Abstract
Background:The polysialylated neural cell adhesion molecule (PSA-NCAM) is expressed in developing brain. Fetal brain damage is caused by different conditions such as seizure and hypoxia. The present study was designed to investigate the effect of maternal seizures on the number of PSA-NCAM positive cells in pup's hippocampus. Methods: Female Wistar rats were divided into four groups: (a) kindled rats which received PTZ (40 mg/kg, i.p.) during pregnancy from embryonic day 14-19 (E14-E19) every 48 h, (b) kindled rats which did not receive PTZ during pregnancy, (c) non-kindle, pregnant rats which received PTZ injection (40 mg/kg, i.p.) during pregnancy from E14 to E19 every 48 h, and (d) non-kindle, pregnant rats which received injection with an equal volume of normal saline as sham controls. At postnatal day 14 (PD14), rat pups were perfused, and their brain were fixed, embedded and coronal sections stained by immunohistochemistry method. The number of PSA-NCAM positive cells per unit area in the pup's hippocampus was counted. Results: The number of PSA-NCAM positive cells in the CA1, CA3, and DG fields of pup's hippocampus, which was obtained from mothers who experienced PTZ injection during pregnancy, was decreased approximately 2.6 (P = 0.001), 2 (P = 0.001), and 2.1 (P = 0.001) times compared with non-PTZ treated maternal groups, respectively. Conclusions: Our study showed that maternal seizures reduced the number of neurons and also PSA-NCAM positive cells per unit area in the offspring hippocampus that it may cause impairment in hippocampal functions.
Key Words: Epilepsy, Polysialylated neural cell adhesion molecule (PSA-NCAM), Hippocampus, Seizures
Introduction
After stroke, epilepsy is the most common neurological disorder (0.5-2% of the general population) and it is estimated that 0.5% pregnant women have epilepsy [1, 2]. Previous studies have been revealed that the children of mothers with epilepsy both treated with anti-epileptic drugs and non-treated, have a higher incidence of intrauterine growth retardation than general population. Thus, it is possible that maternal seizures could have a direct effect on the fetus [3].
Generalized seizures may cause hypoxia, which it can lead to irreversible damages to the central nervous system. A previous study has been reported that maternal seizures in pregnant rats lead to neuronal damage in pup's hippocampus [4]. However, it is unclear that whether seizures alone in the absence of hypoxia can induce damage in nervous system [5].
Cerebral cortex development is a temporo-spatial process and it is carried out by several mechanisms, including proliferation, migration, differentiation, maturation and programmed cell death (apoptosis). Thus, failure in one of these mechanisms can potentially cause an impairment of cortical development [2, 6].
It is recognized that structural alterations in neurons are mediated by expression of molecular changes such as cytoskeletal proteins and cell adhesion molecules. The polysialylated neural cell adhesion molecule (PSA-NCAM) strongly associated with brain plasticity, axonal outgrowth, cell migration and synaptogenesis [7, 8]. The highest expression of PSA-NCAM occurs in the developing nervous system. However, it has been believed that the expression of PSA-NCAM is increased in several cases, including learning and memory [9], stress [10], ischemia and epilepsy [11, 12].
Previously, it has been demonstrated that PSA-NCAM positive cells can be found in neuronal cells of the hilus, CA3 and CA1 regions of hippocampus [13, 14]. Increased neurogenesis in the dentate granule cell layer and increased numbers of PSA-NCAM-positive granule cells have been demonstrated in amygdaloid-kindling [15, 16]. The aim of this study was to determine the effects of maternal seizures on changes in numbers of PSA-NCAM positive cells in rat's hippocampus offspring using immunohistochemical techniques.
MATERIAL AND METHODS
This experimental research was reviewed and approved by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran.
Animals. Eight-week-old Wistar rats (Laboratory Animal Center of Mashhad University of Medical Sciences, Mashhad, Iran), weighing 180-200 g, at the outset were used in this study. The animals were maintained at the animal house under controlled conditions (12 h light and dark cycle, 21°C and 50% relative humidity) with laboratory chow and water provided ad libitum.
