Abstract
Background: Antioxidants such as α-tocopherol (vitamin E) and β-carotene (vitamin A) play an important role in protective effect of repeated brief periods of ischemia, namely ischemic preconditioning (IPC). Values of these antioxidants were investigated and compared after induction of ischemia reperfusion (IR) and kidney IPC in both male and female rats. Methods: Forty eight Wistar rats were divided randomly into six groups of 8: groups A and B (male and female controls, respectively), group C (male IR or IR cases), group D (female IR cases) and groups E and F (male and female IPC cases, respectively). In groups C and D, ischemia was induced by clamping of left renal arteries for 45 min. In groups E and F, rats underwent four cycles of 4 min of arterial clamping and 11 min of de-clamping before final 45 min ischemia induction. Afterward, serum was collected to assess the blood urea nitrogen, creatinine and vitamins E and A values. Renal tissues were obtained for histological assessments. Results: α-tocopherol levels in male and female rats showed a significant increase in IPC compared with IR group (P<0.01) and also in female IPC compared with male IPC group. β-carotene levels had no significant variations. Histological evaluation showed that IR-induced renal injuries were less in female rats. Also, protective effects of IPC were more in female rats (P<0.01). Conclusions: Renal IPC reduced damages in both male and female rats, but tissue injuries in females were decreased much more along with the increase of endogenous vitamin E.
Key Words: α-tocopherol, β-carotene, Antioxidant, Reperfusion, Ischemic preconditioning (IPC)
Introduction
Ischemia reperfusion (IR) injury during kidney transplantation is one of the main causes of transplanted kidney dysfunction that leads to acute renal failure and increased rate of acute and chronic rejection [1]. Acute renal failure caused by renal IR, is an important clinical problem that still results in high morbidity and mortality [2, 3], whereas there have been abundant pathophysiologic studies on renal IR injury. Although there are still few specific and definite treatments [4] in accordance to reperfusion pathophysiology, several interventions have been proposed to decrease the injuries. One of these methods is the manipulation of reperfusion process, namely ischemic preconditioning (IPC). In this process, brief and frequent periods of ischemia are induced prior to a long-term ischemia in the target tissue such as kidney. Researchers have shown that the induction of IPC leads to increased resistance to the injuries, caused by subsequent long-term ischemia, and decreases the IR injury [5].
Difference in sex has a role in intensity of various renal diseases including IR injury. In most cases, the progression of disease is faster in males and also the consequences are worse [6]. However, there is no information about sex differences in the IPC of kidney. Knowing this matter is really important, because if there is a significant difference in the IPC of kidney between males and females, it could be much better and logical to consider a specific gender of donors for kidney transplantation. This issue helps to select the donors based on their genders that respond more to the IPC process. In addition, this matter could really help to the success of kidney transplantation approaches and significantly increase the survival of the transplanted kidney. Moreover, the mechanism of IPC is not completely clear.
Several theories have been proposed and one of them is about the role of antioxidants. The most important antioxidant in this context is α-tocopherol or vitamin E that is an endogenous lipid-soluble chain-breaking antioxidant, which is known to protect cells from the diverse actions of free oxygen radicals by donating its hydrogen atom. Another proposed antioxidant in this context is β-carotene or vitamin A that provides a first line of defense against DNA oxidative damage and acts mainly as a chain-breaking antioxidant lipid per-oxidation of unsaturated fatty acids in the cell membrane [7].
In this study, considering the fact that there are sex differences in the renal IR, but there is no evidence about that in the IPC of kidney, we decided to investigate the gender effects or sex differences in the renal IPC. Also, considering the effects of antioxidants on the IPC process, we investigated and compared the levels and the role of vitamins E and A after IPC in both male and female rats.
MATERIALS AND METHODS
Animals. Experiments were performed on sexually mature male and female Wistar rats, weighing 150-200 grams. The animals were kept at the Animal House of Iran University of Medical Sciences (IUMS, Tehran) in the condition of 12 hours lightness and 12 hours darkness with free access to standard rat chow and tap water. All experiments followed the Principles of Laboratory Animal Care, published by the National Society for Medical Research, and the Guide for the Care and Use of Laboratory Animals, prepared by the Academy of Sciences and published by the National Institutes of Health and were approved by the Governmental Committee on Animal Welfare.
