Abstract
Background: Neutrophil infiltration plays an important role in inflammatory reactions following spinal cord injury (SCI) and these cells cause substantial secondary tissue damage. The purpose of this study was to determine the effect of oleuropein (OE) on myeloperoxidase (MPO) activity as an index of neutrophil infiltration. Methods: Rats were randomly divided into four groups of 7 rats each as follows: sham-operated group, trauma group, and OE treatment groups (20 mg/kg, i.p., immediately and 1 hour after SCI). Spinal cord samples were taken 24 hours after injury and studied for determination of MPO activity. Results: The results showed that MPO activity was significantly decreased in OE-treated rats. Conclusion: On the basis of our findings, we propose that OE may be effective in protecting rat spinal cord from secondary damage by modulating of neutrophil infiltration.
Key Words: Oleuropein (OE), Neutrophil infiltration, Myeloperoxidase
Introduction
Neurological damages after traumatic spinal cord injury (SCI) result from both primary mechanical injury and secondary degeneration process. The outcome of SCI depends on the extent of secondary damage mediated by a series of cellular, molecular and biochemical cascades including calcium ion influx, oxygen free radical-induced lipid peroxidation, inflammatory reaction, autoimmune response, vascular events, and apoptosis [1]. Secondary injury appears to be susceptible to pharmacological interventions including the use of free radical scavengers and anti-inflammatory agents. Neutrophils play a major role in inflammation by releasing reactive oxygen species, pro-inflammatory factors and histolytic enzymes, which lead to severe and irreversible secondary tissue damage [2, 3].
Olive oil is a rich source of phenolic components (such as oleuropein), which have many beneficial health effects in human [4]. On the other hand, hydrolysis of oleuropein (OE) results in the formation of other phenolics including hydroxytyrosol and tyrosol [5]. Experimental studies attributed the beneficial effects of OE and its derivatives such as hydroxytyrosol to a variety of biological activities, including free radical scavenging/antioxidant actions, anti-inflammatory effects, anti-carcinogenic properties, and anti-microbial activities [6-8]. Olive oil phenols have some of protective effects against brain hypoxia-reoxygenation [9, 10], cerebral ischemia [11, 12], brain damage after hypoxia-reoxygenation in diabetic rats [13] and ageing [14]. Although the exact neuroprotective mechanism of olive oil phenols is unclear, the anti-oxidative and anti-inflammatory effects of these phenols are considered to be the main mechanisms leading to this neuroprotective effect.
In the present study, we investigated biochemically the potential anti-inflammatory effect of oleuropein against neutrophil infiltration in the spinal cord after experimental contusion injury.
MATERIALS AND METHODS
Animals. Male adult Sprague-Dawley rats (250-300 g, the Pasteur Institute of Iran, Tehran) were used in this study. The animals were kept under standard conditions according to the Guidelines of the University׳s Animal Care Codes to minimize the animals' suffering.
Contusive SCI using the weight-dropping technique. The animals were anesthetized with ketamine (75 mg/kg i.p.) and xylazine (10 mg/kg i.p.). Laminectomy was performed at T9 level vertebra. The dorsal surface of the cord was then subjected to weight-drop impact using a 10-g weight dropped from a height of 2.5 cm in order to produce contusive SCI [15]. Following the surgery, the recovery of the animals was assisted by administering lactated Ringer׳s solution (12-25 ml) subcutaneously immediately after surgery and cefazolin (50 µg/kg, Jaber Ibn Hayan, Tehran). The urinary bladders were pressed three times a day. The rats were randomly allocated in four groups, each containing 7 rats: (i) sham- operated group, underwent laminectomy alone; (ii) trauma group, which underwent laminectomy followed by SCI and received saline (vehicle); (iii and iv) OE treatment groups, which underwent laminectomy followed by SCI and received a 20- mg/kg single dose of OE (Sigma, USA) i.p. immediately (OE1) and 1 hour (OE2) after trauma.
Biochemical analysis. Twenty four hours after SCI, the rats were euthanized and 1.5-cm traumatized spinal cord sample was removed for biochemical analysis. The obtained samples were thoroughly cleaned of blood and the meninges were carefully removed. Then, the tissue samples were immediately frozen and stored in a -70°C freezer for assays of tissue myeloperoxidase (MPO) activity, an indicator of neutrophil infiltration, as previously described by Mullane [16]. The spinal cord samples were homogenized in a solution containing 0.5% (w/v) hexadecyltrimethyl ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged at 20,000 ×g at 4°C for 30 min. An aliquot of the supernatant was then allowed to react with a solution of 1.6 mM tetramethyl-benzidine and 0.1 mM H2O2. The absorbance of the supernatant was measured by spectrophotometry at 650 nm. The MPO activity was expressed as units of MPO/mg of proteins.
Statistical analysis. Statistical analysis was carried out using SPSS package. Results were presented as mean values (± SEM). The Kolmogorov-Smirnov test was used in order to evaluate the normality of the data. Also, the Tukey׳s multiple comparison tests and the analysis of the variance were used in order to compare each two groups and compare the data among the groups, respectively. A value of P<0.05 was considered significant.
