Abstract
Background: Alzheimer’s disease (AD) is characterized by progressive neuronal loss in hippocamp. Epidermal neural crest stem cells (EPI-NCSC) can differentiate into neurons, astrocytes and oligodendrocytes. The purpose of this study was to evaluate the effects of transplanting EPI-NCSC into AD rat model. Methods: Two weeks after induction of AD by injection of Amyloid-β 1-40 into CA1 area of rat hippocamp, Y-maze and single-trial passive avoidance tests were used to show deficit of learning and memory abilities. EPI-NCSC were obtained from the vibrissa hair follicle of rat, cultured and labeled with bromodeoxyuridine. When Alzheimer was proved by behavioral tests, EPI-NCSC was transplanted into CA3 area of hippocamp in AD rat model. The staining of EPI-NCSC markers (nestin and SOX10) was done in vitro. Double-labeling immunofluorescence was performed to study survival and differentiation of the grafted cells. Results: We showed that transplanted EPI-NCSC survive and produce many neurons and a few glial cells, presenting glial fibrillary acidic protein. Total number of granule cells in hippocamp was estimated to be more in the AD rat model with transplanted cells as compared to AD control group. We observed that rats with hippocampal damage made more errors than control rats on the Y-maze, when reward locations were reversed. Conclusion: Transplanted cells were migrated to all areas of hippocamp and the total number of granule cell in treatment group was equal compared to control group. Transplantation of EPI-NCSC into hippocamp might differentiate into cholinergic neurons and could cure impairment of memory in AD rat model.
Key Words: Alzheimer’s disease, Cholinergic neuron, Hair follicle
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with characteristic clinical and pathological features, but with individual variations for age of onset and pattern of cognitive impairment [1]. No case of AD has been detected under the age of 20, but for people 85 years and older, the incidence is about 30% [2-4]. With each passing year, about four million people in the world develop dementia [2, 5]. Today, it affects nearly 30 million people in the whole world. As the average population increases, the number of AD patients is expected to rise exponentially and there will be 110 million AD patients in the world in 2050 [6]. The characteristic features of AD brains are formation of neurofibrillary tangles and the presence of amyloid plaques. However, the neurotoxicity is believed to be responsible for the neuronal loss and the degeneration of the cholinergic system in AD patients [7, 8]. AD is characterized by neuronal and synaptic loss throughout the brain, involving the basal forebrain cholinergic system, amygdala, hippocamp and several cortical areas [9-11]. Although AD massive neuronal loss occurs only in very few brain structures such as the hippocampal CA1 and CA2 regions, large parts of the brain, the entorhinal cortex and the locus coeruleus, are affected by pathological alterations and decreased neuronal metabolism [12]. Current treatments bring only temporary symptomatic relief and do not halt the progression of this disease [13]. AD is also one of the candidate diseases for development of cell-replacement therapy, because transplantation of cells lacking the AD causing mutation can help to replace the lost neurons and reconstitute damaged neuronal connections [14].
Epidermal neural crest stem cells (EPI-NCSC) are multipotent remnants of embryonic NCSC in a postnatal location, the bulge of hair follicles. Sieber-Blum et al. [15] described NCSC in the bulge region of adult murine whisker follicle and designated them as EPI-NCSC. The method of isolation was based on the emigration of cells from explanted bulge region. Emigrated cells expressed SOX10, a marker of neural nrest [16, 17], and intermediate filament protein nestin, a marker of immature and undifferentiated cells [18, 19]. Nestin is also expressed in some cells in the bulge of hair follicle [20, 21]. EPI-NCSC were serially cultured under conditions that favored differentiation, they showed a broad potential for generating cells which express markers appropriate for neurons, glias, smooth muscle cells, chondrocytes and melanocytes. EPI-NCSC responded to neuregulin-1 and BMP2 by generating Schwann cells and chondrocytes, respectively [15, 22].
In the present study, we tried to assess whether the EPI-NCSC grafted in the hippocamp of AD rat model can differentiate to neurons or cholinergic neurons that impaired histology of the cholinergic system after including a lesion in hippocamp. In addition, we focused on spontaneous alternation of AD rat model in Y-maze, which evaluates spatial working memory according to the previous reports.
MATERIALS AND METHODS
Animals and housing conditions. All animal experiments were carried out according to the Guidelines of the Iranian Council for Use and Care of Animals and approved by the Animal Research Ethical Committee of Tehran University of Medical Sciences (Tehran, Iran). Male Wistar rats (n = 40, 250-300 g of body weight) were purchased from the Animal Center of Iran Medical University (Tehran). All rats were maintained in a temperature-controlled environment of 24 ± 1°C with a 12 h dark/light cycle (dark cycle: 8:00 P.M. to 8:00 A.M.) with free access to water and food. The rats were randomly divided into four groups: A) Control group (intact); B) AD group (injected with Amyloid-β 1-40 [Aβ1-40] protein); C) Sham group (injected with deionized/distilled water instead of Aβ1-40 protein) and D) EPI-NCSC-treated group (AD rats receiving EPI-NCSC).
