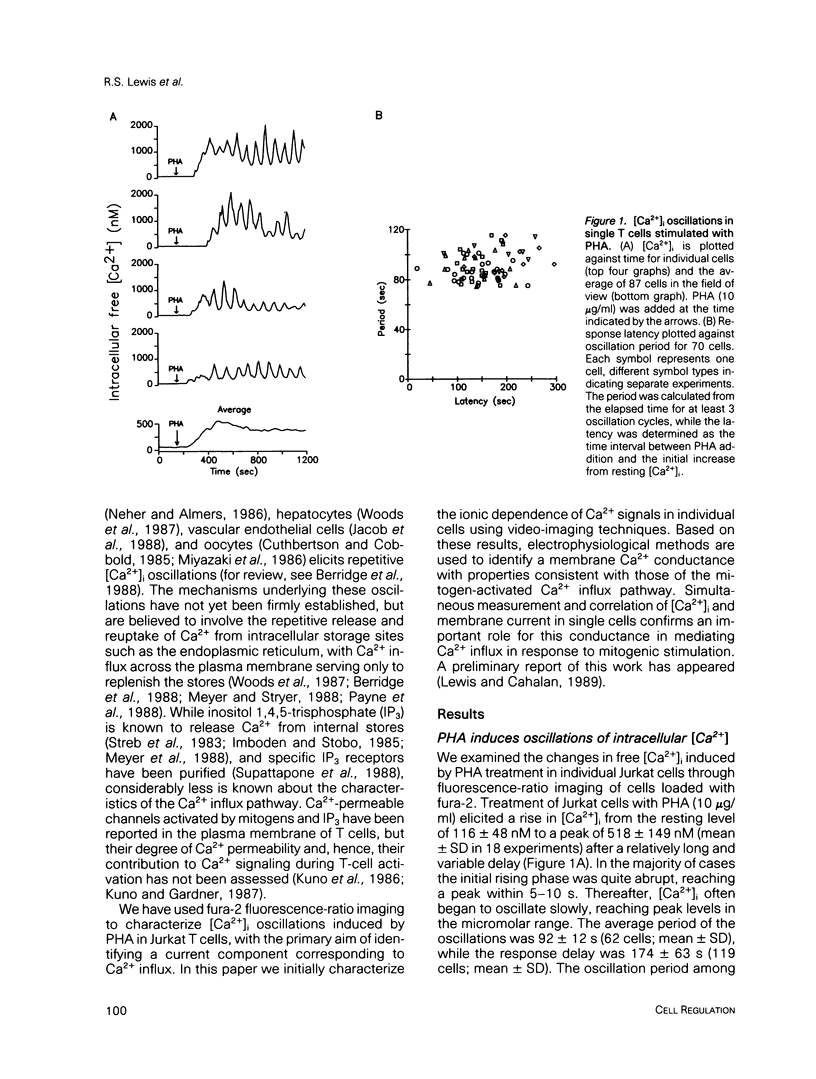
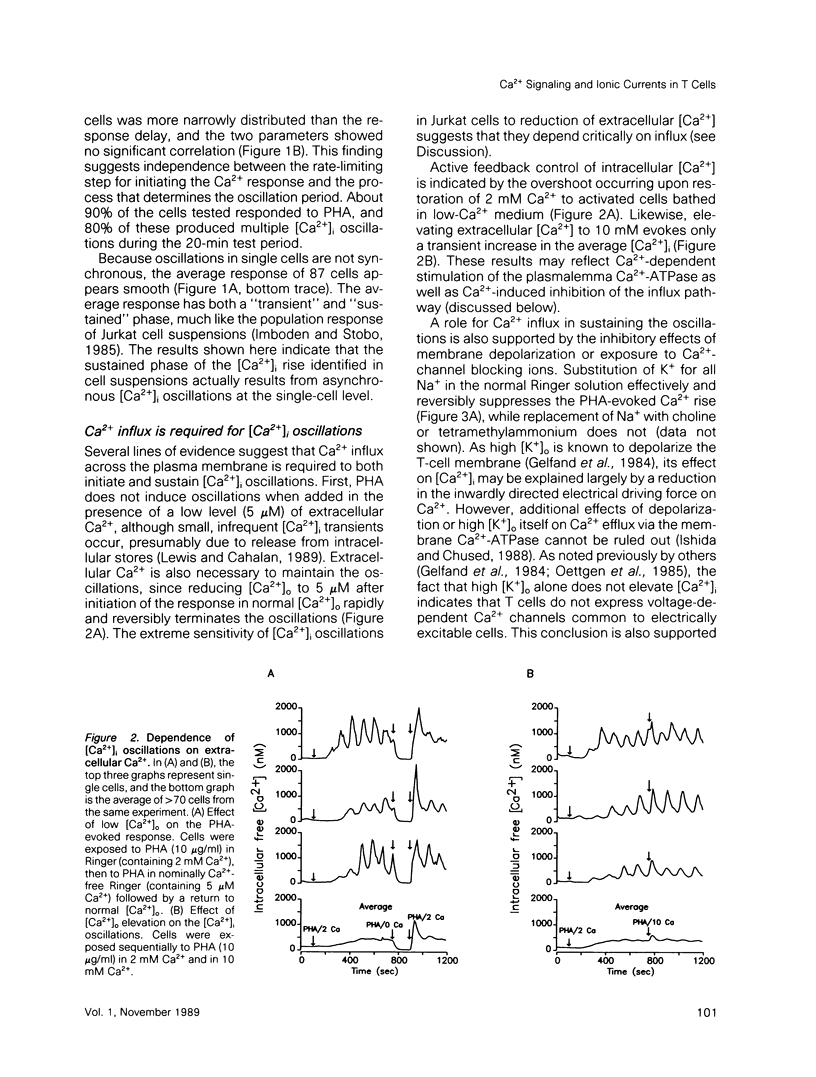
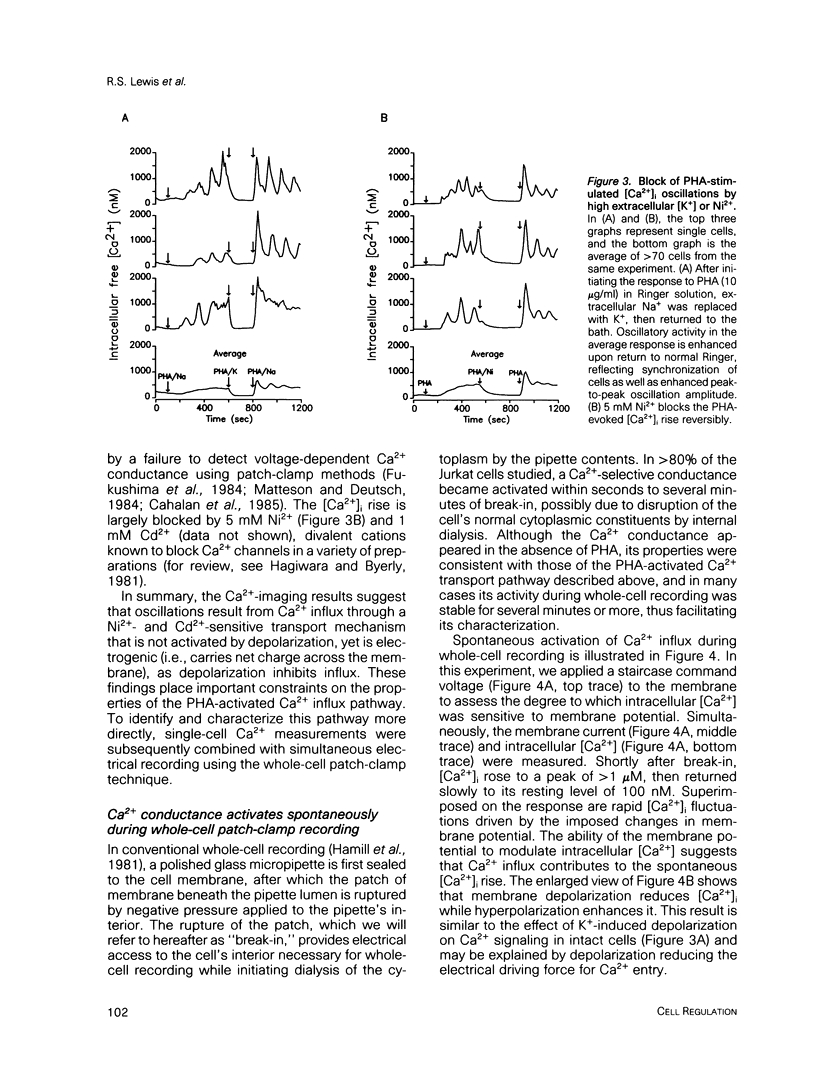
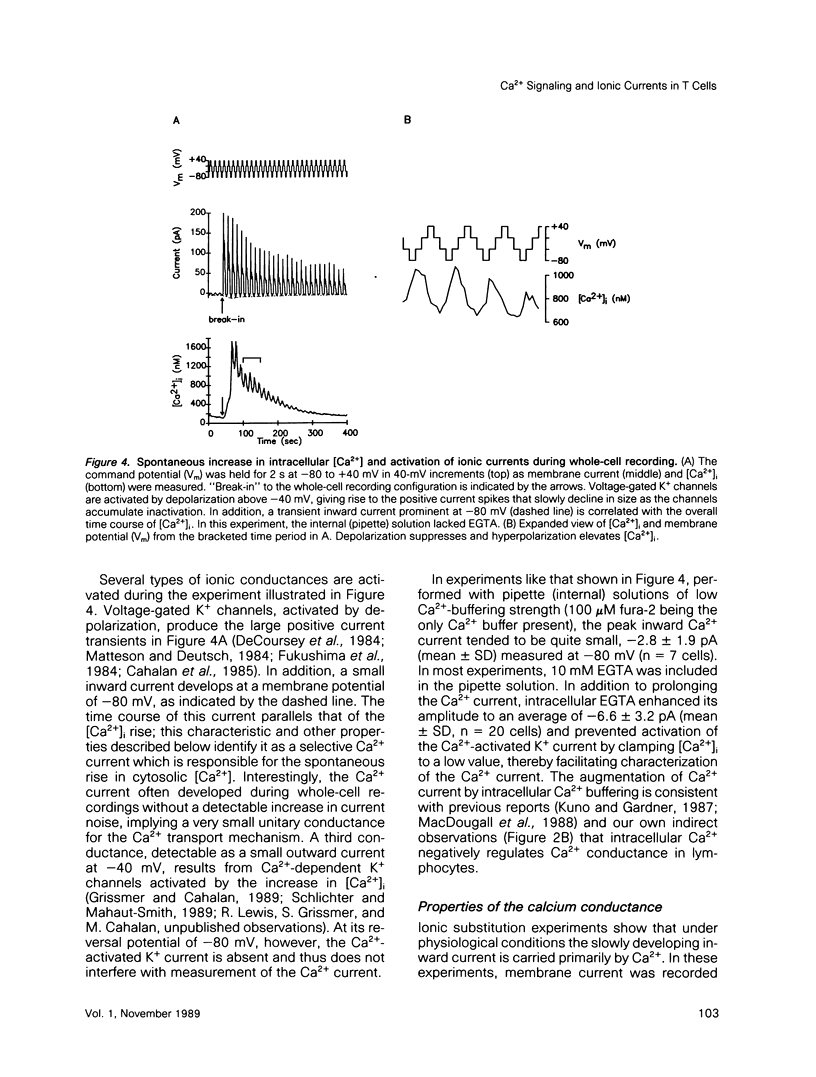
Abstract
A rapid rise in the level of cytosolic free calcium ([Ca2+]i) is believed to be one of several early triggering signals in the activation of T lymphocytes by antigen. Although Ca2+ release from intracellular stores and its contribution to Ca2+ signaling in many cell types is well documented, relatively little is known regarding the role and mechanism of Ca2+ entry across the plasma membrane. We have investigated mitogen-triggered Ca2+ signaling in individual cells of the human T-leukemia-derived line, Jurkat, using fura-2 imaging and patch-clamp recording techniques. Phytohemagglutinin (PHA), a mitogenic lectin, induces repetitive [Ca2+]i oscillations in these cells peaking at micromolar levels with a period of 90-120 s. The oscillations depend critically upon Ca2+ influx across the plasma membrane, as they are rapidly terminated by removal of extracellular Ca2+, addition of Ca(2+)-channel blockers such as Ni2+ or Cd2+, or membrane depolarization. Whole-cell and perforated-patch recording methods were combined with fura-2 measurements to identify the mitogen-activated Ca2+ conductance involved in this response. A small, highly selective Ca2+ conductance becomes activated spontaneously in whole-cell recordings and in response to PHA in perforated-patch experiments. This conductance has properties consistent with a role in T-cell activation, including activation by PHA, lack of voltage-dependent gating, inhibition by Ni2+ or Cd2+, and regulation by intracellular Ca2+. Moreover, a tight temporal correlation between oscillations of Ca2+ conductance and [Ca2+]i suggests a role for the membrane Ca2+ conductance in generating [Ca2+]i oscillations in activated T cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Ambler S. K., Poenie M., Tsien R. Y., Taylor P. Agonist-stimulated oscillations and cycling of intracellular free calcium in individual cultured muscle cells. J Biol Chem. 1988 Feb 5;263(4):1952–1959. [PubMed] [Google Scholar]
