Abstract
Background: It is proved that testis is sensitive to electromagnetic field (EMF) and its damage results in infertility. Exposure to EMF induces reactive oxygen species production and affects on anti-oxidants defense mechanisms. Metallothionein (MT) is a name for a group of low molecular weight (6-7 kDa), sulfhydryl rich proteins. Expression of MT1 and MT2 genes in testis tissue after EMF exposure was aimed in this study. Methods: Male BALB/c mice (8 weeks old) were exposed to 3 MT EMF for 8 weeks, 4 hours/day. After 8 weeks, the mice were sacrificed and the testis tissue was removed. The testis pieces were stained with hematoxylin and eosin and analyzed under an optical microscope. Assessment of MT1 and MT2 genes and also protein expression was performed by real-time PCR and Western-blot, respectively. Results: In light microscopic observation, the number of primary spermatocytes was increased significantly in EMF group (P<0.01). In addition, in interstitial space, the number of leydig cells was increased significantly in EMF group (P<0.01) and basement membrane thickness was increased as well. MT1 and MT2 genes were down-regulated significantly in testis tissue of mice exposed to EMF both in mRNA and protein level compared to control. Conclusion: It is clear that MT is mediated in testis development and spermatogenesis. Down-regulation of MT1 and MT2 after EMF in mouse testis might be followed by some consequences that result in infertility.
Key Words: Testis, Metallothionein (MT), reactive oxygen species (ROS)
Introduction
The biological effects of electromagnetic field (EMF) have been studied extensively in experiments involving both animal and human tissues over the past several decades [1]. Among the tissues, male reproductive system is very sensitive to environment conditions, such as drugs, thermal stress and EMF [1]. Spermatogenesis is affected by the mentioned factors and sometimes results in sub-fertility or infertility in men. In spite of the above words, there are controversies about its impairment effects of EMF [1-4]. A number of studies showed that exposure to EMF did not induce any adverse effects on spermatogenesis and reproductive capacity in experimental animals and human [5-8]. In contrast, other studies showed a clear damage to spermato-genesis [5, 9-11]. The etiology of male infertilities is largely undetermined, and our knowledge of exogenous factors affecting the male reproductive system is still limited. There are a number of data implicated EMF in free radical production, such as superoxide anion in different cells and organs, in macrophages, kidney, liver, and monocytes [12-14]. Indeed, several findings concluded that magnetic field-induced changes in enzyme activity and gene expression affect membrane structure and function and cause DNA damage [15-17].
Metallothionein (MT) is a name for a group of low molecular weight (6-7 kDa), sulfhydryl rich proteins of 61 to 68 amino acid residues. In 1957, it was recognized by Margoshes and Vallee [18] as a cadmium-binding protein in the kidney tissues of horse. These intracellular non-enzymatic ubiquitous polypeptides are distinguished by their extraordinary high cysteine content (up to 30%) and paucity of aromatic amino acids. In addition, MT are named because of their high metal content and extraordinary bio-inorganic structure [19]. Among these four isoforms of MT protein, which has been recognized until now, MT1 and MT2 are inducible forms that their transcription is activated by a variety of stress stimuli, namely metals, glucocorticoids, heat shock, a number of pro-inflammatory cytokines, and reactive oxygen species (ROS). Moreover, MT1 and MT2 play similar biological roles in mammalian cells [20, 21].
It is proved that EMF can induce its hazardous effects through ROS production. Therefore, the present study was designed to evaluate expression of MT1 and MT2 as anti-oxidants at mRNA and protein level in testis tissue after exposure to EMF.
MATERIALS AND METHODS
Mice and irradiation. Male BALB/c mice, 8 weeks old, were used in this experiment. The laboratory was maintained on a 12/12 h light/dark cycle. The mice were placed inside the EMF exposure cage and irradiated for 6 days/week; 4 hours/day from 8:00 AM to 12:00 PM for 8 weeks [17]. After this period, the mice were sacrificed by cervical dislocation and the testis was removed and used for purposes of the study. Animal experiments were approved by the Ethical Committee of Tabriz Medical University and performed in accordance with the guidelines.
Hematoxylin and eosin staining. For light microscopic assessment, the testis pieces were stored in 10% formalin solution for 24 hours and then submitted to the routine process of slide preparation. The 5-µm sections were stained with hematoxylin and eosin. Histological examination on testicular morphology was performed under magnification (40×) in five fields for each slide. Primary spermatocytes were marked with large size cells in seminiferous tubules.