Alzheimer's disease rat model . Synthetic Aβ1-40 amyloid protein (Sigma-Aldrich, USA) was dissolved in deionized/distilled water at 2 nmol/µl, stored at -70C and incubated at room temperature for 2 h before use. Rats were anesthetized with intraperitoneal injection of 10% ketamine (60 ml/kg) and 1% xylazine (20 mg/kg) (both from Alfasan, Woerden, Holland) and mounted in a stereotaxic apparatus (Stoelting Co., USA) for localization of the hippocamp. An incision was made into the scalp and the cranium drilled through with a mini-drill to a depth of 2.6 mm. The point of CA1 region of hippocamp was localized at 2.0 mm lateral and -3.8 mm anterior to the posterior fontanel, followed by Paxinos and Watson atlas. In AD sham group, 4 µl of deionized/distilled water was injected bilaterally into each hippocamp using a 26-gauge needle connected to a micro syringe (Hamilton) over a period of 12 min. The needle was slowly withdrawn after the injection.
Bulge explants and epid ermal neural crest stem cell culture. Bulge explants from whisker follicles of 7-10-week-old Wistar rat were prepared, dissected, cleaned and cut longitudinally and then transversely below and above the bulge region. The bulges were then rolled out of the capsule and placed into collagen-coated culture plates, where they adhered to the substratum within 24 h. Three to four days post-implantation, cells started to emigrate from the bulge explants onto the culture substratum. The culture medium was consisted of 3:1 supplemented mixture medium DMEM/F12 containing 5% fetal bovine serum, antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin and 0.5 µg/ml fungizon), 10 ng/ml epidermal growth factor (Sigma-Aldrich, USA), 10-9 M cholera toxin (Sigma-Aldrich, USA), 0.5 mg/ml hydrocortisone and 5 μg/ml insulin. Three to four days post onset of emigration, bulges were removed and the adhering cells were detached by trypsin treatment and subsequently subcultured (Figs. 1 and 2) [23].
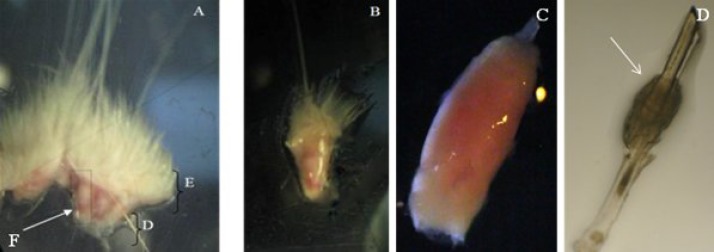
Fig 1.
Isolation and explanation of hair follicle bulge. (A) Whisker pad of rat. E, epidermis; D, dermis and F, follicle (×100); (B) isolated hair follicle (×100); (C) dissected and cleaned hair follicle (×400) and (D) bulge of hair follicle rolled in capsule (×400). Arrows show the bulge.
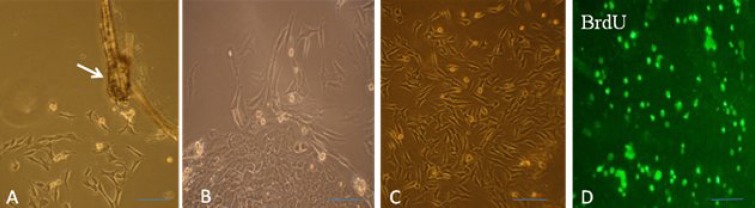
Fig. 2.
Characteristics of epidermal neural crest stem cell (EPI-NCSC) and phase contrast microscopy. (A) Explants 20 h after onset of EPI-NCSC emigration. Migratory cells are present on the collagen substratum; (B) colony formation; (C) proliferation and expanded EPI-NCSC and (D) EPI-NCSC were labeled with BrdU prior to transplantation. Arrow shows the bulge (scale bar = 200 µm).
Immunocytochemistry. Ten days after isolation and culture, cells passaged and plated on collagen-coated cover slips overnight, washed in PBS for 3 × 5 and fixed in 4% paraformaldehyde (PFA) for 10 min. Thefixed cells were washed in PBS for 3 × 5 and incubated in blocking buffer (10% goat serum, Sigma-Aldrich, USA and 0.3% Triton X-100, Fluka, USA) at room temperature for 30 min. They were then incubated at 4°C overnight with the following primary antibodies: mouse anti-nestin monoclonal antibody (1:200, Millipore, USA), mouse anti-SOX10 monoclonal antibody (1:200, Sigma-Aldrich, USA), monoclonal anti β-III tubulin antibody (1:400, Sigma-Aldrich, USA) and monoclonal anti-GFAP (glial fibrillary acidic protein) antibody (1:1000, Invitrogen, USA). The next day, the cells were rinsed for 3 × 5 min to remove unbound primary antibodies. Subsequently, they were incubated at room temperature for 2 h with the following secondary antibodies: goat anti-mouse FITC-conjugate IgG (1:200, Sigma-Aldrich, USA) and Alexa Fluor 546-conjugated goat anti-mouse (1:400, Invitrogen, USA). Cell nuclei were counterstained in PBS with 1 μg/ml diamidino pheylindole dihydro-chloride Sigma-Aldrich, USA) in the dark at room temperature for 1 min. After washing, the cover slips were removed from the 6 wells, mounted on a slide with mounting media and visualized using a fluorescence microscope. To examine the specificity of the nestin antibody, 3T3 fibroblast-like cells were used as negative control cells (Pasteur Institute of Iran, Tehran). For morphological studies, labeled cells were identified using an Olympus photomicroscope (PROVIS AX70, Japan), equipped with a digital camera (DP11, Japan).
