 demonstrated that active synaptic region for produced neurons. Finally, the induction of neuronal phenotype in ADSC using selegiline suggests the possibility of the treatment of neurodegenerative diseases by selegiline (as neuroprotective for neurons) and inducer for ADSC.
ACKNOWLEDGMENTS
We are grateful to Mr. Ali Noorizadeh and Mehri Fayazi for their excellent technical assistance. This work was supported by a research grant (no. 86-N-105) from the Shefa Neuroscience Center, Khatam Al-Anbia Hospital, Tehran, Iran.
References
- 1.Pachón-Peña, G , Yu, G , Tucker, A , Wu, X , Vendrell, J , Bunnell, B.A , Gimble, J.M Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immune-phenotypic profiles. J. Cell Physiol. 2011;226:843–51. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuk, P.A , Zhu, M , Ashjian, P , Urgate, D.A , Huang, J.I , Mizuno, H , Alfonso, Z.C , Fraser, J.K , Benhaim, P , Hedrick, M.H Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimble, J.M , Katz, A.J , Bunnell, B.A Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang, T , He, D , Kleiner, G , Kuluz, J Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J. Spinal Cord Med. 2007;1:S35–40. doi: 10.1080/10790268.2007.11753967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang, D.Z , Gai, L.Y , Liu, H.W , Jin, Q.H , Huang, J.H , Zhu, X.Y Transplantation of autologous adipose-derived stem cells ameliorates cardiac function in rabbits with myocardial infarction. Chin. Med. J. (Engl). 2008;120:300–307. [PubMed] [Google Scholar]
- 6.Barzilay, R , Melamed, E , Offen, D Introducing transcription factors to multipotent mesenchymal stem cells: making transdifferentiation possible. Stem Cells . 2009;27:2509–2515. doi: 10.1002/stem.172. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Ramos, J.R. Neural cells derived from adult bone marrow and umbilical cord blood. J. Neurosc. Res. 2002;69:880–893. doi: 10.1002/jnr.10337. [DOI] [PubMed] [Google Scholar]
- 8.Safford, K.M , Rice, H.E Stem cell therapy for neurologic disorders: therapeutic potential of adipose-derived stem cells. Curr. Drug Targets. 2005;6:57–62. doi: 10.2174/1389450053345028. [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam, J.D. A double-blind, placebo-controlled trial of the safety and efficacy of selegiline transdermal system without dietary restrictions in patients with major depressive disorder. J. Clin. Psych. 2003;64:208–214. doi: 10.4088/jcp.v64n0216. [DOI] [PubMed] [Google Scholar]
- 10.Magyar, K , Szende, B (-)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicol. 2004;25:233–242. doi: 10.1016/S0161-813X(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 11.Knoll, J. (-)Deprenyl (Selegiline): past, present and future. Neurobiol. 2000;8:179–199. [PubMed] [Google Scholar]
- 12.Esmaeili, F , Tiraihi. T , Movahedin, M , Mowla, S.J Selegiline induces neuronal phenotype and neurotrophins expression in embryonic stem cells. Rejuvenation Res. 2006;9:475–484. doi: 10.1089/rej.2006.9.475. [DOI] [PubMed] [Google Scholar]
- 13.Rodbell, M. Localization of lipoprotein lipase in fat cells of rat adipose tissue. J. Biol. Chem. 1964;239:753–755. [PubMed] [Google Scholar]