RNA extraction. Total RNA from testis tissue was extracted by Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The quantity and quality of RNA were determined by spectrophotometry (ND-1000, Nanodrop, Wilmington, DE, USA) and electrophoresis, respectively.
cDNA synthesis. Reverse transcription was performed by SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) with 1 µg of total RNA, followed by DNaseI (Invitrogen, Carlsbad, CA, USA) treatment and heat inactivation. Semi-quantitative PCR was performed using Taq DNA polymerase (Roche Diagnostic, Germany) in a GeneAmp PCR System 9600 (PerkinElmer Life and Analytical Sciences, Wellesley, MA, USA). After initial denaturation (at 94C for 5 min), cDNA was subjected to 30 cycles of PCR. Primer sets for mouse MT1, MT2 and β-actin have been shown in Table 1. For normalization, expression of β-actin was examined and PCR products were evaluated in a 2% agarose gel. Real-time PCR analysis was performed in a Rotor-Gene RG 3000 (Corbett Research, Sydney, Australia). Amplification was conducted using absolute Syber Green Mix (ABgene, Surrey, UK) according to the manufacturer’s instructions. PCR condition included an initial denaturation at 94C for 15 min, followed by 40 cycles consisting of denaturation at 94C for 30 s, annealing at suitable temperature for 30 s and extension at 72C for 30 s. Threshold cycle values were normalized by β-actin expression. Changes in expression level of the genes were calculated according to Ct (threshold cycle) method.
Table1.
Primers of MT1, MT2 and β-actin genes.
| Genes | Primers | Annealing Temperature (C) |
Accession
Number |
|---|---|---|---|
| MT1 | F primer 5΄-ACC TCC TTG CAA GAA GAG CTG CT-3΄ R primer 5΄-GCT GGG TTG GTC CGA TAC TAT T -3΄ |
60 | NM_013602 |
| MT2 | F primer 5' ´ -CCA TAT CCC TTG AGC CAG AA -3΄ R primer 5΄-ATC GAC GAG AGA TCG GTT TG- 3΄ |
60 | NM_008630 |
| β-actin | F primer 5΄- TTC TAC AAT GAG CTG CGT GTG G -3΄ R primer 5΄-GTG TTG AAG GTC TCA AAC ATG AT-3΄ |
59 | NM_007393.3 |
Western-blot analysis. Total protein was extracted from mouse testis by TriPure Isolation Reagents (Roche Diagnostic, Germany) according to the manufacturer's protocol. Protein concentration was determined by Bio-Rad Protein Assay Kit (Bio-RAD, USA). Same amount of protein from control and exposed samples was heated in 2× SDS-PAGE sample buffer at 95C for 5 min and run on 12% gel. After separation on SDS-PAGE, protein samples were transferred to a polyvinylidene diflouoride (PVDF) membrane over 1.5 h (125 V, Hi-bond Amersham Biosciences, USA) using the Mini Trans-Blot Electrophoretic Transfer Cell system (Bio-Rad, Hercules, CA, USA) in Tris-glycine buffer, pH 8.4, containing 20% (v/v) methanol. Then, the membranes were blocked with a solution containing 5% skimmed milk and 0.1% Tween 20. The blocked membranes were washed with PBS and 0.05% Tween 20. For detection of MT1 and MT2, the membranes were incubated with a monoclonal anti-mouse MT1 and MT2 antibody at room temperature for 1 h. Then, they were washed 4 times with PBS containing 0.1% Tween 20 and incubated with 100 mU/ml horseradish peroxidase-coupled secondary antibody, rabbit anti-mouse horseradish peroxidase ([ab 6728] ABcam, USA) for 1 h. Afterwards, the membranes were washed 4 times with PBS containing 0.1% Tween 20. Finally, diaminobenzidine solution was used to visualize the protein bands (Sigma, USA).
Statistical analysis. The results are expressed as mean ± SD of three independent experiments. Differences were compared using student's t-test.
Results
Histological results. Microscopic observation showed that primary spermatocytes were situated near to the basement membrane of seminiferous tubules and their numbers were increased in EMF exposure group compared to control group (P<0.01). Morphologically, primary spermatocytes are large in size and their counting are easy. According to our results, their numbers in EMF group increased compared to control group (45 ± 8 versus 28 ± 5). Results obtained from hematoxylin and eosin staining showed that nucleus of primary spermatocytes was denser and darker than control group (Fig. 1A). These configurations of nucleus showed apoptosis occurrence in proliferative cells of seminiferous tubules. Moreover, in interstitial space of EMF exposure group, the number of Leydig cells was increased compared to control group (P<0.01). The number of Leydig cells in EMF group was 23 ± 8 versus 13 ± 4 in control group. Another histological finding presents that basement membrane of seminiferous tubules was thickened in EMF group compared to control (Fig. 1B).