General surgical preparation. At first, rats were anesthetized intraperitoneally with ketamine (60 mg/kg) and xylazine (10 mg/kg) [8]. Throughout the anesthesia, the body temperature was monitored by DC temperature controller apparatus (model FHC) by a rectal probe and was maintained using a heating pad at 37C. Temperature control system included: 1) a controlling system, 2) a rectal probe and 3) an electric blanket. This system maintained the body temperature by feedback control. Then, a midline laparotomy was performed and right nephrectomy was performed. After 20 days, the animals were divided into two distinct groups of males and females and each group was divided into three subgroups (8 in each group) as follows: 1) sham group; 2) IR group: 45 min of left renal ischemia followed by reperfusion; 3) IPC group: 4 cycles of 4-min left renal ischemia separated by 11-min reperfusion periods before the final 45 min of left renal ischemia followed by reperfusion. Twenty-four h later, the animals were killed and the renal tissues were extracted for histological assessments. Blood samples were collected from abdominal aorta and serum urea and creatinine levels were measured spectrophoto-metrically using a 704 Hitachi analyzer at 520 nm for BUN and 505 nm for creatinine. Vitamin E and A contents of serum were determined by HPLC as described by Bieri and Tolliver [9]. Briefly, an internal standard, α-tocopheryl acetate (50 µmol/L), was introduced into each serum sample (100µL) in an equal volume of 100% ethanol (10µL). Tocopherols were extracted by the addition of heptane (100µL), followed by vigorous mixing with a vortex mixer. Serum samples were centrifuged to separate the phases, and the organic (top) phase was transferred into a glass test tube. The extracts were evaporated under a stream of nitrogen and then resuspended in diethyl ether (30µL) and methanol (80µL). Fifty milliliters of each extract were injected into the Perkin-Elmer Series 4 HPLC (Norwalk, CT) with a C-18 reverse-phase column (15 cm × 4.6 mm, -3 um; Supelco Inc., Bellefonte, PA). The mobile phase consisted of 100% methanol (flow rate, 1.5 mL/min). Typical retention times were as follows: 3.7 min; α-tocopherol, 4.1 min; α-tocopheryl acetate, 5.0 min. Tocopherols were monitored at 292 nm (Perkin-Elmer LC-75 UV detector). Sample α-tocopherol concentrations were calculated from peak area responses using a standard curve that was established from the chromatography of known amounts of pure α-tocopherol. Standard stock solution of α-tocopheryl acetate (20-200 µmol/L ethanol) was used to establish calibration curves. Linear regression analysis showed that the relationship between peak areas to weight of standard α-tocopherol was linear in this concentration range. The limit of detection with this system was 0.2 µg.
Histological examination. At the end of isolated perfusion, kidney samples were taken for histological evaluation. Kidney slices were fixed in buffered formalin overnight. After automated dehydration through a graded alcohol series, the slices were embedded in paraffin, sectioned at 4 µm and stained with hematoxylin-eosin. Blind analysis of morpho-logical assessment was performed by an expert pathologist, who was unaware of the experimental conditions and treatment that the animal had received. The histological parameters evaluated were tubular necrosis, tubular dilatation, interstitial edema, intracellular edema, and tubular cell brush integrity and cell detachment. A minimum of 8-10 fields for each kidney slide were examined and assigned for severity of changes with original magnification ×20. A morphological study was performed using an Olympus photomicroscope (PROVIS AX70, Japan) equipped with a digital camera (DP11, Japan). Histological lesions were graded based on a semi-quantitative scale: 0, no abnormality; 1, mild lesions affecting 10% of kidney samples; 2, moderate lesions, affecting 25% of kidney samples; 3, severe lesions affecting 50% of kidney samples and 4, extreme lesions affecting more than 70% of kidney samples (Table 1).
Table 1.
Histopathological evaluation of kidney damages in different groups based on scored criteria.
| Location | Criteria | M/IPC | F/IPC | M/IR | F/IR |
|---|---|---|---|---|---|
| Tubule | dilatation | 2 | 1 | 3 | 3 |
| luminal digestion | 2 | 0 | 3 | 2 | |
| nucleus changes | 1 | 1 | 3 | 2 | |
| Glomeruli | focal glomerular fragmentation | 2 | 2 | 3 | 2 |
| space in corpuscle | 3 | 2 | 3 | 2 | |
| General | swelling | 1 | 2 | 3 | 2 |
| Apoptosis | cell shrinkage | 2 | 1 | 3 | 1 |
| chromatin condensation | 2 | 2 | 3 | 1 |
Scoring: 0, none; 1, mild; 2, moderate; 3, severe; 4, extreme. M/IPC, male IPC group; F/IPC, female IPC group; M/IR, male IR group and F/IR, female IR group
Statistical analysis. The data were analyzed using two-way ANOVA. The differences between the groups were considered significant at P<0.01. Dunnett's test was used to compare IR and IPC groups with control group, and least statistical difference test was used to compare IR group with IPC group. All results were presented as mean ± SD.
