Abstract
Background: Molecular targeted therapy by different cell death inducers are recently considered in cancer therapy. The aim of this study was to compare the effect of cisplatin and inositol trisphosphate kinase inhibitor (caffeine) on human breast cancer cell line (MCF-7). The pattern of cell death in MCF-7 cells following the exposure to cisplatin and caffeine in individual and combination forms was characterized. Methods: MCF-7cells at late exponential phase were divided into two groups: control and experimental groups. Experimental group was exposed to cisplatin, caffeine and combination of them and control group was treated by vehicle. Forty-eight hours after incubation, floating and attached cells were collected separately. Flowcytometry analysis and electron microscopy were carried out on both attached and floating cells. Results: Two types of apoptotic and non-apoptotic cells were observed in the floating cells as well as in sub G1 cells of both experimental and control groups by electron microscopy. Both early and late stages of apoptosis were characterized and the attached cells remained unaffected. Conclusion: Although two different forms of cell death (apoptosis and non-apoptosis) were appeared in MCF-7 following exposure to cisplatin and caffeine, apoptosis was the major mechanism of cell death. The combination form of anti-cancer drugs with different mechanisms could decrease the dosage of employed anti-cancer drugs.
Key Words: Cisplatin, Caffeine, Apoptosis
Introduction
Cisplatin and its derivatives as active anti-tumor agents are widely used in human chemotherapy. The major target of cisplatin is DNA. Cisplatin becomes active by forming DNA adducts. Formation of cisplatin DNA adducts interferes with cell cycle check points, cell replication and transcription or regulation of signal transduction pathways. The outcome of these alterations is DNA damage and then occurrence of cell death [1]. The efficacy of anti-tumor agents in some cases is low through drug resistance and toxicity. The cellular mechanism of resistance to platinum-based agents includes inhibition of DNA interaction, activity of cell membrane glycoprotein and increasing of DNA adduct repair system [1, 2]. Cisplatin toxicity contributes the limitation of high-dose cisplatin application in clinical practice. One of the major side effects of cisplatin is nephrotoxicity. Despite significant development in chemotherapy, traditional chemotherapy with cisplatin has continued. Today molecular targeted therapy as a combination form may decrease the employed doses of cisplatin. It requires using the agents with different types of biofunction. One method to improve efficacy of cisplatin is to use it in combination with agents that aim intracellular signal pathways [1, 3].
The proliferation of breast cancer cell line (MCF-7) is dependent on estrogen. Three inositol trisphosphate receptor (IP3R) isoforms are identified in MCF-7 cells that cause to release intracellular Ca2+. Intracellular Ca2+ release plays a role in control of cell growth. Caffeine is known as a pharmacological inhibitor of IP3R and IP3 (inositol trisphosphate) kinase that act through intracellular molecular targeted mechanism [4].
Cisplatin and caffeine in individual and combination forms have different intracellular pathways of cell death. In this study, we evaluated and determined the cell death pattern concerning different cell death inducing pathways. Transmission electron microscopy as a golden criteria and flowcytometry were carried out to detect apoptosis and other types of cell death.
MATERIALS AND METHODS
Chemicals. Cisplatin was obtained from clinical formulation of 1 mg/ml. RPMI medium containing L-glutamine, fetal calf serum and antibiotics were purchased from Gibco BRL Laboratories (USA) and caffeine from Sigma Co. (USA).
Cell line. Human MCF-7cells, obtained from the Pasteur Institute of Iran (Tehran), were grown as monolayer in 80 cm2 flasks in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 300 µg L-glutamine, and an antibiotic formulation (100 units/ml penicillin and 100 µg/ml streptomycin). Then, the cells were grown in a humidified atmosphere containing 5% CO2 at 37°C.
Control and experimental groups. For this research project, the cultured cells were selected at late exponential phase. In experimental group, late exponential phase cells were divided into three groups based on employed agents: cisplatin, caffeine and caffeine with cisplatin. In cisplatin group, the cells were treated with 3 µM cisplatin (3 µM cisplatin equals with IC50 cytotoxicity test) and diluted in 0.9% saline for 1 hour. Then, the cells were washed with PBS and incubated in fresh medium containing serum for another 48 hours. In caffeine group, the cells were interacted with 1 µM of caffeine for 48 hours. In cisplatin and caffeine groups, the cells were treated with cisplatin (3 µM) for one hour and then washed. Afterward, the same cells were incubated with caffeine (1 µM) with addition of fresh medium for another 48 hours. In control group, the cells were treated by vehicle (0.9 saline) and incubated in fresh medium. Following incubation, floating and attached cells were harvested separately.
