Heart failure is a highly prevalent, debilitating, and costly condition with generally poor clinical outcomes [1]. Aside from heart transplantation, which is an available treatment option for only a small fraction of patients due to donor organ shortage [2], there is no effective therapy that can reverse the course of this disease. A single episode of myocardial infarction (MI) may result in the loss of 1 billion cardiomyocytes or more (~25% of total cardiomyocytes) [3]. Given the limited intrinsic capacity of the adult heart to repair itself, the goal of cardiac regenerative medicine has centered on strategies to remuscularize the diseased heart.
Conceptually, the functional regeneration of an infarcted heart would entail the replacement of lost myocardium by aligned, electrically coupled, and mature new cardiomyocytes that beat in synchrony with the host myocardium. Beyond achieving this remarkable result, the avoidance of procedure-related complications and other potential adverse events such as tumor formation or cardiac arrhythmia is paramount for the therapy to be considered a success. While the process of finding the most appropriate cell type and delivery approach to achieve this objective has been the holy grail of cardiac regenerative medicine, a growing body of literature has now documented our initial efforts in this area. From these studies, the encouraging finding is that cell transplantation into the diseased heart (via intracoronary, transendocardial, or direct epicardial injection) appears to be reasonably safe. Furthermore, the practicalities of harvesting, expanding, and re-introducing cells back into the patient do not seem too cumbersome. However, the sobering reality we have learned is that tremendous roadblocks exist in achieving significant improvement in long-term cardiac function and bona fide remuscularization after cell transplantation.
We believe the field of cardiac regeneration is at a crossroad. While ongoing debate regarding the most appropriate cell type, timing, route of delivery, and clinical setting will be addressed by further experimentation in animal models and patients, we need to consider whether the premise of cell transplantation as a treatment strategy for diseased hearts is still fundamentally sound and viable for further exploration. As we now enter the second decade of research on cell-based therapy for cardiovascular disease, it is instructive to revisit some of the key findings from published cell transplantation studies in order to better understand what is needed to move the field forward. We will briefly summarize the efforts related to the transplantation of autologous non-cardiac cell populations such as skeletal myoblasts and bone marrow-derived cells (BMCs), as well as recent trials on resident cardiac stem/progenitor cells (Figure 1). We will also discuss whether pluripotent stem cells such as human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) will be able to move into the clinical arena in the next decade, and the advantages and disadvantages of these cells in comparison with autologous adult-derived cells. Ultimately, our pursuit of cardiac regeneration will be looked upon by future generations as akin to Ponce de Leon’s search for the Fountain of Youth, or as one of the greatest success stories in modern medicine. It is our hope that the combined efforts of many dedicated cardiovascular investigators in this area will eventually lead to a durable therapy that can reverse the rising incidence of ischemic heart failure.
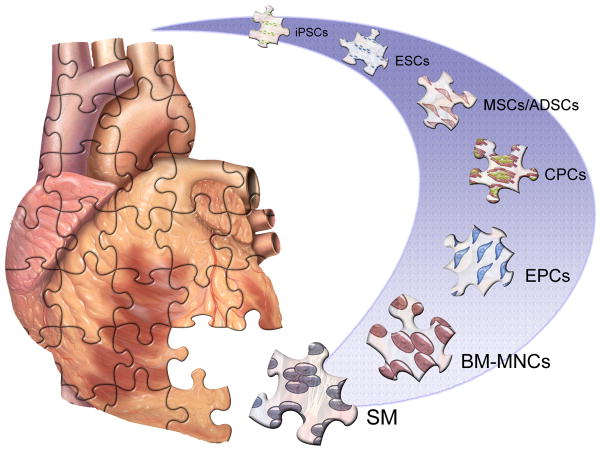
Figure 1. Candidate cell types for cardiovascular regenerative therapy.
A variety of cell sources with differing cardiomyogenic potential and developmental origins are under active investigation for cardiac cell therapy after myocardial infarction. BM-MNCs - Bone marrow mononuclear cells. CSCs -Cardiac stem cells. EPCs - Endothelial progenitor cells. ESC - Embryonic stem cells. iPSC - Induced pluripotent stem cells. MSCs - Mesenchymal stem cells. SM - Skeletal myoblasts.
