Summary
Background:
Hypothyroidism can usually be treated effectively by oral levothyroxine supplementation. There are, however, some rare circumstances, when oral levothyroxine application is not sufficient, for example malabsorption, interactions with food or other medications, or various gastrointestinal diseases.
Case Report:
We present a 42 year old woman with refractory and severe symptomatic hypothyroidism after subtotal thyroidectomy in spite of high dose oral levothyroxine supplementation. By stepwise increasing oral levothyroxine dosage up to 2200 micrograms plus 80 micrograms of thyronine, no sufficient substitution could be achieved. After suspicion of enteral malabsorption due to a pathological D-Xylose-test, subcutaneous levothyroxine supplementation was started. Finally, a sustained euthyroid state could be achieved.
Conclusions:
For selected patients who do not respond to oral treatment subcutaneous application of levothyroxine can be a suitable and effective therapy.
Keywords: thyroxine, thyronine, hypothyroidism, thyroidectomy, subcutaneous
Background
Hypothyroidism is a common problem often resulting from autoimmune thyroid disease or consecutively after thyroid resection [1,2]. In most cases hypothyroidism can be treated effectively by oral thyroid hormone supplementation. When taken in a fasting state, more than 80% of the drug can be absorbed [3], but there are conditions where absorption of thyroxin is limited, e.g. due to taking the medication simultaneously with food, beverages, or co-medication such as estrogen, proton pump inhibitors, calcium products, ferrous sulfate, rifampicin, phenytoin, carbamazepine, lovastatin, and sertraline [4,5]. Furthermore, absorption can be reduced by a variety of diseases of the upper gastrointestinal tract, e.g. gastritis, short bowel syndrome, inflammatory bowel diseases, lactose intolerance, or celiac disease [6]. Besides oral supplementation, intravenous application of levothyroxine is an alternative in cases of myxedema coma when the patient is not able to take his or her medication orally [7]. However, to our knowledge, only one report exists of subcutaneous application of thyroxin to treat chronic hypothyroidism when oral T4 supplementation does not seem to be sufficient [8]. In this context, we describe hypothyroidism resistant to oral administration of levothyroxine effectively treated by subcutaneous injection to increase its awareness among the medical community.
Case Report
A 42 year old Caucasian female was referred to our clinic with persistent symptomatic hypothyroidism due to subtotal thyroidectomy for benign multi-nodular goiter eight years ago despite being treated with an increased dose of 400 μg levothyroxine and 20 μg thyronine. The patient reports having tried several different dosage forms including tablets from different pharmaceutical companies and drops yielding the same result without improving symptoms or laboratory values.
On presentation she complained about a depressive mood with fatigue, cold intolerance, hair loss and progressive weakness. Furthermore, she reported a weight gain of about 50 kg since the operation. According to her past medical history she had been suffering from depression with an unsuccessful suicide attempt 5 years ago.
Baseline thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), and free thyroxine (fT4) were 19.1 mU/l (normal range: 0.4–4.0 mU/l), 3.2 ng/ml (normal range: 2.1–4.3 ng/ml), and 6.67 ng/l (normal range: 8–18 ng/l), respectively, revealing primary hypothyroidism. Autoimmune serology was negative for microsomal, thyroideaperoxidase, or TSH-receptor antibodies. Further, creatinkinase (171 U/l; normal range up to 170 U/l) and total cholesterol (234 mg/dl) were slightly elevated. Diabetes mellitus was ruled out with an HbA1c level of 5.3% and a normal oral glucose tolerance test. To exclude other endocrinological reasons for weight gain a 24-hour urine collection was performed in order to exclude hypercortisolism. Further laboratory tests were normal.
On physical examination the patient had a body temperature of 35.6°C, her blood pressure was 90/60 mmHg, her heart rate was 72 beats per min. Her weight was 150 kg resulting in a body mass index of 53 kg/m2. Further examination was consistent with hypothyroidism with the patient having dry skin and nonpitting edema on feet and lower legs as well as on the back of her hands. Her deep tendon reflexes showed delayed and weak relaxation. The remainder of the examination was uneventful. Ultrasound was consistent with thyroidectomy showing small residual tissue on both sides of 2.1 ml and of hypoechoic structure.