Electron microscopy. The harvested cells were fixed in 1.6% glutaraldehyde in 0.1 M cacodylate buffer and centrifuged at 4°C to obtain cell pellets. Cell pellets were post-fixed in 1% osmium tetraoxide for an hour, dehydrated through a graded ethanol series and infiltrated and embedded in Epon-Araldite resin. Electron microscopy was performed on thin sections of cell pellets stained with Uranyl acetate and lead citrate, and examined by a transmission electron microscope (Loe, Germany and England).
Cell cycle analysis by flowcytometry. The harvested cells were collected and fixed in 70% ethanol and stored at 4°C until flowcytometry analysis. The cells were resuspended in 800 ml PBS and 100 µl propidium iodide solution (100 µg/ml), incubated with 100 µl RNase (1µg/ml) at 37°C for 30 minutes and analyzed
using Epics XL coulter counter (USA). Data were analyzed using Multicycle cell cycle software (version V).
Results
In experimental groups, the cells in exponential phase showed a decline in cell growing. This appearance was observed independent of cisplatin and caffeine treatment. In control group, the cells were grown until confluent phase. Attached cells did not show any alterations and some of them were detached from attachment surface and floated to medium by cisplatin and caffeine.
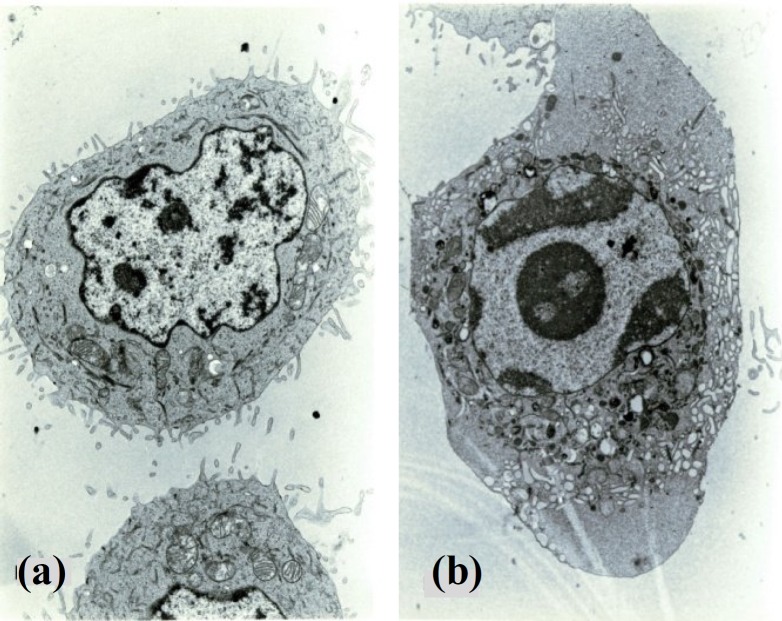
Electron microscopy revealed that the attached MCF-7cells in control and experimental groups are associated with numerous plasma cell membrane extensions with many microvilli. In addition, cellular organelles distributed homogeneously throughout the cytoplasm (Fig. 1a).
Fig. 1.
Electron micrographs of MCF-7floating cells showing (a) normal cell (×9000) and (b) apoptotic cell (×9000).