Results
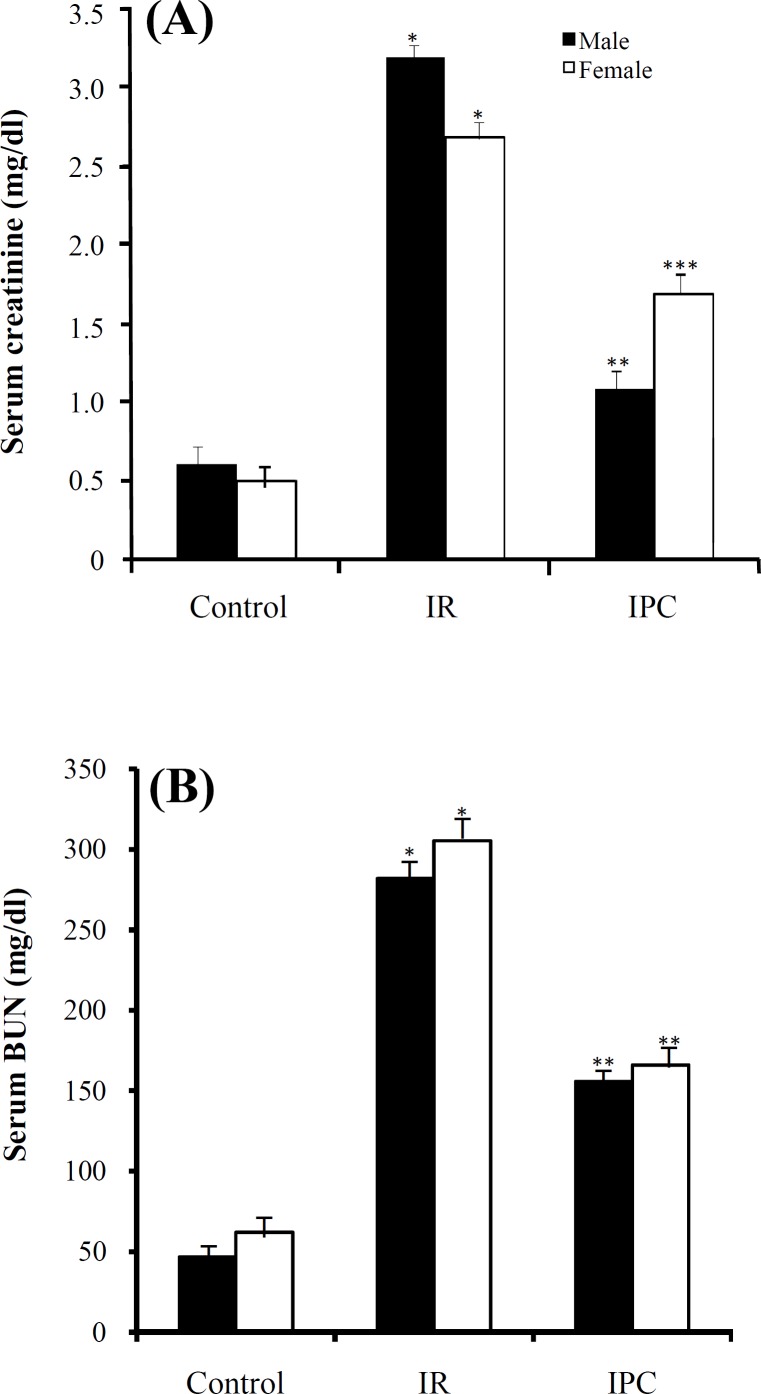
Creatinine changes. In male rats, serum creatinine levels increased significantly in IR group in comparison with control group (P = 0.0001) and decreased significantly in IPC group in comparison with IR group (P = 0.0001). In female rats, the same results were seen (female-IR vs. female-control, P = 0.0001 and female-IPC vs. female-IR, P = 0.002). Creatinine level differences between males and females in the similar groups (male-IPC vs. female-IPC) were not statistically significant (Fig. 1A).
Fig. 1.
Mean ± SD of creatinine (A) and BUN (B) levels in male and female control, ischemia reperfusion (IR) and ischemic preconditioning (IPC) groups. The values are represented as a mean ± SD for 8 rats per group. *Significantly different (P = 0.0001) from the co-gender control group. **Significantly different (P = 0.0001) from co-gender IR group. ***Significantly different (P = 0.002) from co-gender IR group.
Blood urea nitrogen changes. In male rats, serum BUN level showed a significant increase in IR group in comparison with control group (P = 0.0001) and a significant decrease in IPC group in comparison with IR group (P = 0.0001). In female rats, the same results were seen (IR vs. control, P = 0.0001 and IPC vs. IR, P = 0.0001). Differences in BUN level between males and females in the similar groups were not significant (Fig. 1B).
