Abstract
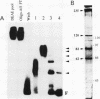
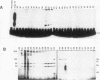
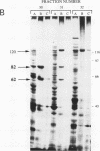
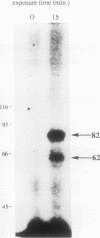
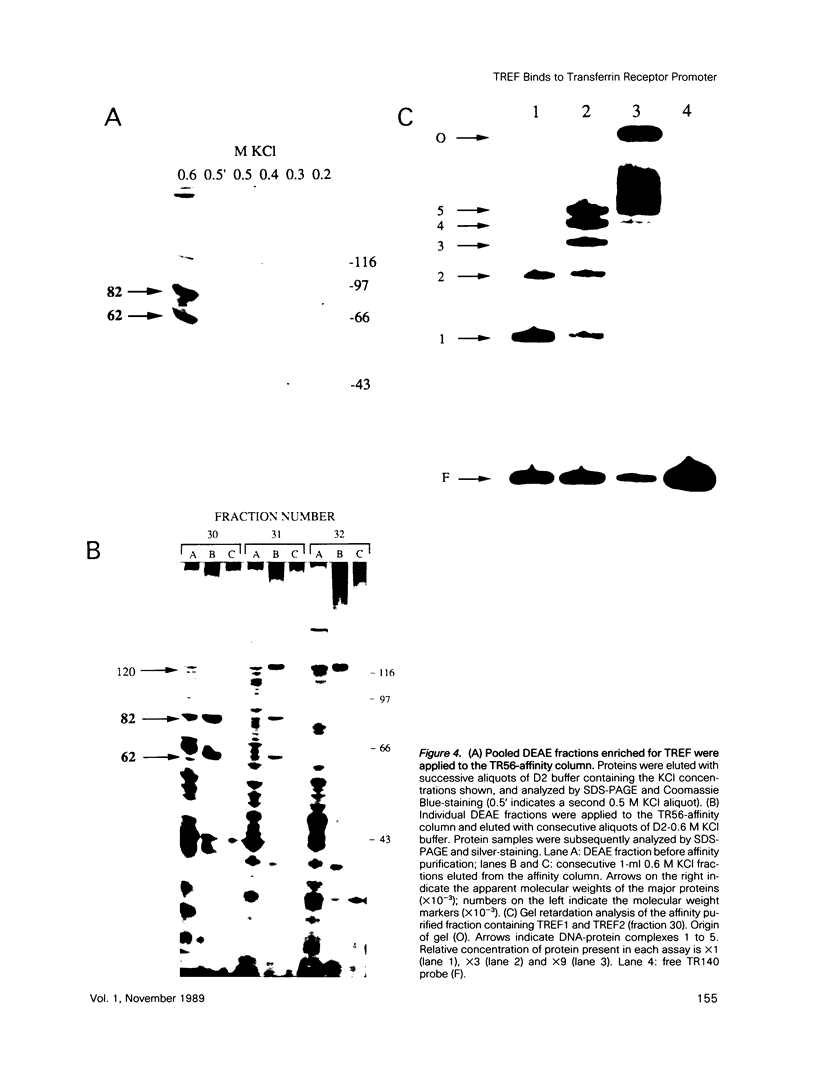
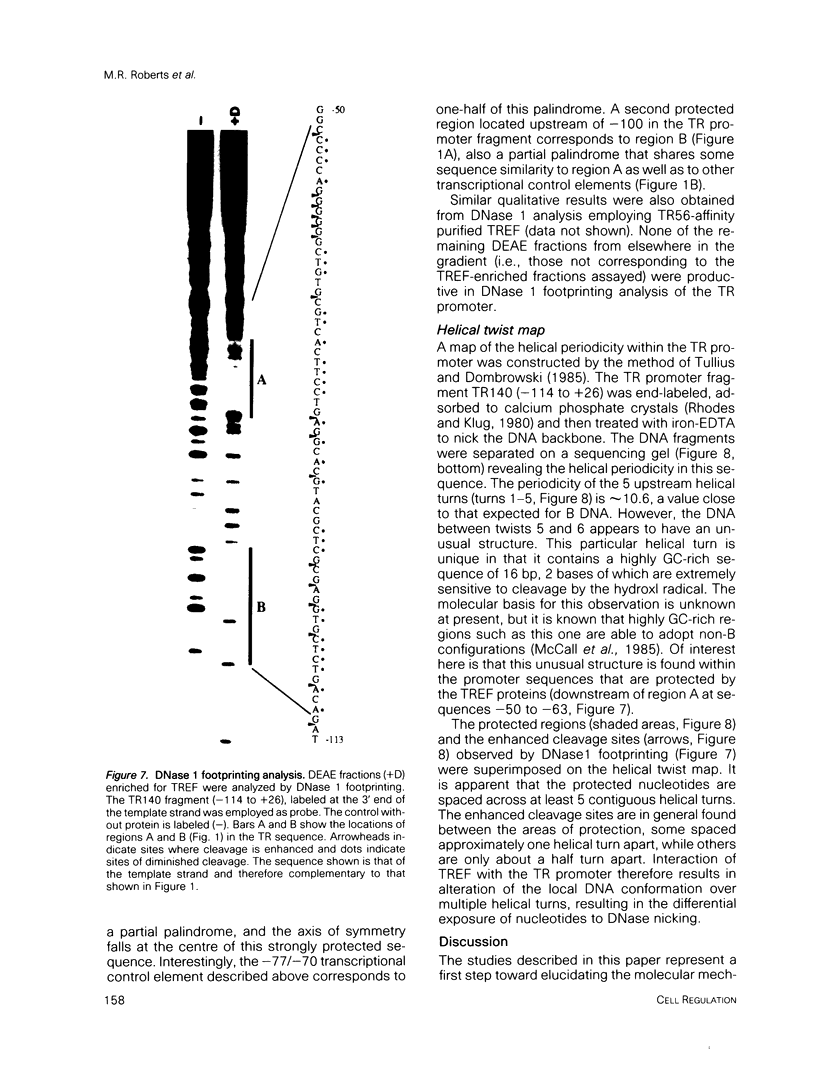
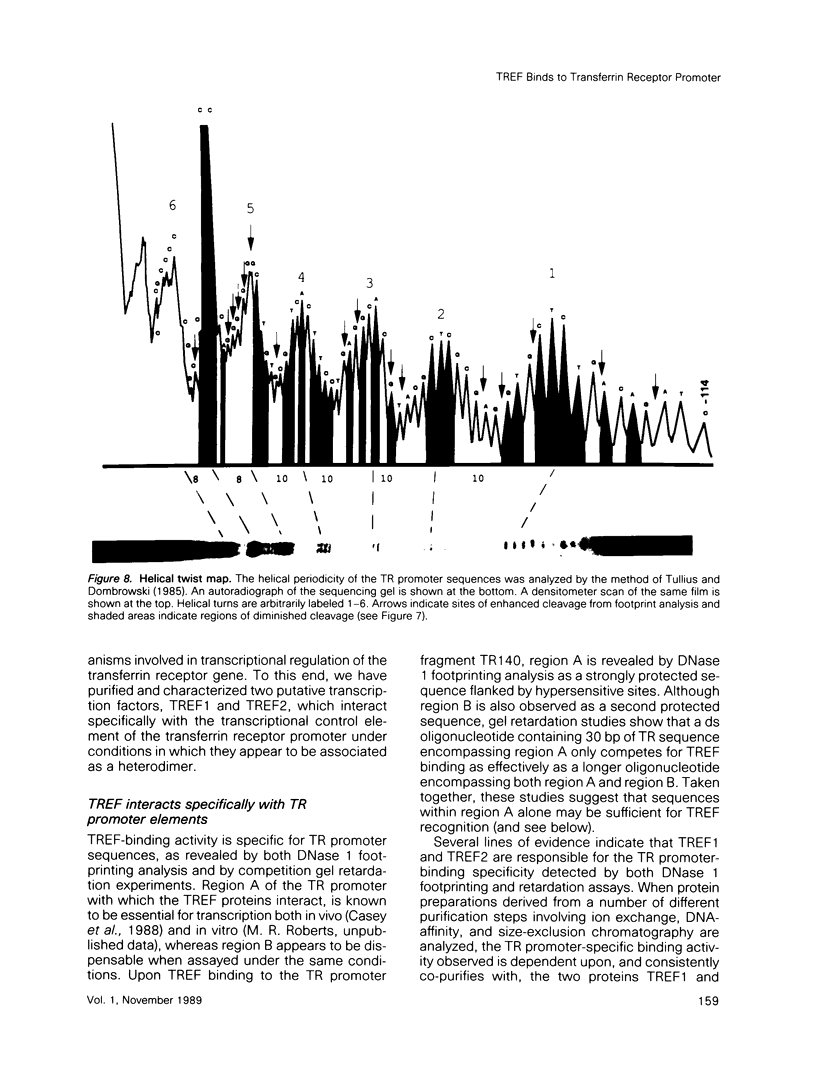
Two novel proteins that bind specifically to the transferrin receptor (TR) promoter, have been isolated from HeLa cell nuclear extract using a combination of ion exchange and oligonucleotide-affinity chromatography. TREF1 and TREF2, which have apparent molecular weights of 82 and 62 kDa, respectively, appear to be associated as a heterocomplex (TREF), and both proteins are able to contact target DNA directly. TREF interacts specifically with a region of the TR promoter which contains the TR transcriptional control element. This region is similar in sequence to the cAMP-responsive and phorbol ester-responsive elements found in several viral and cellular genes. Binding of TREF to the TR promoter results in modification of DNA topology over multiple helical turns, including a sequence revealed by a helical periodicity map as having an unusual structure.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Bohmann D., Tsuchie H., Tjian R., Vogt P. K. v-jun encodes a nuclear protein with enhancer binding properties of AP-1. Cell. 1988 Mar 11;52(5):705–712. doi: 10.1016/0092-8674(88)90408-4. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Di Jeso B., Rao K. K., Rouault T. A., Klausner R. D., Harford J. B. Deletional analysis of the promoter region of the human transferrin receptor gene. Nucleic Acids Res. 1988 Jan 25;16(2):629–646. doi: 10.1093/nar/16.