Fig 1.
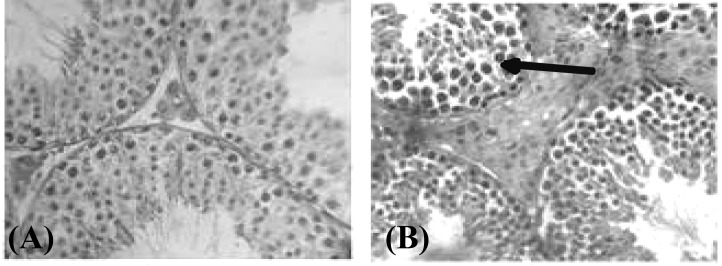
Hematoxylin and eosin staining of testis tissue in control (A) and EMF-exposed groups (B). Arrow shows dense nucleus.
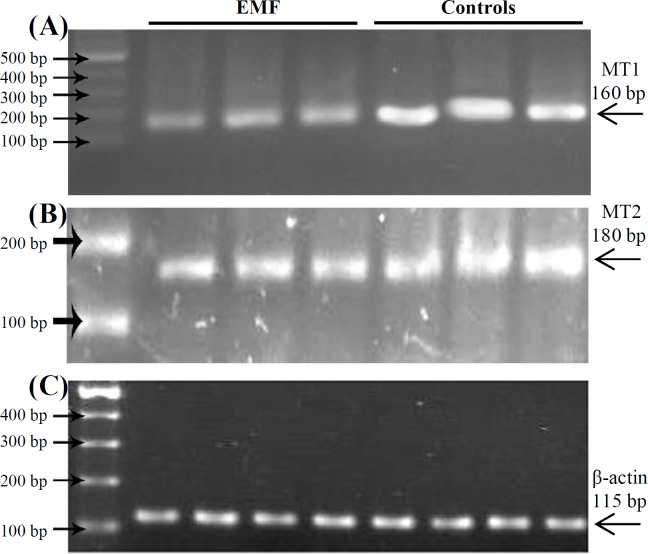
Downregulation of MT1 and MT2 in mouse testis after exposure to EMF. To determine whether EMF could change MT1 and MT2 gene expression, mice were exposed to EMF exposure and the gene expression was assessed by real-time PCR and Western-blot. Firstly, we performed semi-quantitative RT-PCR. MT1 and MT2 genes were expressed in control samples. This result indicates that expression of MT1 and MT2 genes in testis is necessary for normal physiology of the cells. However, expression of MT1 and MT2 were down-regulated in the exposed samples compared to the normal (Fig. 2).
Fig 2.
MT1 and MT2 gene expression determined by RT-PCR in control and EMF-exposed groups. Lanes 1, 2 and 3 show EMF-exposed group and lanes 4, 5 and 6 show controls. Down-regulation of MT1 and MT2 has been shown in (A) and (B), respectively. (C) Shows β-actin expression in both groups. The experiment has been shown in triplicate.
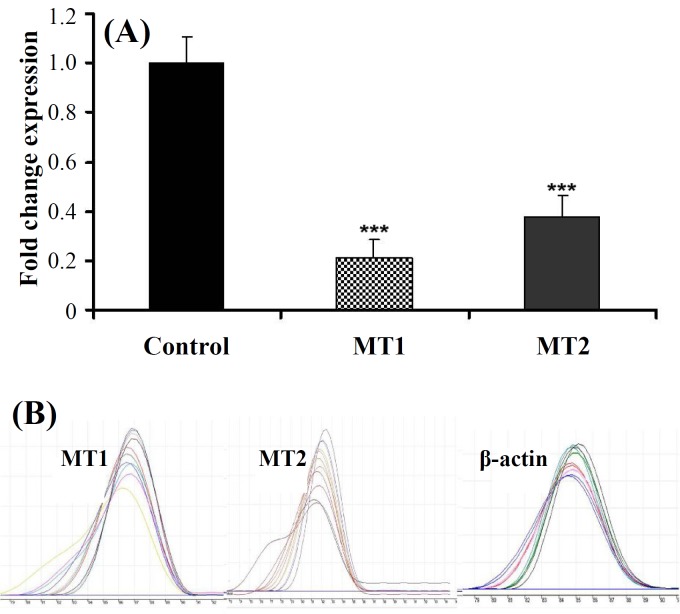
Secondly, we quantified MT1 and MT2 expression by real-time PCR. MT1 expression was decreased about 5 folds (5 ± 0.07, P<0.001) in EMF exposure group compared to control. Similarly, MT2 expression was also decreased in EMF exposure group about 3 folds (3 ± 0.091, P<0.001) compared to control samples (Fig. 3A). Melting curve of MT1, MT2 and housekeeping gene (β-actin) have been shown in Figure 3B.