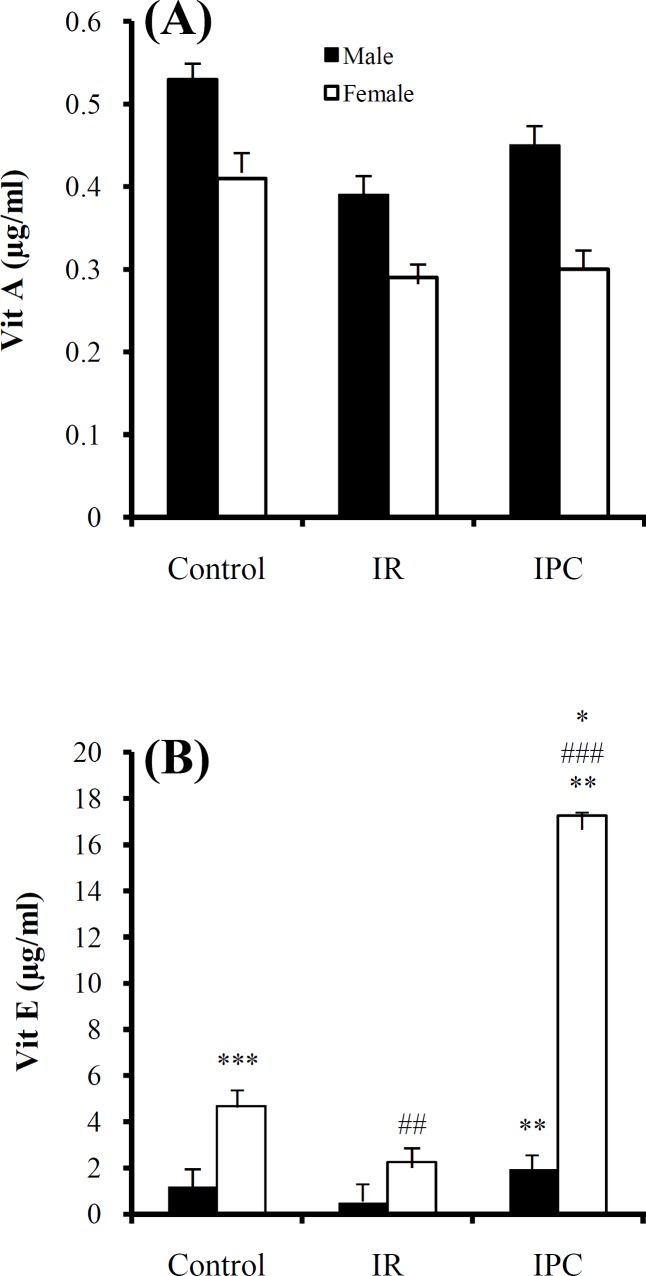
Vitamin A changes. In male rats, serum vitamin A levels did not show significant variations between IR group and control group (P = 0.03) and also between IPC group and IR group (P = 0.3). In female rats, the same results were seen (IR vs. control, P = 0.14 and IPC vs. IR, P = 0.1). Differences in serum vitamin A level between males and females in the similar groups were not statistically significant (Fig. 2A).
Fig. 2.
Mean ± SD of vitamin A (A) and vitamin E (B) levels in male and female control, ischemia reperfusion (IR) and ischemic preconditioning (IPC) groups. The values are represented as mean ± SD for 8 rats per group. *Significantly different (P<0.01) from the co-gender control group. **Significantly different (P = 0.0001) from the co-gender IR group. ***Significantly different (P = 0.001) from the opposite control group. ##Significantly different (P = 0.0001) from the opposite IR group. ###Significantly different (P = 0.0001) from the opposite IPC group.
Vitamin E changes. α-tocopherol as serum vitamin E levels in male rats was increased significantly in IPC group in comparison with IR group (P = 0.0001). In female rats, serum vitamin E levels did not show significant variations between IR and control groups (P = 0.3), but showed a significant increase in IPC group in comparison with the both control and IR groups (both P = 0.0001). Serum vitamin E level differences between males and females in the similar groups were statistically significant as follows: vitamin E value showed a significant increase in female control group in comparison with male control group (P = 0.001), in female IR group in comparison with male IR group (P = 0.0001) and in female IPC group in comparison with male IPC group (P = 0.0001) (Fig. 2B).