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh L. A., Baldwin A. S., Carthew R. W., Sharp P. A. Human CCAAT-binding proteins have heterologous subunits. Cell. 1988 Apr 8;53(1):11–24. doi: 10.1016/0092-8674(88)90483-7. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Carthew R. W., Sharp P. A. A single polypeptide possesses the binding and transcription activities of the adenovirus major late transcription factor. Mol Cell Biol. 1986 Dec;6(12):4723–4733. doi: 10.1128/mcb.6.12.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Drogula C., Krönke M., Waldmann T. A., Greene W. C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A. Regulation of transferrin receptor expression in concanavalin A stimulated and Gross virus transformed rat lymphoblasts. J Cell Physiol. 1982 Oct;113(1):40–46. doi: 10.1002/jcp.1041130109. [DOI] [PubMed] [Google Scholar]
- Hearing P., Shenk T. The adenovirus type 5 E1A enhancer contains two functionally distinct domains: one is specific for E1A and the other modulates all early units in cis. Cell. 1986 Apr 25;45(2):229–236. doi: 10.1016/0092-8674(86)90387-9. [DOI] [PubMed] [Google Scholar]
- Hen R., Sassone-Corsi P., Corden J., Gaub M. P., Chambon P. Sequences upstream from the T-A-T-A box are required in vivo and in vitro for efficient transcription from the adenovirus serotype 2 major late promoter. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7132–7136. doi: 10.1073/pnas.79.23.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H. Y., Gardner J., Aisen P. Inducibility of transferrin receptors on friend erythroleukemic cells. Science. 1977 Aug 5;197(4303):559–561. doi: 10.1126/science.267327. [DOI] [PubMed] [Google Scholar]