Skeletal myoblasts
The initial observation that skeletal myoblasts can be harvested and cultured ex vivo from muscle biopsies and then transplanted into an infarcted animal heart sparked the interest of basic and translational investigators that cell-based therapy may be a potentially viable strategy for cardiac remuscularization [4]. The appeal for using skeletal myoblasts as a donor cell source is their autologous origin, ability to rapidly expand in culture, and propensity to generate muscle cells by spontaneous differentiation. Furthermore, these cells appear to engraft into the injured heart with remarkable efficiency and undergo in situ differentiation into striated muscle bundles. While the earliest clinical studies conducted in small numbers of patients using autologous skeletal myoblasts reported significant improvements in cardiac function [5], a subsequent trial with a larger number of participants found no demonstrable benefit (as measured by ejection fraction) and a high prevalence of ventricular tachyarrhythmia requiring the implantation of defibrillators [6]. This early effort on skeletal myoblast transplantation illustrates our capability to move quickly from basic discovery to human studies in the cell therapy arena. However, the finding of potentially lethal arrhythmia in the remuscularized hearts suggests the requirement of cell-to-cell coupling between the transplanted graft and endogenous cardiomyocytes to minimize electrical heterogeneity within the diseased heart. Indeed, the introduction of connexin 43, a cell junction protein involved in cardiomyocyte coupling, into mouse skeletal myoblasts reduced arrhythmia following cell transplantation [7].
Bone marrow-derived stem cells
The finding that hematopoietic cells may harbor greater developmental plasticity than previously suspected had spurred the subsequent investigation of these cells for regenerative studies in other tissues including the heart [8]. Studies on hematopoietic cells have most often utilized unselected mononuclear cells isolated directly from the bone marrow or from peripheral blood, as well as a more refined subset of marrow-derived or circulating cells termed endothelial progenitor cells. This latter subset has been reported to induce neovascularization in animal models [9], and are enriched in cell populations possessing the cell surface markers CD34, CD133, and/or receptors for vascular endothelial growth factor. Conceptually, the introduction of autologous cells that exhibit stem cell features into a damaged heart is highly appealing. Furthermore, results from clinical trials have supported the safety and feasibility of intracoronary delivery of bone marrow and circulating stem cells. However, despite encouraging results in animal models, the efficacy of bone marrow cell transplantation in patients has been modest overall and inconsistent between studies [10–17]. Nevertheless, these initial studies have illustrated three key issues that warrant further consideration: 1. The low retention rate of bone marrow cells in the heart, 2. The questionable efficiency of cardiomyocyte transdifferentiation, and 3. The uncertain mechanisms of functional improvement by the delivery of bone marrow cells.
It appears that the chosen cell type, as well as the route of administration may play a role in the issue of low cell retention in the heart after transplantation. One human PET imaging study demonstrated that CD34+ cells homed to the infarcted myocardium with a roughly 10-fold greater efficiency compared to unselected BMCs after intracoronary cell transfer [18]. Additionally, emerging data suggests that direct intramuscular injection may be slightly more effective than intracoronary delivery [19, 20]. For intracoronary injections, the requirement for diapedesis through the coronary arterial wall may account for the limited amount of cell retention. Hence, innovative strategies aimed at increasing the targeting efficiency of transplanted cells and methods promoting cell survival following transplantation into the heart would prove highly beneficial [21].
The efficiency of cardiomyocyte transdifferentiation from bone marrow-derived cells still poses significant challenges. While the initial findings appeared highly encouraging, subsequent studies identified several confounding issues, such as cell fusion and imaging artifacts that may account for some, if not all, of the apparent hematopoietic cell transdifferentiation into cardiomyocytes [22–25]. While it is not improbable that bone marrow cells can transdifferentiate into cardiomyocytes with the introduction of appropriate epigenetic modifiers, the exact conditions and molecular factors required to achieve this in vivo are far from clear.