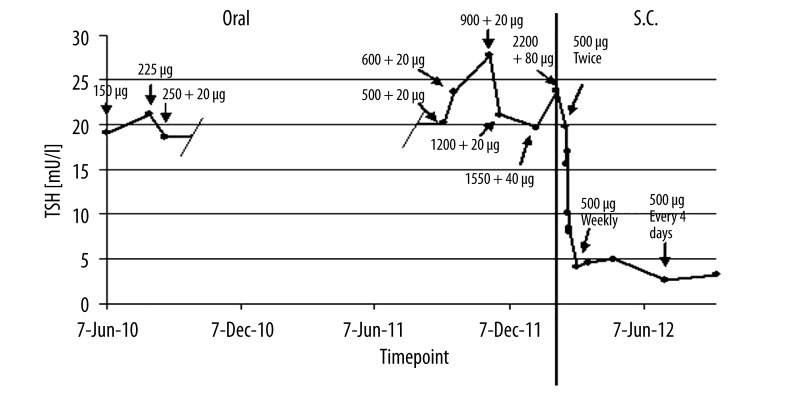
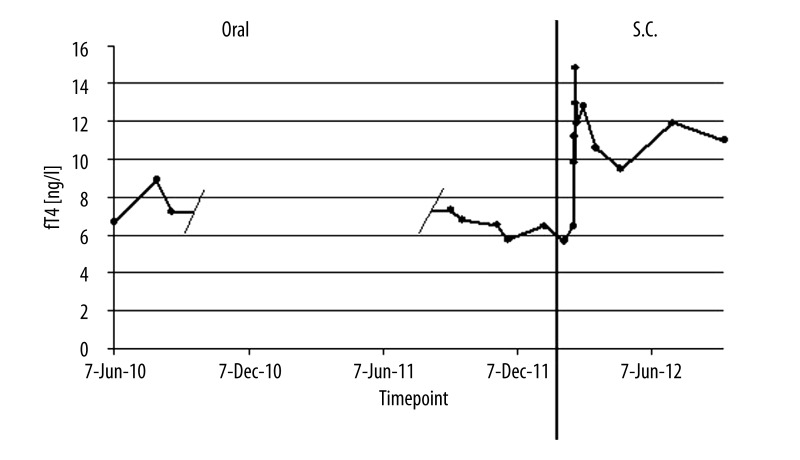
First, the patient was instructed how to take her medication. During a stay on an endocrinological ward it was proven that the patient was taking her prescribed medication as recommended, at least thirty minutes before breakfast with water only. Care was taken that the thyroxin medication was taken in a fasting state without concomitant therapy. No tablets could be detected in her mouth after swallowing, making sure the tables were taken correctly. After four weeks of treatment while having no effect on blood values and symptoms, the intake was delayed to bedtime, being likely to increase absorption in some cases [9]. This, however, did neither improve TSH nor fT4 values. Next, the dosage of thyroxin was increased about 100 μg every 4 weeks over the first two months of treatment. However, with a dosage of 600 μg of levothyroxine and 20 μg of thyronine, no improvement of symptoms or in TSH, fT4, and fT3 levels was observed. Therefore, a more rapid dosage regime was chosen and the dosage was increased about 200 μg every 4 weeks resulting in a daily dose of 2200 μg per day of levothyroxine after eight months and 80 μg thyronine was added to the medication. However, neither the patient’s complaints nor laboratory values improved during therapy (Figures 1 and 2).
Figure 1.
TSH values over time. Above the dots, doses resulting in the indicated TSH values are given. On the left: oral application, levothyroxine dose + thyronine dose. On the right s.c. application of levothyroxine. s.c.: subcutaneous.
Figure 2.
fT4 values over time. Doses at different timepoints as stated in Figure 1.
Additionally, to rule out gastro-intestinal disease, a gastroscopy was performed showing antrum gastritis as the only pathological result and excluding celiac disease with biopsies and antibody tests. Moreover, an oral D-xylose absorption test revealed decreased levels of D-xylose one and two hours after application of 25 g D-xylose in the blood as well as low urinary excretion implicating malabsorption (Table 1).