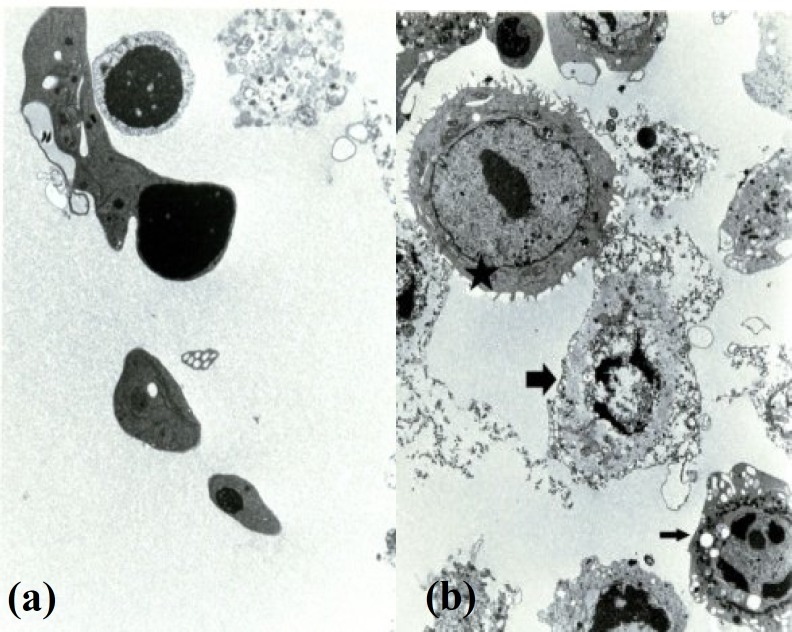
Apoptosis was found in the floating cells but not in the attached cells either in control or experimental group by electron microscopy. Moreover, apoptotic cells were found in early and late stages. The early morphological changes were characterized by smoothing of plasma membrane, elimination of microvilli, appearance of intracytoplasmic vacuoles, rounding of the nucleus and condensation of chromatin (Fig. 1b). At late stage, the apoptotic changes were seen into two different morphological forms. The first one (late changes) was associated with an increasing number of intracytoplasmic vacuoles, attachment of vacuoles to cell membrane, entrapment of organelles in a membrane structure (apoptotic body), and eventually the separation of apoptotic bodies from apoptotic cells (Fig. 2a). The second one was observed with damaged cell membrane and condensed nucleus. Finally, the damaged cells were completely lysed. In experimental and control groups, three types of cells were observed including apoptotic, normal and non-apoptotic cells (Fig. 2b).
Fig. 2.
Electron micrographs of MCF-7cells showing (a) apoptotic bodies (×11,000) and (b) three types of cells: normal cell (٭), apoptotic cell (C) and secondary necrotic cell (D) (×7500).
In flowcytometric analysis, the floating cells of both control and experimental groups have shown the appearance of a sub G1 peak as an indication of apoptosis in DNA histogram. No sub G1 peak has been shown with the attached cells either in experimental or control groups (Fig. 3).
Fig. 3.
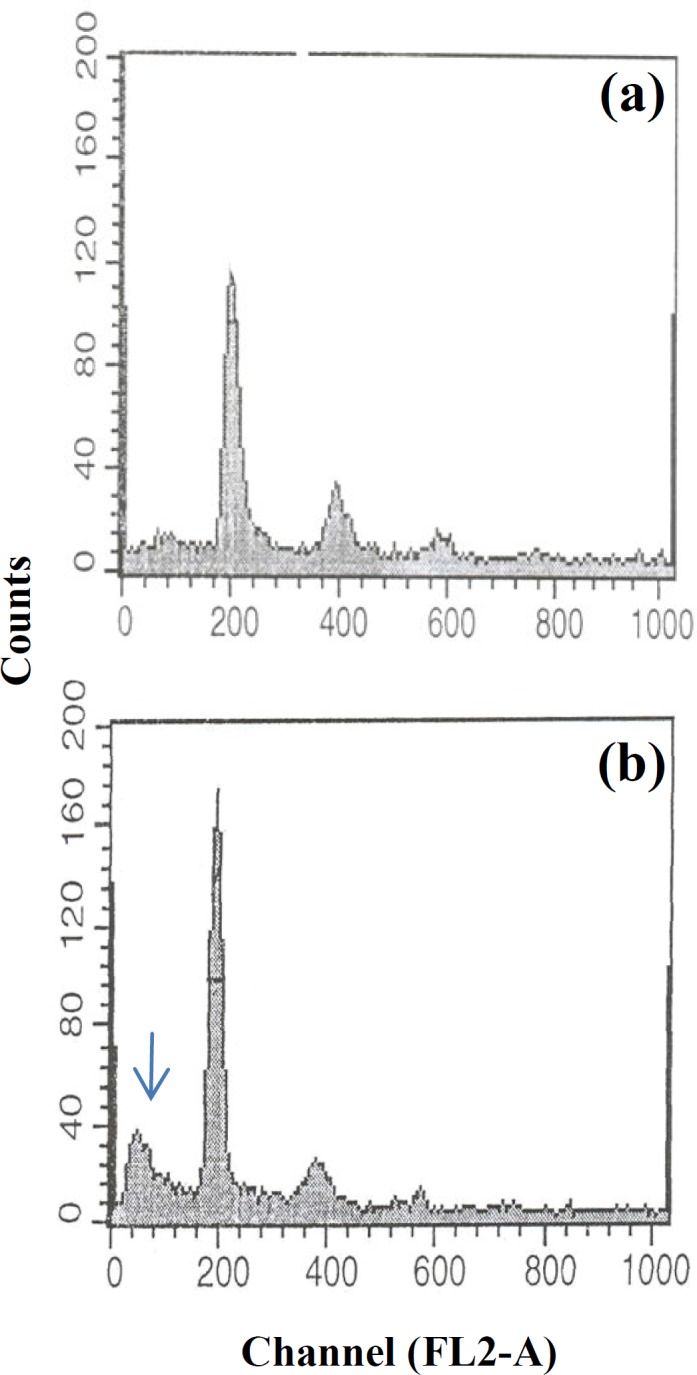
DNA histograms of attached and floating MCF-7cells. (a) Attached cells showing normal histogram without any subG1 peak; (b) Floating cells with sub G1 peak (arrow) indicating apoptosis.