Despite the low efficiency of cell retention and cardiomyocyte transdifferentiation, there appears to be a modest, but statistically significant (~3–5%), rise in ejection fraction after bone marrow cell transplantation compared with control [14, 26]. Head to head comparisons and meta-analyses suggest this effect has not been reserved to a particular cell type, although most studies used unselected bone marrow mononuclear cells rather than mobilized circulating cells or other selected cell populations [27–29]. There is a growing consensus that the beneficial effects are mediated by paracrine action from either the process of cell injection alone, regardless of cell type, or specific factors secreted by the transplanted cells, or both [30, 31]. The presence of these factors may exert a favorable remodeling effect, augment neovascularization, and/or stimulate the expansion of endogenous cardiac progenitor cells. In support of this, Loffredo et al. showed recentlythat bone marrow-derived c-kit+ cells stimulate cardiomyogenesis by increasing the number of stem/progenitor cell-derived cardiomyocytes [31]. Furthermore, the number of proliferative BrdU+ cardiomyocytes increased in bone marrow c-kit positive but not c-kit negative cell-treated hearts. These results suggest that the identification of specific paracrine factor(s) and the targeted cell population mediating endogenous cardiomyogenesis may allow us to achieve at least as comparable a regenerative response as bone marrow cell transplantation, but in a cell-free manner.
Mesenchymal stem cells
Mesenchymal stem cells, or MSCs, are multipotent stromal cells that can differentiate into a variety of mesodermally derived tissues and constitute another potential candidate for cell-based therapy. For cardiac applications, they have been most commonly isolated from the bone marrow or adipose tissue and defined by their ability to adhere to plastic culture dishes during in vitro propagation. In vitro, MSCs may be induced to exhibit cardiomyocyte-like features in the presence of the demethylating agent 5-azacytidine or when co-cultured with cardiomyocytes [32–34]. They also enjoy the potential advantages of possessing low immunogenicity, as well as the ability to home to the site of injury within the myocardium [35]. The combination of these attributes, in theory, could allow for intravenous allogeneic delivery of cell therapy via an ‘off the shelf’ approach without the requirement for administering concomitant immunosuppression.
In this regard, the results from the recently reported POSEIDON study showed that transendocardial allogeneic MSC transplantation was associated with a favorable safety profile when compared to autologous MSCs transplantation [36]. Also, in an earlier randomized trial, the intravenous application of allogeneic MSCs after acute MI resulted in an improvement in global symptom score at 6 months and a small but significant improvement in ejection fraction in patients with large MI’s [37]. As with bone marrow-derived stem cell transplantation, the low frequency of MSC engraftment and cardiomyogenic differentiation in the heart suggests that the functional improvement observed in preclinical models and human trials is likely related to paracrine effects of the injected cells as opposed to generation of de novo cardiomyocytes [38]. Several other ongoing clinical trials including PROMETHEUS, TAC-HFT, ADVANCE, and PRECISE will add important information as to safety and efficacy of bone marrow or adipose derived MSC therapy in cardiac disease.
Endogenous cardiac stem cells
While the lesson from bone marrow cell transplantation studies may be found in the presence of a paracrine-mediated effect in cardiac repair, the goal of achieving cardiomyogenesis by direct cell introduction into the injured heart remains elusive. As the search for a true cardiomyogenic cell population continues, several laboratories have reported an endogenous population of cardiac stem/progenitor cells (CSCs) residing in the postnatal heart. Adult CSCs have been isolated based on the expression of surface markers or functional features such as c-Kit, Sca-1, MDR-1/ABCG2 (a.k.a. side population), and aggregational properties (i.e. cardiosphere) [39–42]. The capacity of these CSCs to self-renew in a clonal fashion in vitro and differentiate into multiple cardiovascular lineages both in vitro and in vivo suggests the potential therapeutic benefit of these cells following transplantation into the injured heart [43]. The first study (SCIPIO) evaluating the safety and feasibility of c-Kit+ CSCs in a clinical context was recentlyreported [44]. The encouraging finding is that harvesting and ex vivo expansion of c-Kit+ CSCs appears to be feasible, and no overt toxicity has been found thus far. While not pre-specified as the primary endpoints, LV systolic function increased and infarct size reduced following intracoronary infusion of autologous ex vivo expanded c-Kit+ cardiac cells in ischemic heart failure patients. Further studies will be needed to determine whether the functional benefit achieved is due to new cardiomyogenesis or paracrine effects as observed in bone marrow cell transplantation. In addition, as the identity and the cardiomyogenic potential of cardiac c-Kit+ cells becomes clarified between different groups [45, 46], it will be important to have additional confirmation of the results in SCIPIO by other investigators in order to help sustain the interest of cardiovascular clinicians and scientists in this approach.