Kindling procedure. Female rats were received a dose of 40 mg/kg pentylentetrazol (PTZ, Sigma, USA) dissolved in 1ml normal saline i.p., every 48 h. In total, 13 doses of PTZ were applied. The convulsive behavior was observed after each PTZ-injection for 20 min. The seizures were classified according to Racine [17] as follows: stage 0, no response; stage 1, ear and facial twitching; stage 2, myoclonic jerks without upright position; stage 3, myoclonic jerks, upright position with bilateral forelimb clonus; stage 4, clonic-tonic seizures; stage 5, generalized clonic-tonic seizures, loss of postural control. To check the maintenance of kindling state, the animals were challenged with a sub-convulsive PTZ dose 10 days after the last kindling injection. Only the rats showing generalized clonic-tonic seizures were used in this study as kindled. The rats injected with a equal volume of normal saline were used as sham controls.
Breeding protocol and study groups. Female rats were placed with “stud” males in the late afternoon (4-5 PM) and removed the next morning (9-10 AM). The day on which spermatozoa were found in a vaginal smear was designated as embryonic day 0 (E0). After confirmation of kindling and pregnancy, the kindled pregnant rats were divided into two experimental groups randomly (6 rats in each group): (a) kindled rats which received PTZ injection (40 mg/kg) i.p. from E14 to E19 every 48 h; (b) kindled rats which did not receive PTZ during pregnancy. The rats who were not received PTZ before pregnancy (non-kindled) were divided into two control groups randomly (6 rats in each group): (c) non-kindled, pregnant rats which received PTZ injection (40 mg/kg) i.p. from E14 to E19 every 48 h, and (d) pregnant non-kindled rats injected with equal volume of normal saline as sham controls.
Tissue processing. Rat pups at postnatal day 14 (PD14) were anesthetized via inhalation of ether and perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffered solution, pH 7.4. Brains were removed and fixed in the paraformaldehyde 1% solution (pH 7.4) for 2 days. The brain tissues were processed by routine histological methods and embedded in paraffin blocks. Paraffin blocks were placed in rotary microtome (Leitz 1512, Germany) and serial coronal sections of 5 μm thickness were obtained. The boundary of hippocampus was defined in accordance with the atlas of Paxinos and Watson [18]. Next, ten uniform random sampled sections including hippocampus from each animal were chosen and mounted on poly-L-lysine coated slides for immunohistochemistry.
Immunohistochemistry. For immunohistochemical detection of PSA-NCAM, sections were deparaffinized with the xylene, rehydrated through descending concentrations of ethanol and rinsed in 0.1 M PBS for 10 min. The deparaffinized and rehydrated sections were treated with EDTA (pH 8.4) in PBS at 37°C for 15 min to retrieve antigen. Then, they were immersed in a methanol/H2O2 solution (1:100) in dark to block endogenous peroxidase for 30 min and rinsed in 0.05 M PBS plus 0.025% Trition X-100 (3 times for 5 min) at room temperature. Thereafter, the sections were incubated in 10% normal serum with 1% BSA in PBS at room temperature for 2 h. For best result to decrease background staining, all the sections were treated with 10% normal goat serum in PBS (goat as the host of the secondary antibody) for 30 min. The sections were then covered with the anti-PSA-NCAM mouse IgM monoclonal antibody (diluted 1:500 in PBS with 1% BSA) as primary antibody in humidified chamber at 4oC overnight. After incubation, the slides were washed extensively with PBS containing 0.025% Trition X-100 (3 times for 5 min). After washing, the sections were applied with HRP-conjugated secondary antibody (goat anti-mouse IgM, Vector Laboratories, CA, USA) and diluted to the 1:200 concentration in PBS with 1% BSA at room temperature for 2 hours. All the sections were washed extensively with PBS for 3 min and treated with diaminobenzidine solution (0.03 g diaminobenzidine in 100 ml PBS and 200 µl H2O2/100 ml PBS) in dark at room temperature for 30 min. After washing in running water, all the sections were counterstained with a solution of Harris Hematoxylin for 1 min. Finally the sections were dehydrated in increasing graded ethanol, cleared in xylene and mounted in glass slide [19]. The immunostaining sections were evaluated and photographed by a light microscope (Olympus DP12, Japan) and PSA-NCAM positive cells in the hippocampal sections were counted.