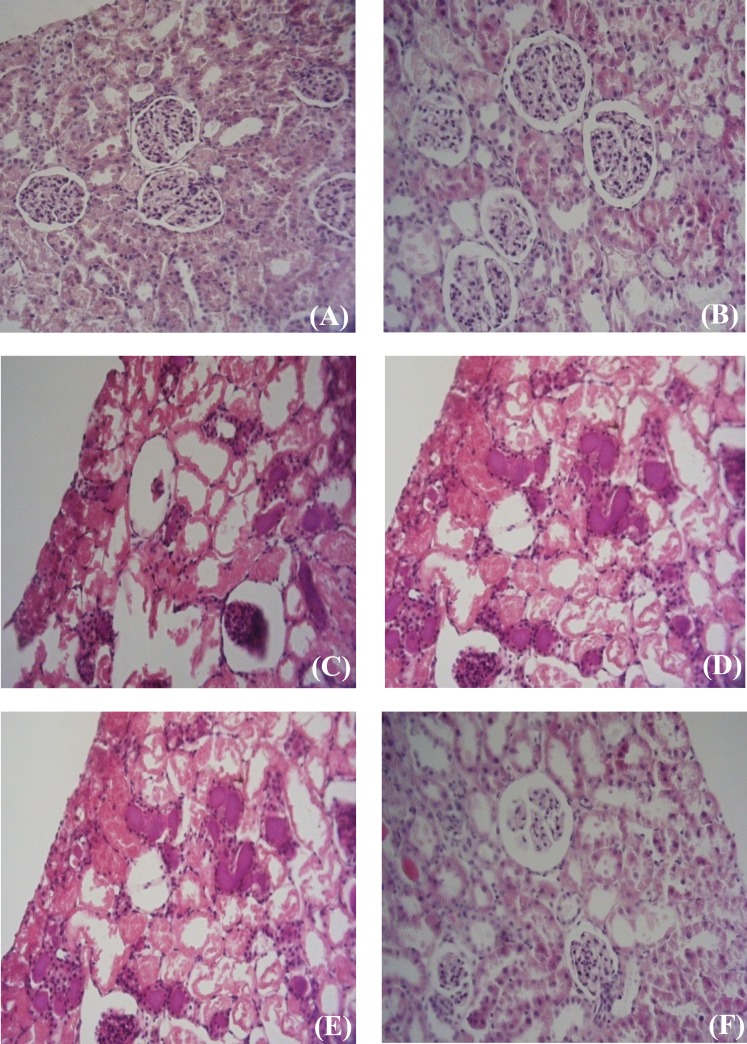
Histological findings. The results of histological examinations in both genders showed that the induction of IPC leads to the improvement in the condition of renal tubules and glomerulus when compared with IR. In controls, we demonstrated normal tubular lumen, glomerular and free space in corpuscle, but in IPC male, demonstrations showed moderate tubular dilatation, focal glomerular necrosis, extent free space in corpuscle, loss of nuclei, predominates over morphological features of apoptosis (chromatin condensation and cell shrinkage), swelling tubular, less lumina congestion, moderate diffuse interstitial edema and dilatation the tubular structure. Also, in IR male, we demonstrated severity in all categories. In IPC females, demonstration showed mild tubular dilatation, focal glomerular necrosis, and extent free space in corpuscle, loss of nuclei, predominates over morphological features of apoptosis (chromatin condensation and cell shrinkage, swelling tubular, lumina congestion, severe diffuse interstitial edema and dilatation of tubular structure. In IR females, we showed severe tubular dilatation, but moderate diffuse interstitial edema and moderate dilatation of the tubular structure (Table 1). Induction of IR in females caused fewer injuries to the kidney in comparison with that in males. Also, the induction of IPC in females led to more obvious improvement when compared with males (Fig. 3).
Fig. 3.
Renal histological microphotographs. Male (A, C and E) and female (B, D and F) kidney sections were taken from sham-operated rats groups (A and B) or ischemic-reperfusion groups (C and D) or ischemic-preconditioned groups (E and F). Sham-operated; control group are normal kidney tissues, normal histological characteristic of glomeruli and tubules and corpuscle’s space were present in male (A) and female (B). In male ischemic-preconditioned group (E), there was moderate tubular dilatation with loss of nuclei in most of tubule, without swelling, luminal congestion (e.g., moderate diffuse interstitial edema and moderate dilatation the tubular structure), focal glomerular necrosis and extent free space in corpuscle and predominates over morphological features of apoptosis (e.g., severe chromatin condensation and cell shrinkage). In female Ischemic-preconditioned group (F), there was mild tubular dilatation with loss of nuclei in some of tubule, with swelling, luminal congestion (e.g. mild diffuse interstitial edema and mild dilatation the tubular structure), focal glomerular fragmentation and extent free space in corpuscle and predominates over morphological features of apoptosis (e.g., moderate chromatin condensation and mild cell shrinkage). In male ischemic-reperfusion group (C), there was severe tubular dilatation, focal glomerular necrosis, and extent free space in corpuscle, loss of nuclei, chromatin condensation and cell shrinkage. In female ischemic-reperfusion group (D), there was severe tubular dilatation, moderate focal glomerular necrosis, and moderate extent free space in corpuscle, moderate loss of nuclei, mild chromatin condensation and mild cell shrinkage.
