Abstract
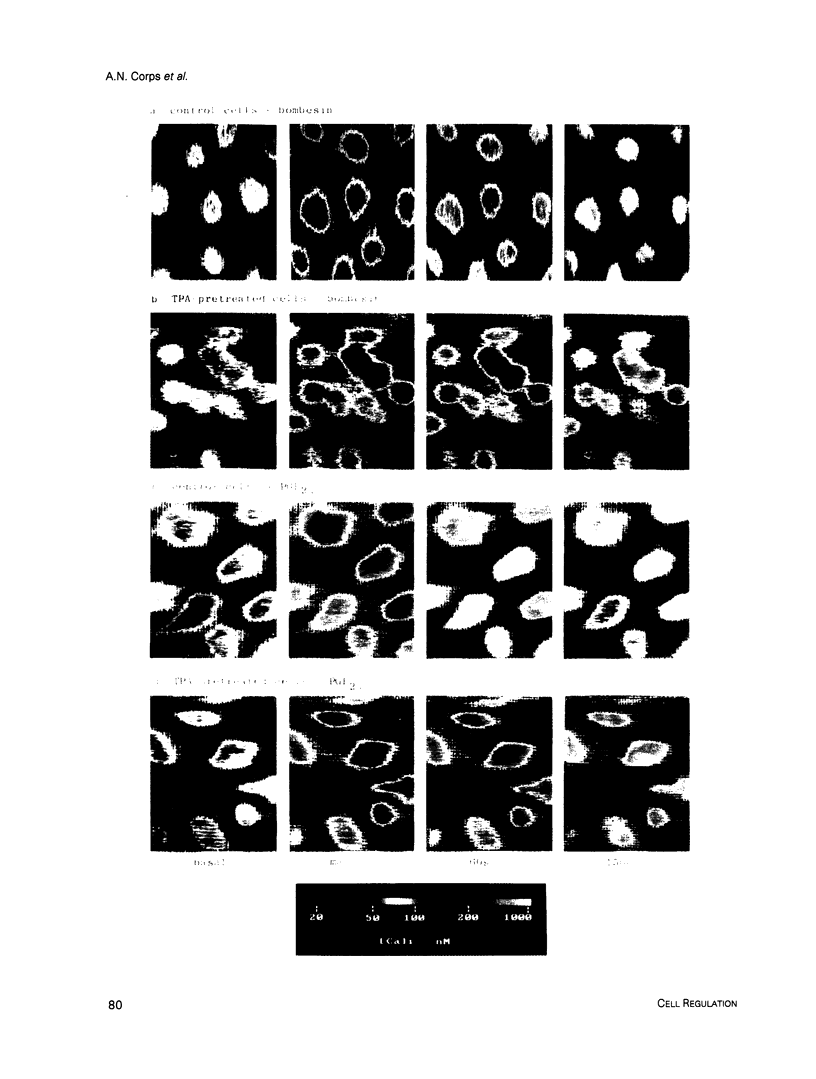
Single-cell fluorescence image analysis has been used to characterize the mitogen-induced increases in intracellular free [Ca2+] ([Ca2+]i) in control and protein kinase C-depleted Swiss 3T3 cells. More than 80% of the control cells exhibited fast, transient responses to bombesin, vasopressin, or prostaglandin F2 alpha (PGF2 alpha). In contrast, the [Ca2+]i responses induced by platelet-derived growth factor (PDGF) were markedly more heterogeneous, slower, and often biphasic, with fewer cells (60-70%) responding. The peak [Ca2+]i values obtained in response to each mitogen showed substantial variation between cells. Brief pretreatment of the cells with 12-O-tetradecanoyl phorbol 13-acetate (TPA) reduced the [Ca2+]i responses to bombesin, but did not affect the responses to PDGF. Long-term pretreatment of the cells with TPA to down-modulate protein kinase C resulted in substantially prolonged [Ca2+]i responses to bombesin, vasopressin, and PGF2 alpha, but had no such effect on the responses to PDGF. We conclude that differences between the [Ca2+]i responses to bombesin and PDGF, previously reported using cell populations, reflect differences occurring in individual cells, and that the [Ca2+]i responses to bombesin, vasopressin, and PGF2 alpha (but not PDGF) are subject to feedback inhibition via protein kinase C.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Gullick W. J. Differences in phorbol-ester-induced down-regulation of protein kinase C between cell lines. Biochem J. 1989 Feb 1;257(3):905–911. doi: 10.1042/bj2570905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler S. K., Poenie M., Tsien R. Y., Taylor P. Agonist-stimulated oscillations and cycling of intracellular free calcium in individual cultured muscle cells. J Biol Chem. 1988 Feb 5;263(4):1952–1959. [PubMed] [Google Scholar]
- Benjamin C. W., Connor J. A., Tarpley W. G., Gorman R. R. NIH-3T3 cells transformed by the EJ-ras oncogene exhibit reduced platelet-derived growth factor-mediated Ca2+ mobilization. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4345–4349. doi: 10.1073/pnas.85.12.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Altin J. G., Karjalainen A., Bygrave F. L. Stimulation of hepatic inositol 1,4,5-trisphosphate kinase activity by Ca2+-dependent and -independent mechanisms. Biochem J. 1988 Dec 15;256(3):697–701. doi: 10.1042/bj2560697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Blakeley D. M., Corps A. N., Brown K. D. Bombesin and platelet-derived growth factor stimulate formation of inositol phosphates and Ca2+ mobilization in Swiss 3T3 cells by different mechanisms. Biochem J. 1989 Feb 15;258(1):177–185. doi: 10.1042/bj2580177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M. Inhibition of the binding of 125I-labelled epidermal growth factor to mouse cells by a mitogen in goat mammary secretions. Biochem J. 1983 May 15;212(2):465–472. doi: 10.1042/bj2120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S., Gross D., Ling Y. C., Morrison G. H. Quantitative imaging of free and total intracellular calcium in cultured cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1870–1874. doi: 10.1073/pnas.86.6.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo D. M., Gaudino G., Naldini L., Comoglio P. M. Receptor for bombesin with associated tyrosine kinase activity. Mol Cell Biol. 1986 Dec;6(12):4641–4649. doi: 10.1128/mcb.6.12.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Lawing W. J., Jr, Majerus P. W. Protein kinase C phosphorylates human platelet inositol trisphosphate 5'-phosphomonoesterase, increasing the phosphatase activity. Cell. 1986 Sep 12;46(6):951–958. doi: 10.1016/0092-8674(86)90077-2. [DOI] [PubMed] [Google Scholar]