Quantification analysis. PSA-NCAM positive cells and the number of neurons per unit area (NA) of the CA1, CA3, DG and hilus subdivisions of the hippocampus were counted. The sections including hippocampus for an unbiased estimation of the number of neurons were obtained by uniform random sampling. The preparations were examined under a light microscope using a ×100 objective lens (UPlanFI, Japan) and images were transferred to computer using a high-resolution camera (BX51, Japan). All sections were digitally photographed and the number of neurons and PSA-NCAM positive cells were counted using a 1000 μm2 counting frame. The mean number of neurons NA in different regions of hippocampus were calculated using the following formula [20]:
Where " " is the sum of counted particles appeared in sections, "a/f" is the area associated with each frame, "∑P" is sum of frame associated points hitting space.
Statistical analysis. Differences between means were determined by one-way analysis of variance (ANOVA) followed by tukey post hoc test using SPSS11.5 software.
Results
PTZ injections . The first few injections of PTZ induced behavioral signs, such as short-term freezing and face automatism (stage 1 of the Racine scale). Repeated injections of PTZ resulted in the clonic-tonic convulsions in rats (stages 4 or 5 of the Racine scale, Fig. 1). Kindling was confirmed 10 days after the last injection of PTZ. Indeed, seizure severity did not differ from that observed after the last injection in the same animals (P = 0.1).
Fig. 1.
Number of PTZ injections required to reach each stage of Racine's scale (mean ± S.D.).
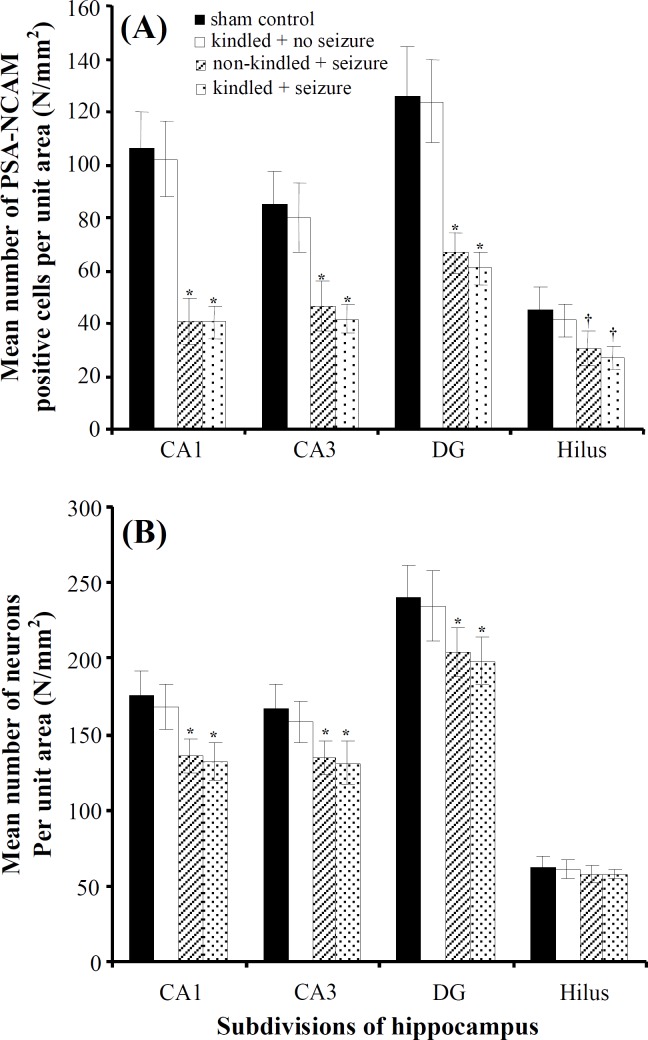
Effect of maternal seizures on the number of PSA-NCAM-positive cells per unit area in hippocampus of rat pups. PSA-NCAM positive cells were found in the various subdivisions of hippocampus in the experimental and sham control groups, but they were less abundant in the groups that received PTZ injection during pregnancy period (Fig. 2). The number of PSA-NCAM positive cells among the experimental and sham control groups presented in Figure 3A. The number of PSA-NCAM positive cells NA,P in the CA1, CA3, and DG subdivisions of hippocampus, obtained from the offspring of kindled mothers who experienced PTZ-induced seizures in pregnancy period, decreased significantly 2.6 (P = 0.001), 2.07 (P = 0.001) and 2.1 (P = 0.001) times compared with sham control groups. Also, in offsprings of non-kindled mothers that they received PTZ during pregnancy this significant reductions were approximately 2.6 (P = 0.01), 1.84 (P = 0.001) and 1.9 (P = 0.01) times compared with sham control groups respectively. Data concerning the number of neurons (including PSA-NCAM positive and non-positive neurons) NA,N in each subdivision of the hippocampus has been presented in Figure 3B. These data demonstrated a moderately significant decrease in the number of neurons NA,N in the various subdivisions of the hippocampus in comparison with sham controls (P = 0.001), in exception of hilus region (P = 0.08).