The overall results of this research show that after induction of IPC in both genders, the kidney functioned better in IPC group in comparison with IR group. Considering the increase of vitamin E in IPC process, we could conclude that vitamin E is effective in the IPC especially in female rats. Also, histological examinations confirm that the induction of IPC decreases the IR injuries more efficiently in females rather than males.
Discussion
The major new finding of this study was that an IPC protocol improved post-ischemic structural recovery in kidney in both female and male rats, but this recovery was more significant in females. IPC is a powerful protective mechanism against ischemic injury in a variety of organ systems, including the heart, brain, spinal cord, retina, liver, lung and skeletal muscles. Also, this phenomenon has protective effects against IR injuries [8]. The difference of IPC effects between male and females has not been assessed very much. Pitcher et al. [10] investigated this difference in the myocardium tissue and results showed that IPC has more protective effects in females rather than males. Also, Song et al. [11] showed that IPC has more protective effects on female mice and showed that these effects disappeared after gonadectomy. Sex difference in the IR injuries has been studied in diverse organs and in most organs like brain, heart and splanchnic, females were more resistant to injuries [12-14]. In contrast, Gasbarrini et al. [15] showed that the liver was more vulnerable to IRI in females rather than males; which resulted in poor outcome of liver transplantation with female donors. Muller et al. [7] revealed that IR injuries in kidney are less in female rather than male rats and also showed that the injuries were decreased after in fertilizing of male rats; probably due to androgen reduction. In two review articles [6, 16], it was showed that the rate of progression of renal disease in males is much more rapid than that in females. Also, there had been worse outcome in chronic renal disease in males. This might be due to differences in kidney structure, glomerular hemodynamic responses to stress and the direct cellular effects of sex hormones. Interestingly, selective estrogen receptor modulators such as raloxifen have shown some renoprotective effects in animals and human [7, 8]. This confirms the beneficial effects of estrogen on kidney. Kher et al. [17] suggested that females are more protected against renal IR injuries in comparison with males. Our study also confirms these findings.
The mechanism of preconditioning still remains obscure with several unknown facts with respect to the intracellular signaling pathways triggered [18]. The opening of mitochondrial KATP channels, with subsequent generation of reactive oxygen species, is considered to be a pivotal step in the mechanism of preconditioning [19]. It is of interest that nitric oxide, guanylate cyclase, cyclic guanyl-mono-phosphate and protein kinase G are significant mediators, which result in the opening of mitochondrial KATP channels [17]. Researchers showed that infusion of free radical scavengers, superoxide dismutase and N-2-mercaptopropionyl glycine prevents preconditioning protection in rabbits and rats [20]. Also, another study revealed that ascorbic acid, which is known to scavenge oxygen-derived free radicals, has also been shown to abolish the benefit of IPC in pigs [21]. Vitamin E as a powerful antioxidant that prevents lipid peroxidation and disruption of membrane integrity has had beneficial effects in the IPC of heart by increasing cGMP [22]. The mediators involved in the mechanism of preconditioning have been extensively investigated in many studies with the use of various models [19]. Another study suggests that prescription of both vitamin E and KATP channel inhibitors reduced the IR injury in the rabbit myocardium [22]. However, regardless of the experimental model, there is a great deal of evidence that intracellular free radicals play a pivotal role, as triggers or mediators, in the observed protection [23]. Apart from its antioxidative properties, vitamin E alters several intracellular agents, interferes in enzymatic activities, promotes apoptosis, and contributes to novel gene expression as reported recently [24-26].
Vitamin E increases NO-dependent relaxation [27]. Also, there are evidences about vitamin E effects on the free radicals [23]. Thus, with respect to free radical generation during preconditioning, it appears that vitamin E does not interfere with lipid peroxidation products, which are elevated by the opening of mitochondrial KATP channels, and therefore does not block the protective effect of preconditioning. Muzakova et al. [28] suggested that in patients with myocardial infarction, the level of antioxidants like vitamins E and A decrease that might be the result of vitamin consumption during ischemia-induced increase in malondialdehyde levels. In spite of these results, Charniot et al. [29] showed that these antioxidants had a usual value during acute heart failure.