Fig. 3.
(A) Down-regulation of MT1 and MT2 expression after exposure to EMF by real-time PCR (mean ± SD, ***P<0.001) and (B) melting curve of MT1, MT2 and β-actin.
Finally, to assess the MT1 and MT2 expression in protein level, Western-blot analysis was performed. Consistent with real-time PCR results, a faint band was observed in exposed samples compared to the controls, indicating down-regulation of MT1 and MT2 protein expression (Fig. 4).
Fig 4.
Western-blot analysis of MT1 and MT2 expression in mouse testis after exposure to EMF. MT1 and MT2 were down-regulated in mouse testis after exposure to EMF [lanes 4, 5 and 6 in (A) and (B)] compared to control group [lanes 1, 2, and 3 in (A) and (B)]. (C) Shows β-actin expression in both mice groups.
Discussion
Increased risks of growth retardation and skeletal system abnormalities in animal fetuses and occurrence of cancer and other diseases in human are the most important reasons for investigation of EMF effects on biological systems [2]. In spite of several investigations about EMF and its harmful effects on different tissues in experimental or clinical level, mechanisms of ROS and anti-oxidants balance have not been completely investigated.
The present study demonstrates that expression of MT1 and MT2 as two anti-oxidants in both RNA and protein level was decreased after EMF exposure and oxidative-anti-oxidative balance in rat testis.
It is proved that EMF induces ROS production in different cells and organs. Down-regulation of MT genes is closely associated with increased formation of ROS and reactive nitrogen species, respectively. Excessive production of these harmful substances along with a reduction in anti-oxidants eventually results in oxidative-anti-oxidative balance [22-24].
Interestingly under normal conditions, the steady-state levels of MT1/MT2 in rodent testes are higher than those found in other organs [25]. Down-regulation of MT1 and MT2 as anti-oxidants more probably is because of free radical production. Our results are consistent with the previous finding regarding the hypothesis that EMF exposure is associated with free radical production [17]. More invasive studies have shown that magnetic field influenced lipid peroxidation and anti-oxidant defense system in rat tissues [19]. Lee and his colleagues [11] suggested that EMF could deteriorate anti-oxidant defensive system by ROS production. It has been proposed that moderate levels of ROS can induce an increase in anti-oxidant enzyme activities, whereas very high-level of these reactants was shown to attenuate anti-oxidant enzyme activities [26]. In our study, down-regulation of MT1 and MT2 could be explained with high-level production of ROS after EMF exposure in mouse testis. Histological results obtained in our study, have confirmed that some pathological changes are occurred in the testis tissue. It has been proved that intracellular metal homeostasis, heavy metal detoxication, and scavenging a wide variety of ROS, including hydroxyl radicals, superoxide, hydrogen peroxide, and nitric oxide are among the accepted roles of MT, though the exact molecular mechanism(s) of function are not fully understood [27, 28]. Interestingly, it has been revealed that the aptitude of MT in scavenging of hydroxyl radicals are mostly in charge of the ROS toxicity and is about three hundred times higher than glutathione, the most plentiful intracellular anti-oxidant [27, 29].
One explanation of how the decreased MT1 level can modulate cellular sensitivity and sensitize germ cells to EMF may be the induction of apoptosis in these cells; however, it is not completely understood. In the present study, the presence of dense nucleus in testis tissue might be a marker of early stages of apoptosis [29]. Many stresses and drugs, such as ultraviolet irradiation, mitomycin C, lucocorticoids, heat shock and heavy-metals were found to induce MT synthesis and lead to the accumulation of the wild-type p53. Furthermore, MT is involved in DNA repair and very recently in programmed cell death [2].
Our results showed that EMF could affect on testis in histological and molecular levels. These changes might result in functional abnormality in testis and subsequent sub-fertility and infertility; however, more investigation must be elucidated.