- Hurst H. C., Jones N. C. Identification of factors that interact with the E1A-inducible adenovirus E3 promoter. Genes Dev. 1987 Dec;1(10):1132–1146. doi: 10.1101/gad.1.10.1132. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Lesley J. F., Schulte R. J. Inhibition of cell growth by monoclonal anti-transferrin receptor antibodies. Mol Cell Biol. 1985 Aug;5(8):1814–1821. doi: 10.1128/mcb.5.8.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall M., Brown T., Kennard O. The crystal structure of d(G-G-G-G-C-C-C-C). A model for poly(dG).poly(dC). J Mol Biol. 1985 Jun 5;183(3):385–396. doi: 10.1016/0022-2836(85)90009-9. [DOI] [PubMed] [Google Scholar]
- Miskimins W. K., McClelland A., Roberts M. P., Ruddle F. H. Cell proliferation and expression of the transferrin receptor gene: promoter sequence homologies and protein interactions. J Cell Biol. 1986 Nov;103(5):1781–1788. doi: 10.1083/jcb.103.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. T., Skuzeski J. T., Lund E., Steinberg T. H., Burgess R. R., Dahlberg J. E. Functional elements of the human U1 RNA promoter. Identification of five separate regions required for efficient transcription and template competition. J Biol Chem. 1987 Feb 5;262(4):1795–1803. [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza C. D., Bleil J. D., Lennox E. S. The control of transferrin receptor synthesis in mitogen-stimulated human lymphocytes. Exp Cell Res. 1984 Oct;154(2):510–520. doi: 10.1016/0014-4827(84)90175-7. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Cohen D. R., Curran T., Bos T. J., Vogt P. K., Bohmann D., Tjian R., Franza B. R., Jr Fos-associated protein p39 is the product of the jun proto-oncogene. Science. 