In this study, we compared the impact of IR in the kidney between male and female rats and results were the same as other studies like the study of Kher et al. [17]. Also, we compared the effect of IPC in the kidney between male and female rats, and found out that IPC is effective in both genders. Previous studies were conducted on male rats and had not investigated the females, but in this study we investigated both male and female rats and compared them [30]. Results showed that there was no significant difference between IR and IPC groups in the kidney function tests such as BUN and creatinine, but in the histological examinations, we found that IR injuries were significantly less in females rather than males and also the protective effects of IPC were more pronounced in females. Vitamin E levels showed significant increase in females in comparison with males after IPC. Nonetheless, in male rats, vitamin E levels were significantly increased in IPC group in comparison with IR group. Unlike these results, we observed no variation in vitamin A levels, because it may release gradually and needs time to be increased in the body, whereas we took samples just 24 hours after our intervention. Our results show that IPC decreases BUN and creatinine levels in both male and female rats; however there was no significant difference between males and females.
In this study, we concluded that after the induction of IPC for kidney in female rats, the value of endogenous vitamin E increased and the histological injuries decreased.
References
- 1.Molitoris BA. Ischemic acute renal failure: Exciting times at our fingertips (editorial) Curr Opin Nephrol Hypertens. 1998 Jul;7(4):405–6. doi: 10.1097/00041552-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Zhao JL, Yang YJ, Cui CJ, You SJ, Gao RL. Pretreatment with simvastatin reduces myocardial no-reflow by opening mitochondrial K (ATP) channel. Br J Pharmacol. 006 Oct;149(3):243–9. doi: 10.1038/sj.bjp.0706862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004 Nov;364(9447):1814–27. doi: 10.1016/S0140-6736(04)17406-0. [DOI] [PubMed] [Google Scholar]
- 4.Yokota N, O'Donnell M, Daniels F, Burne-Taney M, Keane W, Kasiske B, et al. Protective effect of HMG-CoA reductase inhibitor on experimental renal ischemia-reperfusion injury. Am J Nephrol. 2003 Jan-Feb;23(1):13–7. doi: 10.1159/000066301. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002 Jan;11(1):43–8. doi: 10.1097/00041552-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000 Feb;11(2):319–29. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 7.Muller V, Losonczy G, Heemann U, Vannay A, Fekete A, Reusz G. Sexual dimorphism in renal ischemia-reperfusion injury in rats: Possible role of endothelin. Kidney Int. 2002 Oct;62(4):1364–71. doi: 10.1111/j.1523-1755.2002.kid590.x. [DOI] [PubMed] [Google Scholar]
- 8.Torras J, Herrero-Fresneda I, Lioberas N, Riera M, Ma CruzadoJ, Ma GrinyoJ. Promising effects of ischemic preconditioning in renal transplantation. Kidney Int. 2002 Jun;61(6):2218–27. doi: 10.1046/j.1523-1755.2002.00360.x. [DOI] [PubMed] [Google Scholar]
- 9.Bieri JG, Tolliver TJ. On the occurrence of α-tocopherolquinone in rat tissue. Lipids. 1981 Oct;16(10):777–9. doi: 10.1007/BF02535351. [DOI] [PubMed] [Google Scholar]
- 10.Pitcher JM, Wang M, Tsai BM, Kher A, Turrentine MW, Brown JW, et al. Preconditioning: Gender Effects. J Surg Res. 2005 Dec;129(2):202–20. doi: 10.1016/j.jss.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Song X, Li G, Vaage J, Valen G. Effects of sex, gonadectomy, and oestrogen substitution on ischemic preconditioning and ischemia-reperfusion injury in mice. Acta Physiol Scand. 2003 Apr;177(4):459–66. doi: 10.1046/j.1365-201X.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 12.Hale SL, Birnbaum Y, Kloner RA. β-estradiol, but not α-estradiol, reduces myocardial necrosis in rabbits after ischemia and reperfusion. Am. Heart. J. 1996;132:258–62. doi: 10.1016/s0002-8703(96)90419-6. [DOI] [PubMed] [Google Scholar]
- 13.Kim YD, Chen B, Beauregard J, Kouretas P, Thomas G, Farhat MY, et al. 17 β-estradiol prevents dysfunction of canine coronary endothelium and myocardium and reperfusion arrhythmias after brief ischemia-reperfusion. Circulation. 1996;94:2901–8. doi: 10.1161/01.cir.94.11.2901. [DOI] [PubMed] [Google Scholar]