In this regard, a prospective, randomized Phase 1 study of cardiosphere-derived cells (CDCs) (CADUCEUS) was recently reported [47]. Unlike the SCIPIO study where cardiac cells were further purified based on their expression of c-Kit, cardiosphere-derived cells are collected from aggregating cells following right heart biopsy and ex vivo expansion. They express c-Kit in ~20% of the cells [48]. When introduced into patients with systolic heart failure, CDC-treated patients showed an apparent reduction in scar size and a corresponding increase in heart muscle mass. Regional contractility was increased as well. Interestingly, the end-diastolic and end-systolic volume, and the overall left ventricular ejection fraction (LVEF) were not changed compared to control patients. It remains to be seen whether the transplantation of CDCs results in new cardiomyocyte generation, given the reported cardiomyogenic potential of these cells, or whether an endogenous cardioprotective mechanism becomes activated to prevent myocyte loss. While LVEF is an imperfect measurement of systolic function, the lack of a significant increase in this parameter, despite the reported reduction in scar size and increase in heart muscle mass, suggests that even if new cardiomyocytes were generated from the transplanted CDCs, their ability to contribute to positive force generation during systole is somewhat limited. Further studies that address the extent of CDC engraftment in the transplanted heart, the degree of their cardiomyocyte differentiation, and the extent of electrical coupling with native cardiomyocytes will help clarify the apparent disconnect between the increase in muscle mass and the lack of improvement in LVEF.
Pluripotent stem cells
Thus far, our efforts to regenerate the diseased heart have focused on identifying a population of cells that is both autologous and most likely to harbor cardiomyogenic potential. The immunocompatibility and the relative accessibility of autologous cells have been chosen over cardiomyogenic efficiency. This bias has been deliberate since it allows us to move from preclinical studies to human trials in a relatively short period of time. As we speculate on where cell therapy for cardiac disease might be in another decade, it is worth revisiting our original goal of in vivo cardiomyocyte replacement. There is a general agreement that pluripotent stem cells such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) have unambiguous ability to differentiate into most, if not all, major cell types within the body. Since their initial discovery in 1998 [49], human ESCs have been touted as one of the most promising cell sources for regenerative therapies. The ability of human ESCs to self-renew indefinitely in vitro and differentiate into cell types of interest with the supplementation of selected growth factors has stimulated the development of various differentiation strategies to increase the efficiency of cardiomyocyte generation and purification [50].
One of the main reasons for the need to generate a high degree of cardiomyocyte purity is the alarming frequency of teratoma formation when undifferentiated ESCs are introduced into the heart [51, 52]. Indeed, the issue of tumorigenicity has raised the threshold for FDA approval of human ESC products so high that one well-recognized company in this area had to eliminate its entire program. There are currently active trials on Stargardt’s disease and adult macular degeneration using human ESC-derived retinal epithelial cells. It remains to be seen whether teratoma formation will be an issue in these early applications of human ESC-derived products.
For cardiac diseases, a number of human ESC-derived cardiomyocyte (hESC-CM) preclinical studies have been published [21, 53]. In these studies, the transplantation of hESC-CMs into the injured rodent heart has resulted in small-sized grafts that are largely electrically isolated. As a consequence, the functional benefit observed early (4–6 weeks) after transplantation was not sustained at 12 weeks or beyond. An encouraging finding from these studies is that no teratoma formation was observed despite the presence of ~20% non-cardiomyocytes within the cardiomyocyte-enriched cell population that underwent transplantation. However, since these studies were performed as xenografts in immunocompromised rodent hosts and the number of the engrafted cells has been very small, it remains to be seen if teratomas would be found when a larger number of these cells are transplanted into humans.