References
- 1.Juutilainen, J , Saali, K Development of chick embryos in 1 Hz to 100 kHz magnetic fields. Radiat. Environ. Biophys. 1986;25:135–140. doi: 10.1007/BF01211737. [DOI] [PubMed] [Google Scholar]
- 2.Zusman, I , Yaffe, P , Pinus, H , Ornoy, A Effects of pulsing electromagnetic fields on the prenatal and postnatal development in mice and rats: in vivo and in vitro studies. Teratology. 1990;42:157–170. doi: 10.1002/tera.1420420207. [DOI] [PubMed] [Google Scholar]
- 3.Huuskonen, H , Juutilainen, J , Komulainen, H Effects of lowfrequency fields on fetal development in rats. Bioelectromagnetics. 1993;14:205–213. doi: 10.1002/bem.2250140304. [DOI] [PubMed] [Google Scholar]
- 4.Rommereim, D.N , Kaune, W.T , Buschbom, R.L , Phillips, R.D , Sikov, M.R Reproduction and development in rats chronologically exposed to 60-Hz electric fields. Bioelectromagnetics. 1987;8:243–258. doi: 10.1002/bem.2250080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan, B.M , Symanski, R.R , Pomeranz, L.E , Johnson, T.R , Gauger, J.R , McCormick, D.L Multigeneration reproductive toxicity assessment of 60-Hz magnetic fields using a continuous breeding protocol in rats. Teratology. 1999;59:156–162. doi: 10.1002/(SICI)1096-9926(199903)59:3<156::AID-TERA7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 6.Lundsberg, L.S , Bracken, M.B , Belanger, K. Occupationally related magnetic field exposure and male subfertility. Fertil. Steril. 1995;63:384–391. doi: 10.1016/s0015-0282(16)57373-7. [DOI] [PubMed] [Google Scholar]
- 7.Kowalczuk, C.I , Robbins, L , Thomas, J.M , Saunders, R.D Dominant lethal studies in male mice after exposure to a 50 Hz magnetic field. Mutat. Res. 1995;328:229–237. doi: 10.1016/0027-5107(95)00012-8. [DOI] [PubMed] [Google Scholar]
- 8.Heredia-Rojas, J.A , Caballero-Hernandez, D.E , Rodriguez-de la Fuente, A.O , Ramos-Alfano, G , Rodriguez-Flores, L.E Lack of alterations on meiotic chromosomes and morphological characteristics of male germ cells in mice exposed to a 60 Hz and 2.0 mT magnetic field. Bioelectromagnetics. 2004;25:63–68. doi: 10.1002/bem.10184. [DOI] [PubMed] [Google Scholar]
- 9.Al-Akhras, M.A , Elbetieha, A , Hasan, M.K , Al-Omari, I , Darmani, H , Albiss B Effects of extremely low frequency magnetic field on fertility of adult male and female rats. Bioelectromagnetics. 2001;22:340–344. doi: 10.1002/bem.59. [DOI] [PubMed] [Google Scholar]
- 10.Ramadan, L.A , Abd-Allah, A.R , Aly, H.A , Saad-el-Din, A.A Testicular toxicity effects of magnetic field exposure and prophylactic role of coenzyme Q10 and L-carnitine in mice. Pharmacol. Res. 2002;46:363–370. doi: 10.1016/s1043661802001718. [DOI] [PubMed] [Google Scholar]
- 11.Lee, J.S , Ahn, S.S , Jung, K.C , Kim, Y.W Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J. Androl. 2004;6:29–34. [PubMed] [Google Scholar]
- 12.Kula, B , Sobczak, A , Kuska, R Effects of static and ELF magnetic fields on free-radical processes in rat liver and kidney. Electron. Magnetobiol. 2000;19:99–105. [Google Scholar]
- 13.Simko, M , Droste, S , Kriehuber, R , Weiss, D.G Stimulation of phagocytosis and free radical production in murine macrophages by 50 Hz electromagnetic field. Eur. J. Cell Biol. 2001;80:562–566. doi: 10.1078/0171-9335-00187. [DOI] [PubMed] [Google Scholar]
- 14.Lupke, M , Rollwitz, J , Simko, M Cell activating capacity of 50 Hz magnetic fields to release reactive oxygen intermediates in human umbilical cord blood-derived monocytes and in Mono Mac 6 cells, Free. Radic. Res. . 2004;38:985–993. doi: 10.1080/10715760400000968. [DOI] [PubMed] [Google Scholar]
- 15.Lewy, H , Massot, O , Touitou, Y Magnetic field (50 Hz) increases N-acetyltransferase, hydroxy-indole-O-methyltransferase activity and melatonin release through an indirect pathway. Int. J. Radiat. Biol. 2003;79:431–435. doi: 10.1080/0955300031000140757. [DOI] [PubMed] [Google Scholar]