RESULTS AND DISCUSSION
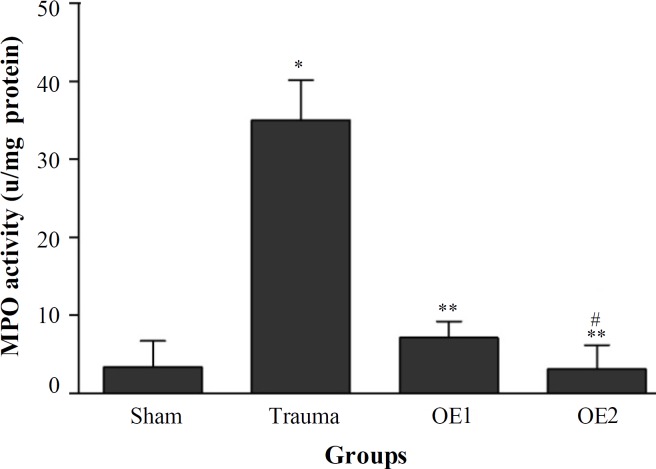
The histogram of the MPO activity for all groups at 24 hours post-injury has been shown in Figure 1. Induction of SCI in trauma group produced a significant elevation (P<0.05) in MPO activity compared to the sham-operated group. The MPO activity in OE treatment groups was significantly lower than trauma group (P<0.05), while the differences between OE1 and OE2 were not significant (P>0.05).
Fig. 1.
Effects of oleuropein on myeloperoxidase (MPO) activity. The histogram shows the activity of MPO at 24 hours after SCI. MPO activity was expressed as units of MPO/mg of proteins. *P<0.05 versus sham; **P<0.05 versus trauma; #P>0.05 versus OE1 group.
Post-traumatic inflammation is characterized in part by the accumulation of activated leukocytes, especially neutrophils which are the first leukocytes to arrive within the traumatized spinal tissue, peak at 24 hours post-injury [17].
Some evidences suggested that these cells play an important role in the pathogenesis of secondary degeneration of SCI by releasing of inflammatory mediators, including cytokines, chemokines, proteases, and free radicals, which can cause neuronal and glial toxicity [17]. Tissue MPO, a well-known oxidative enzyme, is a exclusive indicator of the extent of post-traumatic neutrophil infiltration [18]. On the other hand, MPO generates hypochlorous acid that damages nearby tissues. It is well-documented that a decrease in MPO activity correlates with reduction in traumatic spinal cord damage and better functional outcome after SCI in rats [19]. In the present study, we have observed that the MPO activity reduced significantly in OE-treated rats when compared with non-treated rats. Although the most famous and widely renowned properties of olive phenolics have long been attributed to the antioxidant and free radical scavenging effects, emerging evidences have shown the anti-inflammatory effects of these phenolics [20, 21]. In this regard, it has been documented that OE strongly inhibited the enzyme MPO in the inflamed tissue [20]. Plasma levels of the pro-inflammatory cytokines were also significantly reduced by OE in mice subjected to collagen-induced arthritis [22]. Visioli and colleagues [23] reported that OE inhibits the respiratory burst of neutrophils and hypochlorous acid-derived radicals. Moreover, other study has shown that olive oil polyphenols inhibit endothelial-leukocyte adhesion molecule expression [24].
Finally, our results showed that administration of OE immediately and 1 hour after SCI significantly attenuated MPO activity. This finding indicates a reduction of the neutrophil influx in the injured spinal tissue and possibly neuroprotective effects of OE after SCI.
Acknowledgment
This work was supported by Razi Herbal Medicines Research Center (Tehran, Iran).