Table 1.
D-Xylose test. Values of D-Xylose in blood before, one and two hours after an oral dose of 25 g of D-Xylose as well as urine excretion over five hours.
| D-Xylose | Normal range | |
|---|---|---|
| Blood: | ||
| Baseline | Negative | Patient specific |
| After 1 hour | 181 mg/l | >200 mg/l |
| After 2 hours | 135 mg/l | >200 mg/l |
|
| ||
| Urine over 5 hours: | 2.2 g/5 h | >4.0 g/5 h |
However, since doses up to 2200 μg levothyroxine and 80 μg of thyronine were insufficient to achieve any improvements it was decided to inject 500 μg of levothyroxine (L-Thyroxin Henning® inject, Henning, Berlin, Germany) subcutaneously. Replacement therapy with subcutaneous thyroxin injection was continued with 500 μg on a once weekly basis. Due to the high sample volume of 10 ml it was recommended to split this dosage on two injection sites. After 14 days of subcutaneous injection treatment fT4 (12.77 ng/l; normal range: 8–18 ng/l), and fT3 (2.93 ng/l; normal: 2.0–4.2 ng/l) were within normal range and TSH improved considerably, dropping to 4.11 mU/l (normal range: 0.4–4.0 mU/l) (Figures 1 and 2). Five months later, TSH was finally normalized at 2.66 mU/l and remained so on another follow-up visit seven months after the first subcutaneous injection with fT3 and fT4 always staying within normal range. Furthermore, not only laboratory values were normalized but fatigue and depressed mood improved significantly. Due to alternating relapse of these symptoms over the weekly period after injection, injection intervals were truncated to 500 μg every four days resulting in sustained clinical improvement.
Discussion
Mode of application
In this case, we report that subcutaneous application of levothyroxine might be an effective therapeutical approach in rare cases where patients do not respond to oral therapy on a long-term basis. In our patient rapid improvement of hypothyroidism with regard to clinical symptoms and laboratory data concerning TSH, fT4, and fT3 was evident after changing the method of application from oral to subcutaneous. In most cases with refractory hypothyroidism, intravenous application was a successful treatment option [7]. To our knowledge, only one report exists describing off label subcutaneous use of thyroxin inject [8]. In that report, however, subcutaneous injections were described as painful most likely deriving from the large sample volume of 10 ml per injection suggesting substitution via intramuscular application [8]. However, in our patient the split dose regimen with two injections of 5 ml each was well tolerated over a period of seven months of follow-up. The advantage of subcutaneous injections is that patients can easily perform it themselves.
Reasons for failing therapy (compliance, pseudomalabsorption, malabsorption)
Some antidepressants can interfere with TSH levels. Since our patient did not take any other medication besides levothyroxine and thyronine, this is unlikely to be the cause for her high TSH values.
The most frequent cause of hypothyroidism in the setting of excessive oral supplementation of levothyroxine may be poor compliance with medication [6,10]. In this context, pseudomalabsorption of levothyroxine has been noticed as clinical problem itself, which can be examined by controlled supplementation of levothyroxine [11]. In our case the patient was taught about the way of levothyroxine intake several times and was additionally treated for hypothyroidism in hospital. Therefore, it was unlikely to be the cause of persistent hypothyroidism.
However, true malabsorption, especially in obese patients, can be subtle and difficult to recognize due to its superficial clinical appearance [11]. Besides gastrointestinal diseases like celiac disease, Helicobacter pylori infection, atrophic gastritis, lactose intolerance, chronic inflammatory bowel diseases, or parasitic diseases like Giardiasis [6], pancreatic and liver diseases, dietary interference with different kinds of juice, coffee or cereals [6], or interference due to medications such as calcium formulations [12], proton pump inhibitors, ion or cation exchange resins, bile acid sequestrants, ferrous sulfate, orlistat, or aluminium magnesium hydroxide [6], previous GI surgeries with resulting short bowel syndrome, congestive heart failure, and pregnancy are the most frequent causes for a malabasorption syndrome [13]. Also, the required thyroxine dose is increased in obese patients [6]. Therefore, after ruling out compliance problems and drug interactions, fecal fat excretion and a gastroscopy should be performed to rule out these causes [11]. Other causes for malabsorption of levothyroxine could be different preparations of the medication, though absorption differences turned out to be as small as 10–15% [14], making this theory unlikely in our patient. In other cases, levothyroxine taken at bedtime lead to higher blood concentrations of thyroid hormones and lower TSH than taken in the morning, probably due to better absorption over night [9]. This, however, also remained without effect in the presented case.