- 16.Yokus, B , Cakir, D.U , Akday, M.Z , Sert C , Mete, N Oxidative DNA damage in rats exposed to extremely low frequency electromagnetic fields. Free Radic. Res. 2005;39:317–323. doi: 10.1080/10715760500043603. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi Roushandeh, A , Halabian, R , Mozafari, P , Soleimani Rad, J , Sadeghzadeh Oskouei, B , Samadi Kuchaksaraei, A , Habibi Roudkenar, M Down-Regulation of lipocalin 2 expression in mouse testis after exposure to electromagnetic field. IJMS . 2009;34 [Google Scholar]
- 18.Margoshes, M , B. L. Vallee A cadmium protein from equine imaging hyperintensity in Alzheimer’s disease: Correlation with kidney cortex. J. Am. Chem. Soc. 1957;79:4813–4814. [Google Scholar]
- 19.Miles, A.T , Hawksworth, G.M , Beattie, J.H , Rodilla, V Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit. Rev. Biochem. Mol. Biol. 2000;35:35–70. doi: 10.1080/10409230091169168. [DOI] [PubMed] [Google Scholar]
- 20.Penkowa, M , Tio, L , Giralt, M , Quintana, A , Molinero, A , Atrian, S Specificity and divergence in the neurobiologic effects of different metallothioneins after brain injury. J. Neurosci. Res. 2006;83:974–984. doi: 10.1002/jnr.20790. [DOI] [PubMed] [Google Scholar]
- 21.Lu, H , Hunt, D.M , Ganti, R , Davis, A , Dutt, K , Alam, J , Hunt, R Metallothionein protects human retinal pigment epithelial cellsaganist apeptosis and oxidative stress. Exp. Eye Res. 2002;74:83–92. doi: 10.1006/exer.2001.1101. [DOI] [PubMed] [Google Scholar]
- 22.Chin, J.L , Banerjee, D , Kadhim, S.A , Kontozoglou, T.E , Chauvin, P.J , Cherian, M.G Metallothionein in testicular germ cell tumors and drug resistance. Cancer. 1993;72:3029–3035. doi: 10.1002/1097-0142(19931115)72:10<3029::aid-cncr2820721027>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Kontozoglou, T.E , Banerjee, D , Cherian, M.G.G Immunohistochemical localization of metallothionein in human testicular embryonal adenocarcinoma. Virchows. Arch [A] 1989;45:545–549. doi: 10.1007/BF00718648. [DOI] [PubMed] [Google Scholar]
- 24.Lee, B.C , Johng, H.M , Lim, J.K , Jeong, J.H , Baik, K.Y , Nam, T.J , Lee, J.H , Kim, J , Sohn, U.D , Yoon, G , Shin, S , Soh, K.S Effects of extremely low frequency magnetic field on the antioxidant defense system in mouse brain: a chemiluminescence study. J. Photochem. Photobiol. B. 2004;23:43–48. doi: 10.1016/j.jphotobiol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Salehi-Ashtiani, K , Widrow, R.J , Markert, C.L , Goldberg, E Testis-specific expression of a metallothionein I-driven transgene correlates with undermethylation of the locus in testicular DNA. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8886–8890. doi: 10.1073/pnas.90.19.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brydon, L , Petit, L , Delagrange, P , Strosberg, A.D , Jockers, R Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology. 2000;142:4264–4271. doi: 10.1210/endo.142.10.8423. [DOI] [PubMed] [Google Scholar]
- 27.Kaina, B , Lohrer, H , Karin, M , Herrlich, P Overexpressed human metallothionein IIA gene protects Chinese hamster ovary cells from killing by alkylating agents. Proc. Natl. Acad. Sci. USA. 1990;87:2710–2714. doi: 10.1073/pnas.87.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy, D , McGown, A.T , Crowther, D , Mander, A , Fox, B.W Metallothionein levels in ovarian tumours before and after chemotherapy. Br. J. Cancer. 1991;63:711–717. doi: 10.1038/bjc.1991.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritsche, M , Haessler, C , Brander, G Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agent. Oncogene . 1993;8:307–318. [PubMed] [Google Scholar]