- 14.Squadrito F, Altavilla D, Squadrito G, Campo GM, Arlotta M, Arcoraci Vetal. The involvement of tumor necrosis factor- α in the protective effects of 17 β-oestradiol in splanchnic ischemia-reperfusion injury. Br J Pharmacol. 1997 Aug;121(8):1782–8. doi: 10.1038/sj.bjp.0701288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasbarrini A, Addolorato G, Di CampliC, Simoncini M, Montemagno S, Castagneto M, et al. Gender affects reperfusion injury in rat liver. Dig Dis Sci. 2001 Jun;46(6):1305–12. doi: 10.1023/a:1010679716435. [DOI] [PubMed] [Google Scholar]
- 16.Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5((Suppl 1)):S3–10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia–reperfusion injury. Cardiovasc Res. 2005 Sep;67(4):594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg O, Qin Q, Krieg T, Yang XM, Philipp S, Critz SD. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoKATP channel opening and leads to cardioprotection. Am J Physiol Heart Circ Physiol. 2004 Jan;286(1):H468–76. doi: 10.1152/ajpheart.00360.2003. [DOI] [PubMed] [Google Scholar]
- 19.Schulz R, Cohen MV, Behrends M, Downey JM, Heusch G. Signal transduction of ischemic preconditioning. Cardiovasc Res. 2001 Nov;52(2):181–98. doi: 10.1016/s0008-6363(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 20.Patel HH, Hsu A, Moore J, Gross GJ. BW373U86, a delta opioid agonist, partially mediates delayed cardio-protection via a free radical mechanism that is independent of opioid receptor stimulation. J Mol Cell Cardiol. 2001 Aug;33(8):1455–65. doi: 10.1006/jmcc.2001.1408. [DOI] [PubMed] [Google Scholar]
- 21.Skyschally A, schulz R, Gres P, Korth H, Heusch G. Attenuation of ischemic preconditioning in pigs by scavenging of free oxyradicals with ascorbic acid. Am J Physiol Heart Circ Physiol. 2003 Feb;284(2):H698–703. doi: 10.1152/ajpheart.00693.2002. [DOI] [PubMed] [Google Scholar]
- 22.Ingold KU, Webb AC, Witter D, Burton GW, Metcalfe TA, Muller DP. Vitamin E remains the major lipid-soluble, chain-breaking antioxidant in human plasma even in individuals suffering severe vitamin E deficiency. Arch Biochem Biophys. 1987 Nov;259(1):224–5. doi: 10.1016/0003-9861(87)90489-9. [DOI] [PubMed] [Google Scholar]
- 23.Andreadou I, Iliodromitis EK, Tsovolas K, Aggeli IK, Zoga A, Gaitanaki C, et al. Acute administration of vitamin E triggers preconditioning via K(ATP) channels and cyclic-GMP without inhibiting lipid peroxidation. Free Radic Biol Med. 2006 Oct;41(7):1092–9. doi: 10.1016/j.freeradbiomed.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Ricciarelli R, Zingg J, Azzi A. Vitamin E: protective role of a Janus molecule. FASEB J. 2001 Nov;15(13):2314–25. doi: 10.1096/fj.01-0258rev. [DOI] [PubMed] [Google Scholar]
- 25.Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002 Oct;76(4):703–16. doi: 10.1093/ajcn/76.4.703. [DOI] [PubMed] [Google Scholar]
- 26.Rimbach G, Minihane AM, Majewicz J, Fischer A, Pallauf J, Virgli F, et al. Regulation of cell signaling by vitamin E. Proc Nutr Soc. 2002;61:415–25. doi: 10.1079/pns2002183. [DOI] [PubMed] [Google Scholar]
- 27.Car AC, Zhu BZ, Frei B. Potential antiatherogenic mechanisms of ascorbate (vitamin C) and α-tocopherol (vitamin E) Circ Res. 2000 Sep;87(5):349–54. doi: 10.1161/01.res.87.5.349. [DOI] [PubMed] [Google Scholar]
- 28.Muzakova V, Kandar R, Vojtisek P, Skalicky J, Vankova R, Cegan A. Antioxidant vitamin levels and glutathione peroxidase activity during ischemia-reperfusion in myocardial infarction. Physiol Res. 2001;50(4):389–96. [PubMed] [Google Scholar]
- 29.Charniot JC, Vignat N, Albertini JP, Bogdanova V, Zerhouni K, Monsuez JJ, et al. Oxidative stress in patients with acute heart failure. Rejuvenation Res. 2008 Apr;11(2):393–8. doi: 10.1089/rej.2008.0663. [DOI] [PubMed] [Google Scholar]
- 30.Kadkhodaee M, Aryamanesh S, Faghihi M, Zahmatkesh M. Protection of rat renal vitamin E levels by ischemic-preconditioning. BMC Nephrol. 2004 Apr;5:6–13. doi: 10.1186/1471-2369-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]