Fig. 2.
Photomicrograph of the PSA-NCAM positive cells (Arrows) in hilus area of rat offspring hippocampus in postnatal day 14. Sham control (A); hippocampus obtained from the offspring of kindled mothers who did not experience epileptic seizures during pregnancy (B); hippocampus obtained from the offspring of non-kindled (C) and kindled (D) mothers who experienced epileptic seizures during pregnancy.
Fig. 3.
Mean number of (A) PSA-NCAM positive cells (NA,P) and (B) neurons (NA,N) per unit area in the subdivisions of hippocampus (mean ± S.D). *P = 0.001, †P = 0.01.
Although the number of neurons NA,N in hilus region among all groups was not different significantly, the number of PSA-NCAM positive cells in this region had shown a significant decrease in the groups which received PTZ-induced injection in comparison with the groups did not receive it (P = 0.01).
As shown in Figure 3, there was no statistically significant difference in the number of neurons NA,N and PSA-NCAM positive cells NA,P in hippocampus obtained from the offspring of kindled mothers who did not experienced PTZ-induced seizures during pregnancy when compared with sham control group (P = 0.08).
Discussion
This study evaluated the effect of maternal epileptic seizures during pregnancy on a number of neurons (NA,N) and PSA-NCAM positive cells (NA,P) in the rat pup's hippocampus in PD14. Our results showed that maternal seizures affected the number of neurons NA,N and PSA-NCAM positive cells NA,P in the rat pup's hippocampus.
PSA-NCAM plays an important role in synaptogenesis and migration of neural precursors [21]. It is well established that developing and migrating neurons in hippocampus are PSA-NCAM positive cells [22]. Previous studies have suggested that PSA-NCAM-induced plasticity is essential for learning [23, 24]. Therefore, any changes in the number of PSA-NCAM-positive cells may cause an impairment of hippocampal development.
The results of this study showed that the effect of maternal seizures on the number of PSA-NCAM positive cells NA,P was not similar in various subdivisions of hippocampus. Indeed, CA1 region showed a 2.6 fold decrease in the number of PSA-NCAM positive cells in the experimental pup's hippocampus in comparison with sham controls, whereas CA3 and DG regions showed approximately two fold decrease (Fig. 3A).
Iwai et al. [11] have shown a reduction of PSA-NCAM immunoreactivity in the granular cell layer of dentate gyrus following induction of ischemia and suggested that production of polysialylic acid is reduced under the influence of ischemia [11]. In addition, Uyanikgil et al. [25] have investigated the effect of maternal seizures during pregnancy on nestin expression in the immature rat cerebellum. They have demonstrated that nestin positive cells was increased in the experimental epilepsy group and also the epileptic seizures during pregnancy may cause an impaired neurogenesis. Some researchers have reported a reduction numbers of PSA-NCAM positive cells after seizures in pediatric and adult patients with temporal lobe epilepsy [26, 27].
In contrast, some researchers have reported an increase in the number of PSA-NCAM positive cells in the subventricular zone and hippocampus after general seizure in kindled rats [12, 28]. However, the mentioned studies have investigated the effect of epileptic seizures, but not maternal seizures.
It is established that the majority of cortical neurogenesis in rodent occurs at second week of prenatal period. In the rat, formation of hippocampus is completed during the first 2 weeks of postnatal life [29, 30]. Therefore, maternal seizures might affect neurogenesis that is required for the hippocampal development. In this study, we observed that the number of neurons NA,N decreased in the hippocampus obtained from the offspring that their mothers experienced epileptic seizures during pregnancy in comparison with sham controls.