Bromodeoxyuridine (BrdU) labeling. BrdU, a thymidine analogue that is incorporated into the DNA of dividing cells during S phase, was used to label newly synthesized DNA. Forty eight to 72 h before cell transplantation, BrdU (5 µmol/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to flask of cultured cells. For checking cell labeling with BrdU, 48 h after cell labeling, the labeled cells that had been on collagen-coated cover slips were washed in PBS for 3 × 5 min and fixed in 4% PFA for 10 min. Then, the fixed cellswere washed in PBS for 3 × 5 min and incubated in 2N HCL at 60C for 45 min and washed 2 times in 0.1 M borate buffer (pH 8.3). After washing in blocking buffer (10% goat serum, Sigma-Aldrich, USA/0.3% Triton X-100 Fluka, USA and 1% BSA) at room temperature for 60 min, the incubated cells were again incubated with the primary antibody anti-BrdU (1:500, Sigma-Aldrich, USA) at 4°C overnight. The next day, the cells were rinsed in PBS for 3 × 5 min to remove unbound primary antibodies. Subsequently, they were incubated at room temperature for 1 h with secondary antibody: goat anti-mouse FITC conjugate IgG (1:200, Abcam, Cambridge, UK), washed in PBS for 3 × 10 min, mounted with mounting media and visualized using a fluorescence microscope. (Fig. 2D).
Transplantation of epi dermal neural crest stem cells . Fourteen days after induction of AD model, Y-maze and single-trial passive avoidance tests were carried out. Then, EPI-NCSC were injected bilaterally into rats hippocampus of EPI-NCSC-treated group (a total of 200-300,000 cells were injected into each hippocamp in a total of 4 µl solution containing 50-60,000 cells per µl). The site of injection was CA3 region with depth of 3.8 mm, 2.6 mm lateral and -4.30 mm anterior to posterior fontanel, followed by Paxinos and Watson atlas.
Behavioral measurements:
Y-maze task . Rats were examined in the behavioral assessment Y-maze test, initiated 14 day after Aβ injection and 4 weeks after cell delivery. All testing was carried out from 3 p.m. to 6 p.m. Behavioral testing was conducted in an enclosed Plexiglas Y-maze. The Y-maze is a three-arm horizontal maze with 40 cm long, 30 cm high and 15 cm wide in which the arms are symmetrically disposed at 120° angles from each other. The maze floor and walls were constructed from dark opaque polyvinyl plastic as described previously. Rats were initially placed within one arm, and the sequence and number of arm entries were recorded manually for each rat over an 8-min period. Alternation was defined as successive entries into the three arms on overlapping triplet sets. The alternation percentage was calculated as the ratio of actual to possible alternations (defined as the total number of arm entries minus two) ×100. The number of arm entries serves as an indicator of locomotors activity [24].
Single-trial passive avoidance test: This test was performed 16 days post surgery and 4 weeks after cell delivery.
Apparatus. The apparatus for the step-through passive inhibitory avoidance test (BPT Co., Tehran, Iran) consisted of an illuminated (base side, 20 × 20 cm; floor side, 13.5 × 10 cm and height 30 cm) and a dark (base side, 20 × 20 cm; floorside, 15.5 × 10 cm and height 30 cm) compartments. These two compartments were divided by a wall that had either a guillotine door or hole (5-10 cm) that connected them. The dark compartment had a removable cover made of the same material. A lamp (20 W, positioned 20 cm above the apparatus) was used to illuminate the side of the light compartment.
Procedure. The test was conducted on 4 consecutive days. All rats were adapted to the apparatus on the first and second days of testing for 5 min. In the acquisition trial, the rats were gently placed into the illuminated compartment, facing away from the dark compartment. Then, the door to the dark compartment was opened and the rats were allowed to step into it with all four paws. On the third day, the rats individually entered the light compartment and remained there for 2 min then the guillotine door was opened and the rat entered the dark compartment and received a 2 s, 1 mA electric foot shock. To retest, 24 h later, each rat was again placed in the light compartment. The latency to step through the dark compartment (maximum 600 s) was measured and recorded as index for the passive avoidance behavior. The behavioral observations were carried out between 12:00 M.D. to 15:00 P.M. [25].
Histological procedure. Animals were anesthetized with ketamine (100 mg/kg) and xylazine (20 mg/kg) mixture 4 weeks after cell transplantation and perfused transcardially with 0.9% saline, followed by 4% PFA. Brains were post-fixed in 4% PFA in phosphate buffer overnight. Then, the brains were removed and fixed in the similar solution for 24 h. Following routine processing in paraffin embedded, a total of 16 coronal brain sections (8 μm) were collected from each brain with a rotary microtome (Leitz, 1512, Germany). Every section was spaced by 5 sections to ensure that the examined sections were at a similar level between control and experimental rats. For cell count, neuron numbers were counted in all regions of molecular layer cells in the CA1 region of hippocamp on Nissl stained in the light microscope (magnification ×40). The results were expressed as density (number/mm2) and calculated as percentage of control rats. One of the five sections of each rat was subjected to modified Bielschowsky staining to determine amyloid plaques, neurofibrillary tangles and Nissl dye staining for cell count. Some brain sections from all transplanted animals were stained by hematoxilin and eosin to assess possible tumor formation.