Assuming the teratoma concern can be eliminated in the near future, a patient who undergoes hESC-CM treatment would still require immune suppression given the allogeneic source of the engrafted hESC-CMs. Despite intensive efforts to generate patient-specific hESCs by somatic cell nuclear transfer (SCNT), there has so far been no bona fide hESC line created by this approach. The interest in pursuing the generation of patient-specific human ESCs from SCNT waned when it was discovered by Takahashi and Yamanaka that the forced expression of Oct4, Sox2, Klf4, and c-Myc can induce a pluripotent cell phenotype from somatic cells [54, 55]. These so-called induced pluripotent stem cells (iPSCs) exhibit properties highly similar, but not identical, to hESCs and can readily generate cardiomyocytes by in vitro differentiation using protocols similar to those used for hESCs. While the generation of iPSCs from patients’ own fibroblasts should, in principal, obviate the need for immunosuppression when cardiomyocytes derived from these cells are transplanted, a recent study reported the triggering of an immune response against autologous iPSC-derived teratoma in mice, raising the possibility of rare antigen expression in iPSCs that is not normally expressed in ESCs [56, 57]. It would be important to clarify whether these antigens are unique to teratoma per se or are present in all iPSC-derived cells. If these antigens are expressed ubiquitously in iPSCs and their progenies, the advantage of autologous human iPSCs over allogenic hESCs would be limited.
An additional consideration that will undoubtedly play a role in pluripotent stem cell-based therapy for cardiac disease is the phenotype of the iPSC/ESC-derived cardiomyocytes. Myocytes in the heart are naturally diverse, with highly specialized physiological attributes and regional diversity that are essential for normal cardiac function. Currently, all protocols for human iPSC/ESC differentiation generate a mixture of atrial and ventricular cardiomyocytes and nodal-like cells. These cells are largely immature and do not resemble the mature rod-shaped, and often bi-nucleated, cardiomyocytes found in an adult mammalian heart. Their fetal ion channel expression and electrophysiological properties may potentially be arrhythmogenic if electrically coupled with endogenous mature cardiomyocytes. If so, toxicity issues will significantly diminish their translational potential in clinical applications [58, 59]. In this respect, efforts addressing factors that regulate ventricular vs atrial vs nodal-specific differentiation will be highly valuable [60]. Furthermore, understanding the key roadblocks that prevent electromechanical maturation of in vitro differentiated iPSC/ESC-derived cardiomyocytes will help to minimize cardiotoxicity from cardiomyocyte transplantation. Ultimately, it will be important to determine the exact degree of cardiomyocyte maturation needed to enable the most optimal engraftment, expansion, and maturation after transplantation. It might be the case that transplanting cardiomyocyte progenitor cells can lead to greater cell engraftment and survival in the diseased heart but these cells mature poorly and form a heterogeneous population of cardiovascular cells that becomes arrhythmogenic. On the other hand, transplanting fully mature cardiomyocytes may result in poor overall engraftment and survival due to their greater demand for oxygen and cell-cell contact despite being more phenotypically compatible with endogenous adult cardiomyocytes. Additional studies in large animal disease models using human ESC/iPSC-derived cardiac progenitor cells, immature cardiomyocytes, and mature ventricular cardiomyocytes should help to resolve some of these dilemmas.