Hallak et al. [5] have reported several fetal neuronal damage, such as shrinkage of cells, nuclear pyknosis, and karyorrhexis in the hippocampus, obtained from the offspring that their mothers experienced epileptic seizures during pregnancy. In addition, McCabe et al. [31] has found a reduction in new granular cells in DG after recurrent seizures in the neonatal rats.
The results of our study showed that the number of neurons NA,N was significantly decreased in the CA1, CA3 and DG subdivisions of hippocampus in offspring of mothers who experienced epileptic seizures during pregnancy in comparison with sham controls. Although there are evidences showing that DG is more vulnerable than other subdivisions of hippocampus to seizures and ischemia during postnatal life [32, 33], the results of our study show that CA1 region appears to be particularly vulnerable to maternal seizures.
In this study, despite reduction of the number of PSA-NCAM positive cells NA,P in hilus region, there was no significant difference in the number of neurons NA,N among all groups in this region (Fig. 3B). In addition, in our study, there were no significant changes in both the number of neurons NA,N and PSA-NCAM positive cells NA,P in the hippocampi, obtained from the offspring of kindled and non-kindled mothers who did not experience PTZ-induced seizures in pregnancy period. On the other hand, this experiment showed that kindling did not affect the number of neurons NA,N and PSA-NCAM positive cells NA,P in various subdivisions of hippocampus. Therefore, the only factor involved in reduction of PSA-NCAM-positive cells was PTZ-induced seizures during pregnancy period.
In agreement with the present study, Siddiqui and Joseph [34] evaluated neuronal cell loss in various subdivisions of hippocampus at 16 weeks post-status epilepticus rats. They have reported that the granule cells in dentate gyrus were very resilient to kainate-induced status epilepticus. In hilus region, they have showed that at 16 weeks post-status epilepticus, there was no significant difference in neuronal loss between kainate and control groups; while the number of neurons in the CA3 and CA1 regions at 16 weeks post-status epilepticus showed significant decrease in kainate-induced epileptics in comparison with controls. Finally they have reported that the CA1 region was the greatest affected area in hippocampus.
Naseer et al. [35] evaluated effects of maternal epileptic seizures induced by PTZ on the neuronal cell apoptosis in the rat pup's hippocampus. They have demonstrated that PTZ-induced seizures during pregnancy period increased neuronal apoptosis by elevating intracellular cytochrome C and Caspase-3 expressions. In addition, they have reported that pyramidal neurons of the CA1 region were scattered and shrunken, and the nuclei were condensed after inducing of seizure in pregnancy period. It seems that there is an imbalance between cell proliferation and cell apoptosis in the developing hippocampus after induction of seizure in pregnancy period.
This experiment showed that maternal seizure activity was associated with neuronal loss in the rat offspring hippocampus. Indeed, maternal epileptic seizures adversely impair normal postnatal hippocampal development, leading to alterations in hippocampal cell number. Reduction of PSA-NCAM positive cells in the experimental group might be suggested an impairment of postnatal neurogenesis and retardation of maturation. However, further studies are needed to investigate the effect of maternal seizures on the plastic changes of cells and their biological functions in the hippocampus.
Acknowledgment
The provided data in this paper are from a Ph.D. student thesis that was supported financially (grant No.88637) by Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran. The authors would like to thank Ms. F. Motejadded for her excellent technical assistance.