Immunohistochemistry. Paraffin sections were stained with Envision G12 double-stain system kit (DAKO, USA), according to the kit protocol. Following primary antibodies were used in this protocol: monoclonal anti β-III tubulin antibody (1:400, Sigma-Aldrich, USA), monoclonal anti-GFAP antibody (1:1000, Invitrogen, USA), monoclonal anti-ChAT antibody (1:200, Sigma-Aldrich, USA) and monoclonal anti-BrdU antibody (1:500, Sigma-Aldrich, USA). The secondary antibodies in this protocol were horse reddish peroxidase and alkaline phosphatase that were detected by diaminobenzidine (DAB) and permanent red staining.
Statistical analysis. Results were expressed as mean ± S.E.M. in the passive avoidance task and Y-maze task. Data were analyzed by one-way analysis of variance (ANOVA), followed by post hoc analysis, and Student’s t-test was used whereas it was appropriate. Differences were considered significant at the level of P<0.05.
Results
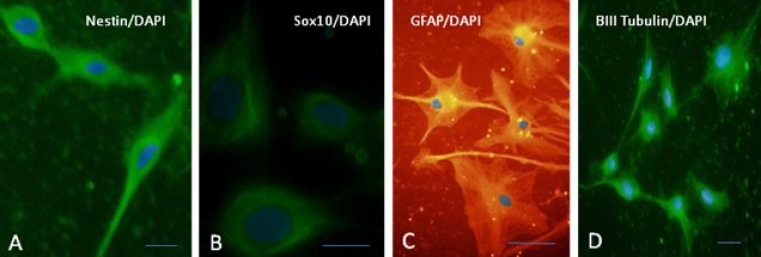
Epidermal neural crest stem cell are self-renewing and multipotent. We first characterized the EPI-NCSC used in this study in vitro to determine if they are capable of self-renewal and multipotent; giving rise to all neural lineages (neurons and glias). Immuno-histochemical analysis of undifferentiated EPI-NCSC revealed coexpression SOX10 and nestin, well-established markers of these hair follicle stem cells (Fig. 3). In contrast, adding mitogen and neuronal differentiation induces expression of neuronal and glial markers (Fig. 3). Therefore, the EPI-NCSC used represent multipotent, self-renewing stem cells.
Fig. 3.
Epidermal neural crest stem cell (EPI-NCSC) multipotency and self-renewing. (A) Expression of immature and undifferentiated cells marker, nestin; (B) expression of neural crest marker, SOX10. EPI-NCSC differentiated into neuron and glia in vitro; (C) expression of marker of glias (glial fibrillary acidic protein) and (D) expression of neuron-like marker (β-ІІІ tubulin). Nuclei were counterstained using diamidino pheylindole dihydrochloride (scale bar = 100 µm).
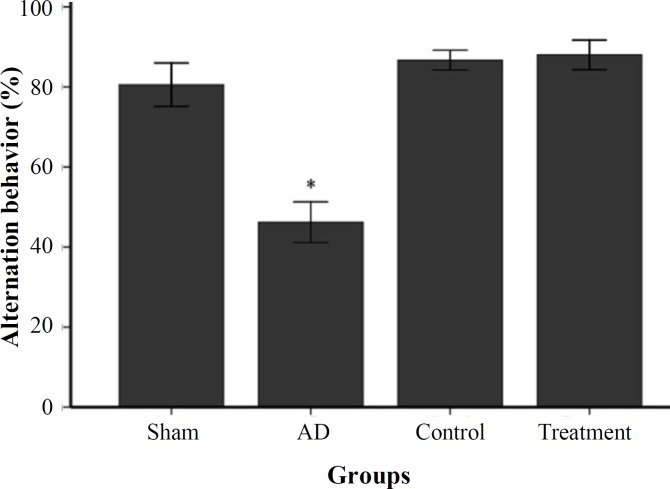
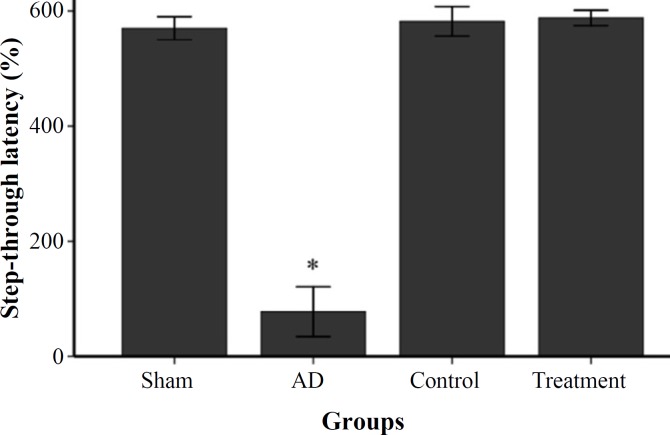
Transplanted NSC rescue cognitive deficits . We determined cognition deficit in rats with well-established plaque and tangle pathology and behavioral deficits. In this study, short-term spatial memory was examined by Y-maze task. AD group showed a significant reduction in alternation behavior compared to control and sham groups (P<0.05, Fig. 4). There was no significant difference between sham and control groups. In passive avoidance test, the AD group rats indicated a significant damage in retention. The mean of acquisition in passive avoidance test showed a non-significant decrease in sham group compared to control group. This result exhibited significantly decreased acquisition to passive avoidance response in AD group compared to control and sham groups (P<0.05, Fig. 5). One month after EPI-NCSC delivery, rats were again habituated, trained, and tested on 2 hippocampal-dependent behavioral tasks. As shown in Figures 4 and 5, the learning and memory impairments rescued in treatment group and was significant compared to AD group.