1988 May 20;240(4855):1010–1016. doi: 10.1126/science.3130660. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Voulalas P. J., Franza B. R., Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988 Dec;2(12B):1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
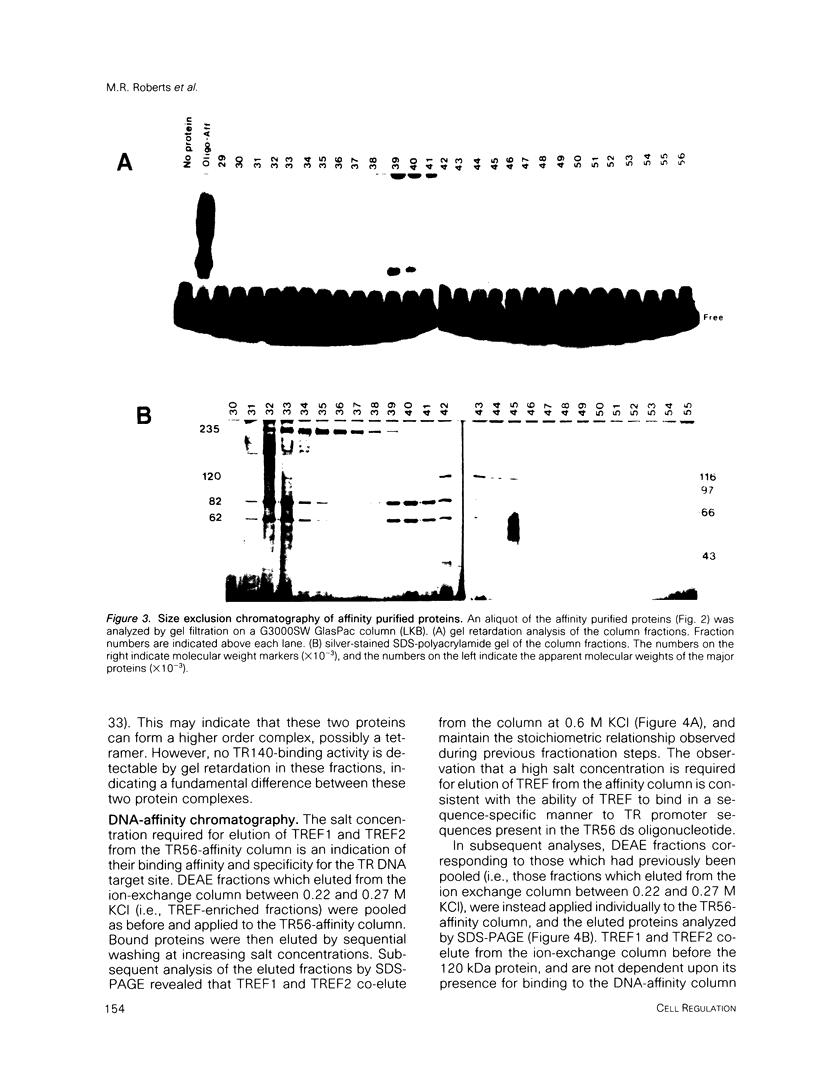
- Rhodes D., Klug A. Helical periodicity of DNA determined by enzyme digestion. Nature. 1980 Aug 7;286(5773):573–578. doi: 10.1038/286573a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Marshall J., Hayman M. J., Ponka P., Beug H. Control of erythroid differentiation: possible role of the transferrin cycle. Cell. 1986 Jul 4;46(1):41–51. doi: 10.1016/0092-8674(86)90858-5. [DOI] [PubMed] [Google Scholar]
- Selsing E., Wells R. D., Alden C. J., Arnott S. Bent DNA: visualization of a base-paired and stacked A-B conformational junction. J Biol Chem. 1979 Jun 25;254(12):5417–5422. [PubMed] [Google Scholar]
- Tei I., Makino Y., Sakagami H., Kanamaru I., Konno K. Decrease of transferrin receptor during mouse myeloid leukemia (M1) cell differentiation. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1419–1424. doi: 10.1016/s0006-291x(82)80157-5. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1175–1179. doi: 10.1073/pnas.79.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullius T. D., Dombroski B. A. Iron(II) EDTA used to measure the helical twist along any DNA molecule. Science. 1985 Nov 8;230(4726):679–681. doi: 10.1126/science.2996145. [DOI] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Wu B. J., Kingston R. E., Morimoto R. I. Human HSP70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]