References
- 1.Pitkanen, A Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 2009;14:16–25. doi: 10.1016/j.yebeh.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 2.do Vale, T.G , da Silva, A.V , Lima, D.C , de Lima, E , Torres, L.B , Cossa, A.C , de Oliveira, E.M , Cabral, F.R , Cavalheiro, E.A , da Graça, M , Amado, D Seizures during pregnancy modify the development of hippocampal interneurons of the offspring. Epilepsy Behav. 2010;19:20–25. doi: 10.1016/j.yebeh.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Battino, D , Kaneko, S , Andermann, E , Avanzini, G , Canevini, M.P , Canger, R , Croci, D , Fumarola, C , Guidolin, L , Mamoli, D , et al. Intrauterine growth in the offspring of epileptic women: a prospective multicenter study. Epilepsy Res. 1999;36:53–60. doi: 10.1016/s0920-1211(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 4.LaJoie, J , Moshé, S.L Effects of seizures and their treatment on fetal brain. Epilepsia. 2004;45:48–52. doi: 10.1111/j.0013-9580.2004.458007.x. [DOI] [PubMed] [Google Scholar]
- 5.Hallak, M , Kupsky, W.J , Hotra, J.W , Evans, J.B Fetal rat brain damage caused by maternal seizure activity: prevention by magnesium sulfate. Am. J. Obstet. Gynecol. 1999;181:828–834. doi: 10.1016/s0002-9378(99)70309-1. [DOI] [PubMed] [Google Scholar]
- 6.Bentivoglio, M , Tassi, L , Pech, E , Costa, C , Fabene, P.F , Spreafico, R Cortical development and focal cortical dysplasia. Epileptic Disord. 2003;5:S27–S34. [PubMed] [Google Scholar]
- 7.Van der Borght, K , Brundin, P Reduced expression of PSA-NCAM in the hippocampus and piriform cortex of the R6/1 and R6/2 mouse models of Huntington's disease. Exp. Neurol. 2007;204:473–478. doi: 10.1016/j.expneurol.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Dityatev, A , Dityateva, G , Sytnyk, V , Delling, M , Toni, N , Nikonenko, I , Muller, D , Schachner, M Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J. Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker, C.G , Artola, A , Gerardy-Schahn, R , Becker, T , Welzl, H , Schachner, M The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J. Neurosci. Res. 1996;45:143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Pham, K , Nacher, J , Hof, P.R , McEwen, B.S Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 11.Iwai, M , Hayashi, T , Zhang, W.R , Sato, K , Manabe, Y , Abe, K Induction of highly poly-sialylated neural cell adhesion molecule (PSA-NCAM) in postischemic gerbil hippocampus mainly dissociated with neural stem cell proliferation. Brain Res. 2001;902:288–293. doi: 10.1016/s0006-8993(01)02399-x. [DOI] [PubMed] [Google Scholar]
- 12.Sato, K , Iwai, M , Zhang, W.R , Kamada, H , Ohta, K , Omori, N , Nagano, I , Shoji, M , Abe, K Highly polysialylated neural cell adhesion molecule (PSA-NCAM) positive cells are increased and change localization in rat hippocampus by exposure to repeated kindled seizures. Acta Neurochir. Suppl. 2003;86:575–579. doi: 10.1007/978-3-7091-0651-8_117. [DOI] [PubMed] [Google Scholar]
- 13.Nacher, J , Blasco-Ibáñez, J.M , McEwen, B.S Non-granule PSA-NCAM immunoreactive neurons in the rat hippocampus. Brain Res. 2002;930:1–11. doi: 10.1016/s0006-8993(01)03365-0. [DOI] [PubMed] [Google Scholar]
- 14.Seki, T , Arai, Y Different polysialic acid-neural cell adhesion molecule expression patterns in distinct types of mossy fiber boutons in the adult hippocampus. J. Comp. Neurol. 1999;410:115–125. doi: 10.1002/(sici)1096-9861(19990719)410:1<115::aid-cne10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, W , Wan, Q , Zhang, Z.J , Wang, W.D , Huang, Y.G , Rao, Z.R , Zhang, X Dentate granule cell neurogenesis after seizures induced by pentylenetetrazol in rats. Brain Res. 2003;977:141–148. doi: 10.1016/s0006-8993(03)02438-7. [DOI] [PubMed] [Google Scholar]
- 16.Saegusa, T , Mine, S , Iwasa, H , Murai, H , Seki, T , Yamaura, A , Yuasa, S Involvement of highly polysialylated neural cell adhesion molecule (PSA-NCAM)-positive granule cells in the amygdaloid-kindling-induced sprouting of a hippocampal mossy fiber trajectory. Neurosci Res. 2004;48:185–194. doi: 10.1016/j.neures.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electro-encephalogr. Clin. Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos, G, Watson, C. The rat brain in stereotaxic coordinates. 2007: Academic Press; [DOI] [PubMed] [Google Scholar]