Fig. 4.
Percent of alternation behavior in Y-maze task in studied groups (mean ± SEM). * shows P<0.05 in AD group as compared to sham, control and treatment groups.
Fig. 5.
Single-trial passive avoidance test. Step-through latency of control, sham and treatment groups compared to AD group, *P<0.05.
However, there was no significant difference among sham, control and treatment groups. The mean of acquisition in passive avoidance test showed a non-significant decrease in sham and treatment groups compared to control group. In addition, static evaluation showed that the mean of animal entrance to arms in Y-maze test in four groups (sham group, 17.8; control group, 18.6; AD group, 17 and treatment group, 18) was not significant.
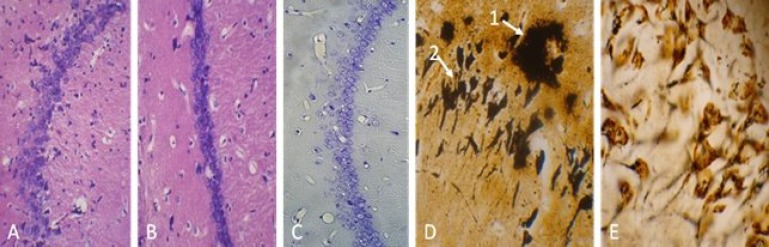
Histological procedure showed pathology characterization of Alzheimer and the cell replacement after EPI-NCSC delivery. In this study, amyloid plaques were detected in the cerebral cortex and hippocamp by Bielschowsky silver staining. The sizes of these plaques were smaller than 210 µm. Also, neurofibrillary tangles were seen in AD and transplantation groups, but not in other groups. Furthermore, Aβ pathology did not alter in the treatment group (Fig. 6D and 6E) and reductions in the neuronal populations were observed in hippocamp. Nissl-stained neuronal densities indicated that AD group had consistently reduced the neuron densities 55.6% in the CA1. An increase in the number of neurons was observed after transplantation, though not up to its normal level (Fig. 6C). Furthermore, hematoxilin and eosin staining revealed that EPI-NCSC did not form tumor, because there were not hypertrophic and dividing nuclei in all transplanted rats 4 week after transplantation (Fig. 6A and 6B).
Fig. 6.
Histology study of hippocamp and hematoxilin and eosin staining. (A) Control group and (B) treatment group did not show hypertrophic and dividing nuclei 4 week after cell transplantation (tumor formation) ( ×400). (C) Nissl staining for cell count in CA1 area (×400). (D) Bielschowsky staining, amyloid plaques (arrow 1) and neurofibrillary tangle (arrow 2) in CA1 area in the AD group and (E) no amyloid plaques and neurofibrillary tangle in control group (×1000).
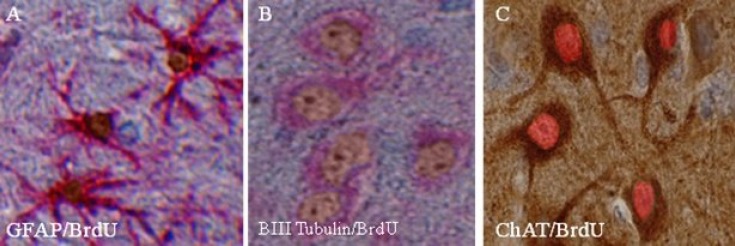
EPI-NCSC-induced cognitive improvement was accompanied not only with an increase in cell number but also with cell differentiation. The increase in cell number was showed by cell count in CA1 area in nissl staining (Table 1). Using double-staining procedure, many glial cells were detected in and around of transplanted site that present BrdU-GFAP; however, many cells were detected that present BrdU-ChAT and BrdU-βIII tubulin too. Using double-staining Envision kit (DAKO, USA), we showed that the nuclei of differentiated cells, that had presented BrdU, were brown and presentation of β-III tubulin or GFAP in cytoplasm was red. For detection of differentiated cholinergic cells, we used DAB staining for ChAT in cytoplasm and permanent red staining for BrdU in nuclei. Therefore, differentiated cholinergic neurons were detected by brownish cytoplasm and reddish nuclei. We did not count differentiated cells, because they were diffused not only in hippocamp but also in the other parts of brain (Fig. 7).