Discussion
The caffeine and cisplatin caused some MCF-7 cells to detach from attachment surface, though the cells that resisted to the drug treatment remained attached. In all groups, the detached cells showed different stages of apoptotic and non-apoptotic cells. Apoptotic MCF-7 cells were revealed two morphological stages: late and early stages. These two apoptosis stages in other cell lines have been defined by transmission electron microscopy [5-7].
At late stage of apoptosis, both apoptotic body and non-apoptotic cells were seen. In addition, non-apoptotic cells were seen in all groups in the study. Therefore, it seems that apoptotic body formation is not endpoint of late stage. There is a question that whether the non-apoptotic cells were different from the necrotic cells. The formation of apoptotic body at late stage of apoptosis has been shown in other reports [5, 7]. Also, secondary necrosis as other form of dead cells has been determined at final stage of apoptosis [8]. Secondary necrosis has been appeared as lysed cells that display disintegration of apoptotic cells, which does not result from primary cell swelling and necrosis [6, 9, 10]. Amin et al. [11] have shown non-apoptotic cells in MCF-7. Morphological data of non-apoptotic cells may be similar to secondary necrosis [11]. Furthermore, the term "programmed necrosis" has been used in some cell lines that were induced by cytokines [12]. A final stage of apoptosis has been reported in several cellular models: apoptotic body, secondary necrosis and non-apoptotic form [10-12, 13]. Therefore, the non-apoptotic form seen in this study is different from necrosis.
The main target of cisplatin is nuclear DNA; however, it shows an affinity to other intracellular components such as cysteine and methionine. DNA adduction of cisplatin may lead to activation of cell cycle check points and then delay in cell cycle progression or inhibition of DNA repair. It has been reported that apoptosis by cisplatin involves several intracellular signal including protein kinase B, protein kinase Cand mitogen-activated protein kinase cascades. They have not been differentiated the signaling pathways of non-apoptotic and apoptotic forms [1]. However, the two forms (apoptosis and non-apoptosis) of cell death were seen in this study by cisplatin application.
Caffeine as an IP3 inhibitor induces apoptosis by Ca2+ release in cells by three forms of IP3R in MCF-7 [4]. There are some controversies over effects of caffeine on apoptosis induction in cancerous cell line.
Caffeine suppresses apoptosis induction in human thyroid papillary carcinoma K1 cells that was treated by cisplatin [14]. However, caffeine induces the cell death in MCF-7 cell line by infrared radiation by G2/M arrest. Caffeine sensitizes MCF-7 to cell death through caspase-3 expression [15]. The caffeine also induced two forms of cell death (apoptotic or non-apoptotic) in individual or combination form in MCF-7 cell line by cisplatin. It seems that the types of cell death (apoptotic and non-apoptotic forms) were not dependent on the used drugs, though it has different signaling pathways.
DNA fragmentation of dead cells was detected by flowcytometry. Apoptotic cells give a "sub G1" peak in DNA histogram [8, 13]. A sub G1 peak in floating MCF-7 cell line which was observed in both control and experimental groups, has confirmed morphological findings. In addition, the sub G1 may indicate both apoptotic and non-apoptotic forms that could not be differentiated in flowcytometry analysis. Therefore, the flowcytometric results solely may not be sufficient to evaluate the drug interactions of cancerous cells.
In conclusion, apoptosis appeared to be the major mechanism of cell death following exposure to cisplatin and caffeine in MCF-7 cell line in this study. End process of apoptotic MCF-7 cells showed two patterns, one was led to form apoptotic bodies and the other one led to non-apoptosis. The non-apoptotic form maybe secondary necrosis that differs from necrosis. IP3 kinase inhibitor acted independently from cisplatin on induction of cell death. Therefore, the combination forms of drugs as molecular targeted therapy can be used in inhibition of cancerous cell growth that this could help to decrease the dose of anti-cancer drugs and the severe side effects.