- Berridge M. J., Cobbold P. H., Cuthbertson K. S. Spatial and temporal aspects of cell signalling. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):325–343. doi: 10.1098/rstb.1988.0080. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch M. K., Rigley K. P., Klaus G. G. Cross-linking of surface immunoglobulin on B lymphocytes induces both intracellular Ca2+ release and Ca2+ influx: analysis with indo-1. Biochem Biophys Res Commun. 1986 May 29;137(1):500–506. doi: 10.1016/0006-291x(86)91238-6. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Chandy K. G., DeCoursey T. E., Gupta S. A voltage-gated potassium channel in human T lymphocytes. J Physiol. 1985 Jan;358:197–237. doi: 10.1113/jphysiol.1985.sp015548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson K. S., Cobbold P. H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature. 1985 Aug 8;316(6028):541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E., Chandy K. G., Gupta S., Cahalan M. D. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984 Feb 2;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S., Henkart M. Potassium current in clonal cytotoxic T lymphocytes from the mouse. J Physiol. 1984 Jun;351:645–656. doi: 10.1113/jphysiol.1984.sp015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P., Alcover A., Kuno M., Moingeon P., Weyand C. M., Goronzy J., Reinherz E. L. Triggering of T-lymphocytes via either T3-Ti or T11 surface structures opens a voltage-insensitive plasma membrane calcium-permeable channel: requirement for interleukin-2 gene function. J Biol Chem. 1989 Jan 15;264(2):1068–1076. [PubMed] [Google Scholar]
- Gelfand E. W., Cheung R. K., Grinstein S., Mills G. B. Characterization of the role for calcium influx in mitogen-induced triggering of human T cells. Identification of calcium-dependent and calcium-independent signals. Eur J Immunol. 1986 Aug;16(8):907–912. doi: 10.1002/eji.1830160806. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Cheung R. K., Grinstein S. Role of membrane potential in the regulation of lectin-induced calcium uptake. J Cell Physiol. 1984 Dec;121(3):533–539. doi: 10.1002/jcp.1041210312. [DOI] [PubMed] [Google Scholar]