Fig. 7.
Differentiation of epidermal neural crest stem cell (EPI-NCSC) after transplantation by using EnVision kit. (A) The cells incubated with bromodeoxyuridine (BrdU) 48 h before transplantation, then paraffin embedded sections immunostained with antibodies against BrdU (diaminobenzidine [DAB], brown) and glial fibrillary acidic protein (permanent red, red). (B) Neuron like, determined by their expression of β-ІІІ tubulin (permanent red, red), had incorporated BrdU (DAB, brown). (C) ChAT positive neuron (DAB, brown) had incorporated BrdU (Permanent red, red) as determined by double labeling with antibodies against ChAT and BrdU (×400).
Discussion
In this study, the experiments were performed on a recently developed AD rat model. Our findings indicated that AD rats have a decreased number of neurons in the CA1 zone. We also found amyloid plaques and neurofibrillary tangles in the hippocamp, cortex and white matter. Using Y-maze and passive avoidance response tests, we demonstrated that AD group had significantly decreased behavior scores compared to control and sham groups. These results are in agreement with the other findings [14, 26-28]. Similar to other studies in this field, it is confirmed that deposition of Aβ in AD brains impairs learning and memory. In addition, the passive avoidance procedure is a quick and simple task to administer this procedure, and is widely used to measure cognitive alterations after drug administration, lesions, and behavioral manipulations [29]. The passive avoidance test has been widely used to evaluate the ability of rodent working memory in association with cortical and hippocampal functions [30]. In our previous study, the latency periods of passive avoidance response in rats were evaluated and the retention of memory was significantly disturbed in the AD groups [31]. In treatment group, memory was improved and there were no significant differences between them and sham and control groups.
Our observations indicate that rat’s EPI-NCSC in hair follicles retain the capacity to differentiate into non-mesenchymal derivatives specifically neurons, suggesting that intrinsic genomic mechanisms of commitment, lineage restriction, and cell fate are mutable. Environmental signals apparently can elicit the expression of pluripotentiality that emphasizes on accepted fate restrictions of cells originating in neural crest-derived germ layers. These adult cells are self-renewing and multipotential [21, 32-35], thereby fulfilling many of the criteria of a stem cell population.
To our knowledge, this is the first report that EPI-NCSC can differentiate into neurons in vivo. EPI-NCSC may be useful in the treatment of a wide variety of neurologic diseases, offering significant advantages over other “stem” cells. The hair follicle stem cells (EPI-NCSC) are readily accessible, overcome the risks of obtaining neural stem cells from the brain, and provide a renewable population. Autologous transplantation overcomes the ethical and immunological concerns in comparative with the use of fetal tissue. Moreover, EPI-NCSC grows rapidly in culture, precluding the need for immortalization, and differentiates into neurons exclusively with use of a simple protocol that we and other used in previous studies [34, 36, 37]. Furthermore, these cells did not have the risk of tumor formation [37]. In this study, grafted cells did not form tumor. In the other studies, tumor formation after stem cell transplantation was evaluated at the same duration of time [27, 38].
In the present study, we have used dual-labeling immunohistochemistry to demonstrate changes in transplanted cell within the hippocampus of AD rat model. We showed that transplanted cells not only survived and migrated in the host tissue but also expressed neuron-like cells, cholinergic cells and GFAP-positive cells. This shows that EPI-NCSC are very likely to differentiate into neuron-like and glial cells in vivo in AD model. It has been demonstrated that EPI-NCSC can differentiate into neurons and neuroglia in vitro and in vivo. Moreover, other studies have indicated that these cells can differentiate into Schwann cells that improved movement in peripheral nerve and spinal cord injury model [37, 39]. In the present study, we observed more neurons that did not present BrdU, showing that neurogenesis was happened. EPI-NCSC integrated with the brain tissue and their differentiation improved the microenviroment to induce host neural stem cell. Clearly, the brain microenvironment has a profound influence on the survival, migration, and differentiation of EPI-NCSC-derived neurons. It will be interesting to characterize the factors that affect these parameters. This highlights the effect of brain on cells. An increase in the number of hippocampal neuron was seen in the AD rat model after cell delivery, though not up to its normal level and like the control group. Our finding is similar to other papers in this field [27, 28].
The beneficial effect of the grafted cells could be attributed to one or both of two functions of the cells in vivo: (i) simple secretion of acetylcholine from transplanted cells or (ii) actual functional integration into the host tissue [40]. We found cholinergic differentiation after transplantation of EPI-NCSC. Some researchers have shown that generation of cholinergic neurons from other cells in experimental animals is possible [27, 41]. Hopefully a similar outcome will be demonstrated for human stem cells. Alternatively, stem cells derived from brain, blood, bone marrow, or skin may be convertible to cholinergic neurons. Immortalized cholinergic neurons may be useful if cell replication after transplant can be controlled. Further studies are required to determine whether newly differentiated neurons are functionally integrated into the injured hippocamp and to characterize the mechanisms underlying stem cell differentiation in the damaged central nervous system.