- 19.Ebrahimzadeh Bideskan, A.R , Hassanzadeh Taheri, M.M , Nikravesh, M.R Lectin histochemical study of vasculogenesis during rat pituitary morphogenesis. IJBMS. 2011;14:35–41. [Google Scholar]
- 20.Howard, C.V, Reed, M.G. Unbiased Stereology. Oxford, UK: BIOS Scientific Publisher Ltd.; 2002. [Google Scholar]
- 21.Seki, T , Arai, Y Highly polysialylated neural cell adhesion molecule (NCAM-H) is expressed by newly generated granule cells in the dentate gyrus of the adult rat. J. Neurosci. 1993;13:2351–2358. doi: 10.1523/JNEUROSCI.13-06-02351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempermann, G , Jessberger, S , Steiner, B , Kronenberg, G Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Van der Borght, K , Wallinga, A.E , Luiten, P.G , Eggen, B.J , Van der Zee, E.A Morris water maze learning in two rat strains increases the expression of the polysialylated form of the neural cell adhesion molecule in the dentate gyrus but has no effect on hippocampal neurogenesis. Behav. Neurosci. 2005;119:926–932. doi: 10.1037/0735-7044.119.4.926. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, K.J , O'Connell, A.W , Regan, C.M Repetitive and transient increases in hippocampal neural cell adhesion molecule polysialylation state following multitrial spatial training. J. Neurochem. 1996;67:1268–1274. doi: 10.1046/j.1471-4159.1996.67031268.x. [DOI] [PubMed] [Google Scholar]
- 25.Uyanıkgil, Y , Baka, M , Yurtseven, M , Turgut, M The effect of experimental epilepsy induced by penicillin administration during pregnancy on nestin expression in the immature rat cerebellum. Childs Nerv. Syst. 2004;20:176–182. doi: 10.1007/s00381-003-0901-3. [DOI] [PubMed] [Google Scholar]
- 26.Pirttilä, T.J , Manninen, A , Jutila, L , Nissinen, J , Kälviäinen, R , Vapalahti, M , Immonen, A , Paljärvi, L , Karkola, K , Alafuzoff, I , Mervaala, E , Pitkänen, A Cystatin C expression is associated with granule cell dispersion in epilepsy. Ann. Neurol. 2005;58:211–223. doi: 10.1002/ana.20545. [DOI] [PubMed] [Google Scholar]
- 27.Mathern, G.W , Leiphart, J.L , De Vera, A , Adelson, P.D , Seki, T,. Neder, L , Leite, J.P Seizures decrease postnatal neurogenesis and granule cell development in the human fascia dentata. Epilepsia. 2002;43:68–73. doi: 10.1046/j.1528-1157.43.s.5.28.x. [DOI] [PubMed] [Google Scholar]
- 28.Sato, K , Iwai, M , Nagano, I , Shoji, M , Abe, K Expression of highly polysialylated neural cell adhesion molecule in rat subventricular zone with exposure to repeated kindled seizures. Neurosci. Lett. 2002;323:244–246. doi: 10.1016/s0304-3940(02)00139-8. [DOI] [PubMed] [Google Scholar]
- 29.Bayer, S.A. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J. Comp. Neurol. 1980;190:87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- 30.Porter, B.E. Neurogenesis and epilepsy in the developing brain. Epilepsia. 2008;49:50–54. doi: 10.1111/j.1528-1167.2008.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCabe, B.K , Silveira, D.C , Cilio, M.R , Cha, B.H , Liu, X , Sogawa, Y Reduced neurogenesis after neonatal seizures. J. Neurosci. 2000;21:2094–2103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sloviter, R.S. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 33.Matsuyama, T , Tsuchiyama, M , Nakamura, H , Matsumoto, M , Sugita, M Hilar somatostatin neurons are more vulnerable to an ischemic insult than CA1 pyramidal neurons. J. Cereb. Blood Flow Metab. 1993;13:229–234. doi: 10.1038/jcbfm.1993.28. [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui, A.H , Joseph, S.A CA3 axonal sprouting in kainate-induced chronic epilepsy. Brain Res. 2005;1066:129–146. doi: 10.1016/j.brainres.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 35.Naseer, M.I , Shupeng, L , Kim, M.O Maternal epileptic seizure induced by entylenetetrazol: Apoptotic neurodegeneration and decreased GABAB1 receptor expression in prenatal rat brain. Mol. Brain. 2009;2:20. doi: 10.1186/1756-6606-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]