Conclusions
We believe that subcutaneous application of levothyroxine is an effective alternative in cases of hypothyroidism refractory to oral treatment. Further studies are needed to better establish indications, outcomes, and adverse events of this therapy.
Acknowledgments
No conflicts of interest were disclosed by the authors.
References:
- 1.Dar RA, Chowdri NA, Parray FQ, Wani SH. An Unusual Case of Hashimoto’s Thyroiditis with Four Lobed Thyroid Gland. N Am J Med Sci. 2012;4(3):151–53. doi: 10.4103/1947-2714.93881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaturu S, Fontinot J, Rowland T. Mixed medullary thyroid cancer and follicular cancer. Am J Case Rep. 2011;12:1–4. [Google Scholar]
- 3.Hasselstrom K, Siersbaek-Nielsen K, Lumholtz IB, et al. The bioavailability of thyroxine and 3,5,3’-triiodothyronine in normal subjects and in hyper- and hypothyroid patients. Acta Endocrinologica. 1985;110(4):483–86. doi: 10.1530/acta.0.1100483. [DOI] [PubMed] [Google Scholar]
- 4.Khandelwal D, Tandon N. Overt and subclinical hypothyroidism: who to treat and how. Drugs. 2012;72(1):17–33. doi: 10.2165/11598070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009;23(6):781–92. doi: 10.1016/j.beem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Ward LS. The difficult patient: drug interaction and the influence of concomitant diseases on the treatment of hypothyroidism. Arquivos brasileiros de endocrinologia e metabologia. 2010;54(5):435–42. doi: 10.1590/s0004-27302010000500002. [DOI] [PubMed] [Google Scholar]
- 7.Tonjes A, Karger S, Koch CA, et al. Impaired enteral levothyroxine absorption in hypothyroidism refractory to oral therapy after thyroid ablation for papillary thyroid cancer: case report and kinetic studies. Thyroid. 2006;16(10):1047–51. doi: 10.1089/thy.2006.16.1047. [DOI] [PubMed] [Google Scholar]
- 8.Nobre EL, Jorge Z, Anselmo J, et al. [A rare case of malabsorption of thyroid hormones] Acta Medica Portuguesa. 2004;17(6):487–91. [PubMed] [Google Scholar]
- 9.Bolk N, Visser TJ, Kalsbeek A, et al. Effects of evening vs morning thyroxine ingestion on serum thyroid hormone profiles in hypothyroid patients. Clin Endocrinol. 2007;66(1):43–48. doi: 10.1111/j.1365-2265.2006.02681.x. [DOI] [PubMed] [Google Scholar]
- 10.Ain KB, Refetoff S, Fein HG, Weintraub BD. Pseudomalabsorption of levothyroxine. JAMA. 1991;266(15):2118–20. [PubMed] [Google Scholar]
- 11.Ogawa D, Otsuka F, Mimura U, et al. Pseudomalabsorption of levothyroxine: a case report. Endocr J. 2000;47(1):45–50. doi: 10.1507/endocrj.47.45. [DOI] [PubMed] [Google Scholar]
- 12.Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21(5):483–86. doi: 10.1089/thy.2010.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damle N, Bal C, Soundararajan R, et al. A curious case of refractory hypothyroidism due to selective malabsorption of oral thyroxine. Indian J Endocrinol Metab. 2012;16(3):466–68. doi: 10.4103/2230-8210.95716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg M, Distefano JJ. TSH-based protocol, tablet instability, and absorption effects on L-T4 bioequivalence. Thyroid. 2009;19(2):103–10. doi: 10.1089/thy.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]