- Fu T., Okano Y., Nozawa Y. Bradykinin-induced generation of inositol 1,4,5-trisphosphate in fibroblasts and neuroblastoma cells: effect of pertussis toxin, extracellular calcium, and down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1429–1435. doi: 10.1016/s0006-291x(88)81035-0. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Heppel L. A., Gross D. J., Webb W. W., Parries G. The rapid desensitization of receptors for platelet derived growth factor, bradykinin and ATP: studies on individual cells using quantitative digital video fluorescence microscopy. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1205–1212. doi: 10.1016/s0006-291x(88)80494-7. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Heslop J. P., Blakeley D. M., Brown K. D., Irvine R. F., Berridge M. J. Effects of bombesin and insulin on inositol (1,4,5)trisphosphate and inositol (1,3,4)trisphosphate formation in Swiss 3T3 cells. Cell. 1986 Dec 5;47(5):703–709. doi: 10.1016/0092-8674(86)90513-1. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Pattison G. Regulation of inositol 1,4,5-trisphosphate kinase activity after stimulation of human T cell antigen receptor. J Clin Invest. 1987 May;79(5):1538–1541. doi: 10.1172/JCI112986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke C. M., Meisenhelder J., Brown K. D., Gould K. L., Gould S. J., Hunter T. Early phosphorylation events following the treatment of Swiss 3T3 cells with bombesin and the mammalian bombesin-related peptide, gastrin-releasing peptide. EMBO J. 1986 Nov;5(11):2889–2898. doi: 10.1002/j.1460-2075.1986.tb04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives H. E., Daniel T. O. Interrelationship between growth factor-induced pH changes and intracellular Ca2+. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1950–1954. doi: 10.1073/pnas.84.7.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rivas A., Mendoza S. A., Nånberg E., Sinnett-Smith J., Rozengurt E. Ca2+-mobilizing actions of platelet-derived growth factor differ from those of bombesin and vasopressin in Swiss 3T3 mouse cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5768–5772. doi: 10.1073/pnas.84.16.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Millard P. J., Gross D., Webb W. W., Fewtrell C. Imaging asynchronous changes in intracellular Ca2+ in individual stimulated tumor mast cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1854–1858. doi: 10.1073/pnas.85.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monck J. R., Reynolds E. E., Thomas A. P., Williamson J. R. Novel kinetics of single cell Ca2+ transients in stimulated hepatocytes and A10 cells measured using fura-2 and fluorescent videomicroscopy. J Biol Chem. 1988 Apr 5;263(10):4569–4575. [PubMed] [Google Scholar]
- Morris J. D., Metcalfe J. C., Smith G. A., Hesketh T. R., Taylor M. V. Some mitogens cause rapid increases in free calcium in fibroblasts. FEBS Lett. 1984 Apr 24;169(2):189–193. doi: 10.1016/0014-5793(84)80316-6. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A. J., Cheek T. R., Moreton R. B., Berridge M. J., Burgoyne R. D. Localization and heterogeneity of agonist-induced changes in cytosolic calcium concentration in single bovine adrenal chromaffin cells from video imaging of fura-2. EMBO J. 1989 Feb;8(2):401–411. doi: 10.1002/j.1460-2075.1989.tb03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiella A., Vicentini L. M., Meldolesi J. Protein kinase C-mediated feed back inhibition of the Ca2+ response at the EGF receptor. Biochem Biophys Res Commun. 1987 Nov 30;149(1):145–151. doi: 10.1016/0006-291x(87)91616-0. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Jacob R. Calcium oscillations in non-excitable cells. Trends Neurosci. 1989 Feb;12(2):43–46. doi: 10.1016/0166-2236(89)90133-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Murray M., Zachary I., Collins M. Protein kinase C activation enhances cAMP accumulation in Swiss 3T3 cells: inhibition by pertussis toxin. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2282–2286. doi: 10.1073/pnas.84.8.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturani E., Vicentini L. M., Zippel R., Toschi L., Pandiella-Alonso A., Comoglio P. M., Meldolesi J. PDGF-induced receptor phosphorylation and phosphoinositide hydrolysis are unaffected by protein kinase C activation in mouse Swiss 3T3 and human skin fibroblasts. Biochem Biophys Res Commun. 1986 May 29;137(1):343–350. doi: 10.1016/0006-291x(86)91216-7. [DOI] [PubMed] [Google Scholar]
- Valge V. E., Wong J. G., Datlof B. M., Sinskey A. J., Rao A. Protein kinase C is required for responses to T cell receptor ligands but not to interleukin-2 in T cells. Cell. 1988 Oct 7;55(1):101–112. doi: 10.1016/0092-8674(88)90013-x. [DOI] [PubMed] [Google Scholar]