Moreover, we tested the reference memory function of neuronal-transplanted animals. Strengthening of this kind of memory requires generation of new synapses between grafted cells and host neurons, in addition to secretion of acetylcholine. Therefore, we propose that transplanted cells contributed to the improvement of memory function by both making new synapses and acetylcholine secretion.
We have demonstrated that rat EPI-NCSC survives and differentiates into neurons, especially cholinergic neurons for 4 weeks after grafting in the brain of an experimental rat AD and contributes to cognitive functional recovery. The improvement in reference memory function in grafted animals suggests that transplanted cells exert their effect by generating new synapses as well as acetylcholine secretion. The EPI-NCSC display greatly diminished proliferative activity and do not result in obvious tumor formation in the long-term graft, suggesting their potential therapeutic application in Alzheimer's treatment.
ACKNOWLEDGEMENTS
This research was supported by grant No. 756 from Tehran University of Medical Science (Iran). The authors would like to thank the Department of Pharmacology in Medicine Faculty of Tehran University of Medical Science as well as Mrs. Family and Mrs. Hassani for their help and collaboration.
References
- 1.Hodges HM. Alzheimer's disease and neural transplantation as prospective cell therapy. Curr Alzheimer Res. 2005 Jan;2(1):79–95. doi: 10.2174/1567205052772759. [DOI] [PubMed] [Google Scholar]
- 2.Avila J, Insausti R. Memory and neurogenesis in aging and Alzheimer’s disease. Aging Dis. 2010 Aug;1(1):30–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Barten DM, Albright CF. Therapeutic strategies for Alzheimer's disease. Mol Neurobiol. 2008;37:171–86. doi: 10.1007/s12035-008-8031-2. [DOI] [PubMed] [Google Scholar]
- 4.Prince M, Jackson J. Alzheimer’s Disease International World Alzheimer Report 2009. In: Prince M, Jackson J, editors. Alzheimer's Disease International. Alzheimer's Disease International. London: pp. 1–96. [Google Scholar]
- 5.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005 Dec;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sleegers K, Lambert JC, Bertram L, Cruts M, Amouyel P. The pursuit of susceptibility genes for Alzheimer's disease: progress and prospects. Trends Genet. 2010 Feb;26(2):84–93. doi: 10.1016/j.tig.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Blennow K, Zetterberg H. Alzheimer's disease. Lancet. 2006 Jul;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 8.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004 Aug;430(7000):631–9. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turrini P, Casu M, Wong TP, Ribeiro-da-Silva A, Cuello AC. Cholinergic nerve terminals establish classical synapses in the rat cerebral cortex: synaptic pattern and age-related atrophy. Neuroscience . 2001;105(2):277–85. doi: 10.1016/s0306-4522(01)00172-5. [DOI] [PubMed] [Google Scholar]
- 10.Waite JJ, Chen AD, Wardlow ML, Thal LJ. Behavioral and biochemical consequences of combined lesions of the medial septum/diagonal band and nucleus basalis in the rat when ibotenic acid, quisqualic acid, and AMPA are used. Exp Neurol. 1994;130(2):214–29. doi: 10.1006/exnr.1994.1200. [DOI] [PubMed] [Google Scholar]
- 11.Calaminici M, Abdulla FA, Sinden J, Stephenson JD. Plastic changes in the cholinergic innervation of the rat cerebral cortex after unilateral lesion of the nucleus basalis with alpha-amino-3-OH-4-isoxozole propionic acid (AMPA): effects of the basal forebrain transplants into neocortex. Brain Res Bull. 1997;42(2):79–93. doi: 10.1016/s0361-9230(96)00212-2. [DOI] [PubMed] [Google Scholar]
- 12.Givens B, Olton D. Local modulation of basal forebrain:effects on working and reference memory. J Neurosci. 1994;14(6):3578–87. doi: 10.1523/JNEUROSCI.14-06-03578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korecka JA, Verhaagen J, Hol EM. Cell-replacement and gene-therapy strategies for Parkinson's and Alzheimer's disease. Regen Med. 2007;2(4):425–46. doi: 10.2217/17460751.2.4.425. [DOI] [PubMed] [Google Scholar]
- 14.Tsai KJ, Tsai YC, Shen CK. G-CSF rescues the memory impairment of animal models of Alzheimer's disease. J Exp Med. 2007;204(6):1273–80. doi: 10.1084/jem.20062481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231(2):258–69. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- 16.Rehberg S, Lischka P, Glaser G, Stamminger T, Wegner M, Rosorius O. Sox10 is an active nucleocytoplasmic shuttle protein, and shuttling is crucial for Sox10-mediated transactivation. Mol Cell Biol. 2002 Aug;22(16):5826–34. doi: 10.1128/MCB.22.16.5826-5834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001 Jan;15(1):66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990 Feb;60(4):585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 19.Lothian C, Lendahl U. An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur J Neurosc. 1997 Mar;9(3):452–62. doi: 10.1111/j.1460-9568.1997.tb01622.x. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, et al. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Aced Sci USA. 2003 Aug;100(17):9958–61. doi: 10.1073/pnas.1733025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent nestin-expressing hair follicle stem cells. J Dermatol. 2009 Jan;36(1):1–9. doi: 10.1111/j.1346-8138.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 22.Krejci E, Grim M. Isolation and characterization of neural crest stem cells from adult human hair follicles. Folia Biol (Praha) 2010;56(4):149–57. [PubMed] [Google Scholar]