Direct cardiomyocyte reprogramming
The remarkable success of somatic cell reprogramming into iPSCs has generated a renewed interest in direct cell lineage reprogramming since the discovery of MyoD [61]. Indeed, reports of fibroblast conversion into neurons, blood, and liver cells by transcription factor overexpression have been published recently [62–67]. The advantage of somatic cell transdifferentiation into another adult cell without an intermediate state of pluripotency is that it may circumvent the risk of teratoma formation associated with pluripotent stem cell-derived cell transplantation. In this regard, Ieda et al. reported the reprogramming of murine postnatal cardiac and tail tip fibroblasts into cardiomyocyte-like cells by overexpressing a combination of three cardiac transcription factors - Gata4, Mef2c, Tbx5 [68]. Using fibroblasts from αMHC-GFP transgenic mice, approximately 6% of virally infected cells were double positive for GFP and cardiac Troponin-T. In rare instances, spontaneous calcium transients were noted in infected but not control cardiac fibroblasts. While in vitro reprogrammed fibroblasts may constitute a novel source of transplantable cardiomyocytes without the risk of teratoma formation, we believe the reprogramming efficiency must improve significantly (e.g. up to greater than 50% conversion into cardiomyocytes from a starting pool of fibroblasts) for this strategy to be therapeutically relevant, given the issues of cell retention and survival after transplantation discussed above and the lack of ability of these cells to proliferate after transplantation. Nevertheless, the prospect for cellular reprogramming to play a role in cardiac regenerative therapy is quite intriguing and the development of a robust and reproducible protocol for direct conversion of fibroblasts into cardiomyocytes will be important to move the field forward. If this can be achieved, we envision the possibility that one day we might directly reprogram scar fibroblasts in the injured heart without the need for cell transplantation. The recent reports that in vivo reprogramming of scar fibroblasts into cardiomyocytes appears to be more efficient than in vitro reprogramming is a promising first step towards making this a clinical reality [69, 70].
Future perspective
In the past decade, we have witnessed tremendous excitement among basic and translational scientists towards therapeutic strategies that involve direct cell transplantation into the injured heart. From the wealth of preclinical and clinical data gathered, we gained a greater appreciation for the inherent challenges in survival, retention, cardiomyogenic differentiation, and functional integration of cell transplantation. While no durable therapy has arisen from these efforts thus far, the knowledge we gained with regard to cell procurement, processing, and delivery will be very useful for ongoing and future cell transplantation studies and should improve our chances of success with this approach.
Important issues that will continue to require major investigative efforts include the identification of the most effective strategy for cell engraftment and survival, the most efficient delivery technology to accomplish this goal, the most relevant cell type for transplantation that can achieve cardiomyogenesis to the level that directly contributes to positive force generation, and the improvement in hard clinical endpoints such as reduction in mortality and recurrent hospitalization. With regard to translational clinical studies, the challenges of objectively quantifying improvement in cardiac contractility and function in humans will require appropriate trial design and incorporation of technologies that are least susceptible to observer bias. In this regard, it is noteworthy that many of the bone marrow trials reporting improvement in LVEF employed echocardiography as the method of functional assessment whereas studies utilizing cardiac MRI showed less or no improvement after bone marrow cell transplantation. This suggests that meticulous execution of a double-blinded trial design and the incorporation of cardiac MRI in the assessment of LVEF will be optimal in future trials. Given the relatively small change in LVEF after cell transplantation, the absence of data on mortality or major adverse cardiovascular events, and the small number of patients studied in each trial thus far, future clinical studies will likely shift away from patients with acute myocardial infarction towards patients with ischemic heart failure or vascular insufficiency where the benefit from cell therapy may be greater. As we continue the noble pursuit of cardiac regeneration, it will be important to maintain objectivity in the reporting of preclinical and clinical outcomes in order prevent the creation of unrealistic expectations from the public, particularly given the low societal tolerance for medical errors.
We believe the future prospect for cardiovascular cell therapy remains bright. The finding of an effective therapy that can remuscularize a damaged heart will not only be a remarkable achievement in modern medicine–it will be the most effective, if not the only way that we can reverse the growing incidence of heart failure worldwide.
Acknowledgments
Sources of Funding
This work was supported by the German Heart Foundation (M-A.D), the AHA Founders Affiliate and the Lawrence J. & Florence A. DeGeorge Charitable Trust Post-Doctoral Fellowship (A.S), the NIH/NHLBI (S.M.W), NIH/Office of the Director (S.M.W), and the Harvard Stem Cell Institute (S.M.W).