References
- 1.Basu, A, Krishnamurthy, S. Cellular responses to cisplatin-induced DNA damage. J. Nucleic Acids. 2010:1–16. doi: 10.4061/2010/201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart, D. Mechanisms of resistance to cisplatin and carboplatin. Crit. Rev. Oncol. Hematol. 2007;63:12–31. doi: 10.1016/j.critrevonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Bode, A.M, Dong, Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007;247:26–39. doi: 10.1016/j.canlet.2006.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szatkowski, C, Parys, J.B, Ouadid-Ahidouch, H, Matifat, F. Inositol 1,4,5-triphosphate-induced Ca2+ signaling is involved in estradiol-induced breast cancer epithelial cell growth. Mol. Cancer. 2010;9:156–168. doi: 10.1186/1476-4598-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyllie, A.H. Apoptosis and regulation of cell numbers in normal and neoplastic tissues: an overview. Cancer Metastasis Rev. 1992;11:95–103. doi: 10.1007/BF00048057. [DOI] [PubMed] [Google Scholar]
- 6.Bobichon, H, Mayer, P, Carpentier, Y, Desoize, B. HL60 cells exhibit particular ultrastructural features during all-trans retinoic acid-induced apoptosis. 1998;Int. J. Oncol:649–653. doi: 10.3892/ijo.12.3.649. [DOI] [PubMed] [Google Scholar]
- 7.Kerr, J.F, Winterford, C.M, Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Motyl, T, Grzeelkowska, K, Zimowska, W, Skierski, J, Wareski, P, Ploszaj, T, Trzecizkk, L. Expression of bcl-2 and bax in TGF-beta 1-induced apoptosis of L1210 leukemic cells. Eur. J. Biol. 1998;75:367–374. doi: 10.1016/s0171-9335(98)80070-8. [DOI] [PubMed] [Google Scholar]
- 9.Honda, O, Kuroda, M, Joja, I, Asaumi, J, Takeda, Y, Akaki, S, Togami, I, Kanazawa, S, Kawasaki, S, Hiraki, Y. Assessment of secondary necrosis of Jurkat cells using a new microscopic system and double staining method with annexin V and propidium iodide. Int. J. Oncol. 2000;16:283–288. doi: 10.3892/ijo.16.2.283. [DOI] [PubMed] [Google Scholar]
- 10.Herbert, M.J, Takano, T, Holthofer, H, Brady, H.R. Sequential morphologic events during apoptosis of human neutrophils. Modulation by lipoxygenase-derived eicosanoids. J. Immunol. 1996;157:3105–3115. [PubMed] [Google Scholar]
- 11.Amin, F, Bowen, I.D, Szegedi, Z, Mihalik, R, Szende, B. Apoptoticand non-apoptotic modes of programmed cell death in MCF-7 human breast carcinoma cells. Cell Biol. Int. 2000;24:253–260. doi: 10.1006/cbir.2000.0495. [DOI] [PubMed] [Google Scholar]
- 12.Moquin, D, Chan, F.K. The molecular regulation of programmed necrotic cell injury. Trends Biochem. Sci. 2010;35:434–441. doi: 10.1016/j.tibs.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ormerod, M.G, Orr, R.M, Peacock, H.J. The role of apoptosis in cell killing by cisplatin: a flowcytometric study. Br. J. Cancer. 1994;69:93–100. doi: 10.1038/bjc.1994.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deplanque, G, Ceraline, J, Mah-Becherel, C, Cazenave, J.P, Bergerat, J.P, Klein-Soyer, C. Caffeine and the G2/M block override : a concept resulting from a misleading cell kinetic delay, independent of functional p53. Int. J. Cancer. 2001;94:363–369. doi: 10.1002/ijc.1478. [DOI] [PubMed] [Google Scholar]
- 15.Essmann, F, Engels, I.H, Totzke, G, Schulze-Osthoff, K, Janicke, R.U. Apoptosis resistance of MCF-7 breast carcinoma cells to ionizing radiation is independent of p53 and cell cycle control but caused by the lack of caspase-3 and a caffeine-inhibitable event. Cancer Res. 2004;64:7065–7072. doi: 10.1158/0008-5472.CAN-04-1082. [DOI] [PubMed] [Google Scholar]