- 23.Nobakht M, Asalgoo S, Mousavizadeh k, Najafzadeh N. Effects of silibinin on hair follicle stem cells differentiation to neural-like cells. Am J Biochem Mol Biol. 2011;1(2):212–22. [Google Scholar]
- 24.Roghani M, Joghataie MT, Jalali MR, Baluchnejad-mojarad T. Time course of changes in passive avoidance and Y-maze performance in male diabetic rats. Iran Biomed J. 2006 Apr;10(2):99–104. [Google Scholar]
- 25.Kowall NW, Beal MF, Busciglio J, Duffy LK, Yankner BA. An in vivo model for the neurodegenerative effectsof beta amyloid and protection by substance. Proc Natl Acad Sci. 1991 Aug;88(16):7247–51. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, et al. neural stem cells transplantation in cortex in a mouse model of Alzheimers disease. J Med Invest. 2006 Feb;53(1-2):61–9. doi: 10.2152/jmi.53.61. [DOI] [PubMed] [Google Scholar]
- 27.Wu QY, Li J, Feng ZT, Wang TH. Bone marrow stromal cells of transgenic mice can improve the cognitive ability of an Alzheimer’s disease rat model. Neurosci Lett. 2007 May;417(3):281–5. doi: 10.1016/j.neulet.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 28.Li LY, Li JT, Wu QY, Li J, Feng ZT, Liu S, et al. Transplantation of NGF-Gene-Modified Bone Marrow Stromal Cells into a Rat Model of Alzheimer’ Disease. J Mol Neurosci. 2008 Feb;34(2):157–63. doi: 10.1007/s12031-007-9022-x. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu-Sasamata M, Yamamoto M, Okada M, Yamaguchi T, Tamura A. Effects of indeloxazine hydrochloride on behavioral and biochemical changes in chronic phase of focal cerebral ischemia in rats. Arch Int Pharmacodyn Ther. 1992;314:74–89. [PubMed] [Google Scholar]
- 30.Jacqueline NC. Learning and Memory What’s wrong with my mouse? Canada: WLLET-LISS; 2000. [Google Scholar]
- 31.Nobakht M, Hoseini SM, Mortazavi P, Sohrabi I, Esmailzade B, et al. Neuropathological Changes in Brain Cortex and hippocampus in a Rat Model of Alzheimer’s Disease. Iran Biomed J. 2011 Jan-Apr;15(1- 2):51–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hairfollicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005 Apr;102(15):5530–4. doi: 10.1073/pnas.0501263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huisman MA, Löwik CWGM, Frijns JHM. Uncomplicated differentiation of stem cells into bipolar neurons and myelinating glia. Biochem Biophys Res Commu. 2008 Nov;376(2):358–62. doi: 10.1016/j.bbrc.2008.08.166. [DOI] [PubMed] [Google Scholar]
- 34.Jaks V, Kasper M, Toftgard R. The hair follicle—a stem cell zoo. Exp Cell Res. 2010 May;316(8):1422–8. doi: 10.1016/j.yexcr.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Amoh Y, Kanoh M, Niiyama S, Kawahara K, Sato Y, Katsuoka K, et al. Human and mouse hair follicles contain both multipotent and monopotent stem cells. Cell Cycle. 2009 Jan;8(1):176–7. doi: 10.4161/cc.8.1.7342. [DOI] [PubMed] [Google Scholar]
- 36.Nobakht M, Najafzadeh N, Safari M, Delaviz H, Joghataie MT, et al. Bulge cells of rat hair follicles: isolation, cultivation, morphological and biological features. Yakhteh. 2010 Mar;12(1):51–8. [Google Scholar]
- 37.Sieber-Blum M, Schnell L, Grim M, Hu YF, Schneider R, Schwab ME. Characterization of epidermal neural crest stem cell (EPI-NCSC) grafts in the lesioned spinal cord. Mol Cell Neurosci. 2006 May-Jun;32(1-2):67–81. doi: 10.1016/j.mcn.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Alaie H, Karbalaie K, Tanhaei S, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009 Sep-Oct;78(2-3):59–68. doi: 10.1016/j.diff.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, et al. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: an advantageous alternative to ES and iPS cells. J Cell Biochem. 2009 Aug;107(5):1016–20. doi: 10.1002/jcb.22204. [DOI] [PubMed] [Google Scholar]
- 40.Nikolic WV, Hou H, Town T, Zhu Y, Giuntn B, Sanberg CD, et al. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular β-amyloid deposits in Alzheimer’s Mice. Stem Cells Dev. 2008 Mar;17:1–17. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wernig M, Benninger F, Schmandt T, Rade M, Tucker KL, Bussow H, et al. Functional integration of embryonic stem cell-derived neurons in vivo. J Neurosci. 2004 Jun;24(22):5258–68. doi: 10.1523/JNEUROSCI.0428-04.200. [DOI] [PMC free article] [PubMed] [Google Scholar]