- 14.Eslaminejad, M.B , Nikmahzar, A , Taghiyar, L , Nadri, S , Massumi, M Murine mesenchymal stem cells isolated by low density primary culture system. Dev. Growth Differ. . 2006;48:361–370. doi: 10.1111/j.1440-169X.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang, Y , Wang, J , Chen, G , Fan, D , Deng, M Inhibition of Sirt1 promotes neural progenitors toward motoneuron differentiation from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2011;404:610–614. doi: 10.1016/j.bbrc.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J.M , Betz, W.J The color of lactotroph secretory granules stained with FM1-43 depends on dye concentration. Biophys. J. 2008;94:3167–3177. doi: 10.1529/biophysj.107.112573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An, Y , Tsang, K.K Potential of stem cell based therapy and tissue engineering in the regeneration of the central nervous system. Biomed. Mater. 2006;1:R38–R44. doi: 10.1088/1748-6041/1/2/R02. [DOI] [PubMed] [Google Scholar]
- 18.Lindvall, O , Björklund, A Cell replacement therapy: helping the brain to repair itself. NeuroRx. 2004;1:379–381. doi: 10.1602/neurorx.1.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dazzi, F , Ramasamy, R , Glennie, S , Jones, S.P , Roberts, I The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Sylvester, K.G , Loganker, M.T Stem cells. Arch. Surg. 2004;139:93–99. doi: 10.1001/archsurg.139.1.93. [DOI] [PubMed] [Google Scholar]
- 21.Fraser, J.K , Wulur, I , Alfonso, Z , Hedrick, M.H Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Stolzing, A , Jones, E , McGonagle, D Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Lin, L , Shen, Q , Wei, X , Hou, Y , Xue, T , Fu, X , Duan, X Comparison of osteogenic potentials of BMP4 transduced stem cells from autologous bone marrow and fat tissue in a rabbit model of calvarial defects. Calcif Tissue Int. 2009;85:55–65. doi: 10.1007/s00223-009-9250-x. [DOI] [PubMed] [Google Scholar]
- 24.Tapp, H , Hanley, E.N, Patt, J.C , Gruber, H.E Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp. Biol. Med. (Maywood) 2009;234:1–9. doi: 10.3181/0805/MR-170. [DOI] [PubMed] [Google Scholar]
- 25.Scherzed, A , Hackenberg, S , Froelich, K , Radeloff, A , Technau, A , Kessler, M , Hagen, R , Rak, K , Koehler, C , Kleinsasser, N The effect of wound fluid on adipose-derived stem cells in vitro: a study in human cell materials. Tissue Eng. Part C Meth. 2011;17:809–817. doi: 10.1089/ten.TEC.2010.0257. [DOI] [PubMed] [Google Scholar]
- 26.Fraser, J.K , Schreiber, R , Strem, B , Zhu, M , Alfonso, Z , Wulur, I , Hedrick, M.H Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat. Clin. Pract. Cardiovasc. Med. 2006;1:S33–S37. doi: 10.1038/ncpcardio0444. [DOI] [PubMed] [Google Scholar]
- 27.Knoll, J. History of deprenyl: the first selective inhibitor of monoamine oxidase type B. Vopr Med. Khim. 1997;43:482–493. [PubMed] [Google Scholar]
- 28.Ghorbanian, M.T , Tiraihi, T , Mesbah-Namin, S.A , Fathollahi, Y Selegiline is an efficient and potent inducer for bone marrow stromal cell differentiation into neuronal phenotype. Neurol. Res. 2010;32:185–193. doi: 10.1179/174313209X409016. [DOI] [PubMed] [Google Scholar]
- 29.Nagatsu, T , Sawada, M Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson's disease: possible implications of glial cells. J. Neural. Transm. Suppl. 2006;71:53–65. doi: 10.1007/978-3-211-33328-0_7. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Romos, J , Song, S , Cardozo-Pelaez, F , Hazzi, C , Stedeford, T , Willing, A , Freeman, T.B , Saporta, S , Janseen, W , Patel, N , Cooper, D.R , Sanberg, P.R Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 31.Woodbury, D , Schwarz Emily, J , Ira, B Adult Rat and human bone marrow stromal stem cells differentiate into neurons. J. Neurosci. Res. 2002;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.Panahi, M , Al-Tiraihi, T. Morphometric evaluation of the neuroprotective effect of deprenyl on postaxotomic motor neuron losses. Clin Neuropharmacol. 2002;25:75–78. doi: 10.1097/00002826-200203000-00003. [DOI] [PubMed] [Google Scholar]