References
- 1.Amar, A.P , Levy, M.L Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 2.Ducker, T.B , Kindt, G.W , Kempf, L.G Pathological findings in acute experimental spinal cord trauma. J. Neurosurg. 1971;35:700–708. doi: 10.3171/jns.1971.35.6.0700. [DOI] [PubMed] [Google Scholar]
- 3.Taoka, Y , Okajima, K , Uchiba, M , Murakami, K , Kushimoto, S , Johno, M , Naruo, M , Okabe, H , Takatsuki, K Role of neutrophils in spinal cord injury in rats. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 4.Waterman, E , Lockwood, B Active components and clinical applications of olive oil. Altern. Med. Rev. 2007;12:331–342. [PubMed] [Google Scholar]
- 5.Visioli, F , Galli, C , Galli, G Biological activities and metabolic fate of olive oil phenols. Eur. J. Lipid Sci. Technol. 2002;104:677–684. [Google Scholar]
- 6.Visioli, F , Poli, A , Galli, C Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002;22:65–75. doi: 10.1002/med.1028. [DOI] [PubMed] [Google Scholar]
- 7.Cicerale, S , Lucas, L , Keast, R Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010;11:458–479. doi: 10.3390/ijms11020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010;78:133–154. doi: 10.3797/scipharm.0912-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Correa, J.A , Munoz-Marin, J , Arrebola, M,M, , Narbona, F , Lopez-Villodres, J.A , Guerrero, A , De La Cruz, J.P Dietary virgin olive oil reduces oxidative stress and cellular damage in rat brain slices subjected to hypoxia-reoxygenation. Lipids. 2007;42:921–929. doi: 10.1007/s11745-007-3097-6. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Correa, J.A , Navas, M.D , Lopez-Villodres, J.A , Trujillo, M , Espartero, J.L Neuroprotective effect of hydroxytyrosol and hydroxytyrosol acetate in rat brain slices subjected to hypoxia-reoxygenation. Neurosci. Lett. 2008;446:143–146. doi: 10.1016/j.neulet.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 11.Bu, Y , Rho, S , Kim, J , Kim, M.Y , Lee, D.H , Kim, S.Y , Choi, H , Kim, H Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci. Lett. 2007:218–221. doi: 10.1016/j.neulet.2006.08.094. [DOI] [PubMed] [Google Scholar]
- 12.Mohagheghi, F , Bigdeli, M.R , Rasoulian, B , Zeinanloo, A.A , Khoshbaten, A Dietary virgin olive oil reduces blood brain barrier permeability, brain edema, and brain injury in rats subjected to ischemia-reperfusion. Scientific World Journal. 2010;10:1180–1191. doi: 10.1100/tsw.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De La Cruz, J.P , Del Rio, S , Arrebola, M.M , Lopez-Villodres, J.A , Jebrouni, N , Gonzalez-Correa, J.A Effect of virgin olive oil plus acetylsalicylic acid on brain slices damage after hypoxia-reoxygenation in rats with type 1-like diabetes mellitus. Neurosci. Lett. 2010;471:89–93. doi: 10.1016/j.neulet.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Pitozzi, V , Jacomelli, M , Zaid, M , Luceri, C , Bigagli, E , Lodovici, M , Ghelardini, C , Vivoli, E , Norcini, M , Gianfriddo, M , Esposto, S , Servili, M , Morozzi, G , Baldi, E , Bucherelli, C , Dolara, P , Giovannelli, L Effects of dietary extra-virgin olive oil on behavior and brain biochemical parameters in ageing rats. Br. J. Nutr. 2010;103:1674–1683. doi: 10.1017/S0007114509993655. [DOI] [PubMed] [Google Scholar]
- 15.Basso, D.M , Beattie, M.S , Bresnahan, J.C Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transaction. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 16.Mullane, K. Neutrophil-platelet interactions and post-ischemic myocardial injury. Prog. Clin. Biol. Res. 1989;301:39–51. [PubMed] [Google Scholar]
- 17.Fleming, J.C , Norenberg, M.D , Ramsay, D.A , Dekaban, G.A , Marcillo, A.E , Saenz, A.D , Pasquale-Styles, M , Dietrich, W.D , Weaver, L.C The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 18.Taoka, Y , Okajima, K , Uchiba, M , Murakami, K , Kushimoto, S , Johno, M , Naruo, M , Okabe, H , Takatsuki, K Role of neutrophils in spinal cord injury in rats. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 19.Hamada, Y , Ikata, T , Katoh, S , Nakauchi, K , Niwa, M , Kawai, Y , Fukuzawa, K Involvement of an intracellular adhesion molecule-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J. Neurochem. 1996;32:192–194. doi: 10.1046/j.1471-4159.1996.66041525.x. [DOI] [PubMed] [Google Scholar]
- 20.de la Puerta, R , Martinez-Dominguez, E , Ruiz-Gutierrez, V Effect of minor components of virgin olive oil on topical anti-inflammatory assays. Z. Naturforsch. C. 2000;55:814–819. doi: 10.1515/znc-2000-9-1023. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Domingues, E , de la Puerta, R , Ruiz-Gutierrez, V Protective effects upon experimental inflammation models of a polyphenol supplemented virgin olive oil diet. Inflamm. Res. 2001;50:102–106. doi: 10.1007/s000110050731. [DOI] [PubMed] [Google Scholar]
- 22.Impellizzeri, D , Esposito, E , Mazzon, E , Paterniti, I , Di Paola, R , Morittu, V.M , Procopio, A , Britti, D , Cuzzocrea, S Oleuropein aglycone, an olive oil compound, ameliorates development of arthritis caused by injection of collagen type II in mice. J. Pharmacol. Exp. Ther. 2011;339:859–869. doi: 10.1124/jpet.111.182808. [DOI] [PubMed] [Google Scholar]
- 23.Visioli, F , Bellomo, G , Galli, C Free radical scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998;247:60–64. doi: 10.1006/bbrc.1998.8735. [DOI] [PubMed] [Google Scholar]
- 24.Carluccio, M.A , Siculella, L , Ancora, M.A , Massaro, M , Scoditti, E , Storelli, C , Visioli, F , Distante, A , De Caterina, R Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003;23:622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]