- Gray L. S., Gnarra J. R., Sullivan J. A., Mandell G. L., Engelhard V. H. Spatial and temporal characteristics of the increase in intracellular Ca2+ induced in cytotoxic T lymphocytes by cellular antigen. J Immunol. 1988 Oct 1;141(7):2424–2430. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Rink T. J. Agonists stimulate divalent cation channels in the plasma membrane of human platelets. FEBS Lett. 1985 Jul 8;186(2):175–179. doi: 10.1016/0014-5793(85)80703-1. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Weiss A. The T-cell antigen receptor regulates sustained increases in cytoplasmic free Ca2+ through extracellular Ca2+ influx and ongoing intracellular Ca2+ mobilization. Biochem J. 1987 Nov 1;247(3):695–700. doi: 10.1042/bj2470695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Chused T. M. Heterogeneity of lymphocyte calcium metabolism is caused by T cell-specific calcium-sensitive potassium channel and sensitivity of the calcium ATPase pump to membrane potential. J Exp Med. 1988 Sep 1;168(3):839–852. doi: 10.1084/jem.168.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Rabinovitch P. S., Martin P. J., Beatty P. G., Hansen J. A. Distinct patterns of transmembrane calcium flux and intracellular calcium mobilization after differentiation antigen cluster 2 (E rosette receptor) or 3 (T3) stimulation of human lymphocytes. J Clin Invest. 1986 Apr;77(4):1224–1232. doi: 10.1172/JCI112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I., Metzger H. Initial characterization of the calcium channel activated by the cross-linking of the receptors for immunoglobulin E. J Biol Chem. 1984 Aug 25;259(16):10188–10193. [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Kuno M., Goronzy J., Weyand C. M., Gardner P. Single-channel and whole-cell recordings of mitogen-regulated inward currents in human cloned helper T lymphocytes. Nature. 1986 Sep 18;323(6085):269–273. doi: 10.1038/323269a0. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A., Tanguy J. Dependence of intracellular effects of GTP gamma S and inositoltrisphosphate on cell membrane potential and on external Ca ions. Pflugers Arch. 1987 Aug;409(4-5):499–506. doi: 10.1007/BF00583807. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Nabholz M. T-cell activation. Annu Rev Cell Biol. 1986;2:231–253. doi: 10.1146/annurev.cb.02.110186.001311. [DOI] [PubMed] [Google Scholar]
- MacDougall S. L., Grinstein S., Gelfand E. W. Detection of ligand-activated conductive Ca2+ channels in human B lymphocytes. Cell. 1988 Jul 15;54(2):229–234. doi: 10.1016/0092-8674(88)90555-7. [DOI] [PubMed] [Google Scholar]
- Malgaroli A., Milani D., Meldolesi J., Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987 Nov;105(5):2145–2155. doi: 10.1083/jcb.105.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro A. M., Smith M. C. Calcium-dependent activation of lymphocytes by ionophore, A23187, and a phorbol ester tumor promoter. J Cell Physiol. 1983 Jul;116(1):51–56. doi: 10.1002/jcp.1041160109. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Deutsch C. K channels in T lymphocytes: a patch clamp study using monoclonal antibody adhesion. Nature. 1984 Feb 2;307(5950):468–471. doi: 10.1038/307468a0. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Regulation of cytosolic free calcium in fura-2-loaded rat parotid acinar cells. J Biol Chem. 1987 Dec 25;262(36):17362–17369. [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Hashimoto N., Yoshimoto Y., Kishimoto T., Igusa Y., Hiramoto Y. Temporal and spatial dynamics of the periodic increase in intracellular free calcium at fertilization of golden hamster eggs. Dev Biol. 1986 Nov;118(1):259–267. doi: 10.1016/0012-1606(86)90093-x. [DOI] [PubMed] [Google Scholar]
- Neher E., Almers W. Fast calcium transients in rat peritoneal mast cells are not sufficient to trigger exocytosis. EMBO J. 1986 Jan;5(1):51–53. doi: 10.1002/j.1460-2075.1986.tb04176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet-Brown E., Cheung R. K., Lee J. W., Gelfand E. W. Antigen-dependent increase in cytosolic free calcium in specific human T-lymphocyte clones. Nature. 1985 Aug 8;316(6028):545–547. doi: 10.1038/316545a0. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Terhorst C., Cantley L. C., Rosoff P. M. Stimulation of the T3-T cell receptor complex induces a membrane-potential-sensitive calcium influx. Cell. 1985 Mar;40(3):583–590. doi: 10.1016/0092-8674(85)90206-5. [DOI] [PubMed] [Google Scholar]
- Payne R., Walz B., Levy S., Fein A. The localization of calcium release by inositol trisphosphate in Limulus photoreceptors and its control by negative feedback. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 26;320(1199):359–379. doi: 10.1098/rstb.1988.0082. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Treves S., Di Virgilio F., Cerundolo V., Zanovello P., Collavo D., Pozzan T. Calcium and inositolphosphates in the activation of T cell-mediated cytotoxicity. J Exp Med. 1987 Jul 1;166(1):33–42. doi: 10.1084/jem.166.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Daley J. F., Hodgdon J. C., Reinherz E. L. Calcium dependency of antigen-specific (T3-Ti) and alternative (T11) pathways of human T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6836–6840. doi: 10.1073/pnas.81.21.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Requirement for calcium ions in lymphocyte transformation stimulated by phytohemagglutinin. J Cell Physiol. 1972 Dec;80(3):329–337. doi: 10.1002/jcp.1040800303. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Wilson H. A., Greenblatt D., Poenie M., Finkelman F. D., Tsien R. Y. Crosslinkage of B lymphocyte surface immunoglobulin by anti-Ig or antigen induces prolonged oscillation of intracellular ionized calcium. J Exp Med. 1987 Aug 1;166(2):601–606. doi: 10.1084/jem.166.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Zschauer A., van Breemen C., Bühler F. R., Nelson M. T. Calcium channels in thrombin-activated human platelet membrane. Nature. 1988 Aug 25;334(6184):703–705. doi: 10.1038/334703a0. [DOI] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]