Non-standard Abbreviations and Acronyms
- αMHC
alpha myosin heavy chain
- ABCG2
ATP-binding cassette sub-family G member 2
- BMC
bone marrow-derived cell
- CDC
cardiosphere-derived cell
- CSC
cardiac stem cell
- ESC
embryonic stem cell
- GFP
green fluorescent protein
- hESC-CM
human embryonic stem cell-derived cardiomyocyte
- iPSC
induced pluripotent stem cell
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MDR-1
multidrug resistance gene-1
- MI
myocardial infarction
- MSC
mesenchymal stem cell
- PET
positron emission tomography
- Sca-1
stem cell antigen-1
- SCNT
somatic cell nuclear transfer
Footnotes
Disclosures
None.
References
- 1.Krum H, Abraham WT. Heart failure. Lancet. 2009;373:941–55. doi: 10.1016/S0140-6736(09)60236-1. [DOI] [PubMed] [Google Scholar]
- 2.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report-2011. J Heart Lung Transplant. 2011;30:1078–94. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am CollCardiol. 2006;47:1777–85. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–33. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 5.Durrani S, Konoplyannikov M, Ashraf M, Haider KH. Skeletal myoblasts for cardiac repair. Regen Med. 2010;5:919–32. doi: 10.2217/rme.10.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagège AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 7.Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, Doran R, Becher UM, Hwang SM, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI, Fleischmann BK. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–24. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 8.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 9.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 10.Assmus B, Honold J, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 11.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 12.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 13.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–18. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 15.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moyé LA, Simari RD Cardiovascular Cell Therapy ResearchNetwork. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–9. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moyé LA, Simari RD Cardiovascular Cell Therapy Research Network (CCTRN) Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moyé LA, Simari RD Cardiovascular Cell Therapy Research Network (CCTRN) Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380–9. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 19.Li SH, Lai TY, Sun Z, Han M, Moriyama E, Wilson B, Fazel S, Weisel RD, Yau T, Wu JC, Li RK. Tracking cardiac engraftment and distribution of implanted bone marrow cells: Comparing intra-aortic, intravenous, and intramyocardial delivery. J ThoracCardiovasc Surg. 2009;137:1225–33. doi: 10.1016/j.jtcvs.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–6. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 21.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 22.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 23.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 24.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 25.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, Kajstura J, Bolli R. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 26.Malliaras K, Marbán E. Cardiac cell therapy: where we’ve been, where we are, and where we should be headed. Br Med Bull. 2011;98:161–85. doi: 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, Musiałek P, Piwowarska W, Nessler J, Buszman P, Grajek S, Breborowicz P, Majka M, Ratajczak MZ REGENT Investigators. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–21. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 28.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–7. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 29.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–98. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–26. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 33.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Yu X, Lin Q, Deng C, Shan Z, Yang M, Lin S. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42:295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 36.Hare JM, Fishman JE, Gerstenblith G, Difede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of Allogeneic vs Autologous Bone Marrow-Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients With Ischemic Cardiomyopathy: The POSEIDON Randomized Trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 39.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 40.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. ProcNatlAcadSci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–75. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 42.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 43.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–61. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN, Menasché P. Cardiac stem cells in the real world. J ThoracCardiovasc Surg. 2008;135:673–8. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 47.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chimenti I, Smith RR, Li TS, Gerstenblith G, Messina E, Giacomello A, Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 50.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–57. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 52.Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, Geisen C, Xia Y, Lu Z, Duan Y, Kettenhofen R, Jovinge S, Bloch W, Bohlen H, Welz A, Hescheler J, Jacobsen SE, Fleischmann BK. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–27. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.vanLaake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 56.Fairchild PJ. The challenge of immunogenicity in the quest for induced pluripotency. Nat Rev Immunol. 2010;10:868–75. doi: 10.1038/nri2878. [DOI] [PubMed] [Google Scholar]
- 57.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 58.Beqqali A, Kloots J, Ward-van Oostwaard D, Mummery C, Passier R. Genome-wide transcriptional profiling of human embryonic stem cells differentiating to cardiomyocytes. Stem Cells. 2006;24:1956–1967. doi: 10.1634/stemcells.2006-0054. [DOI] [PubMed] [Google Scholar]
- 59.Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–86. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 62.Chambers SM, Studer L. Cell fate plug and play: direct reprogramming and induced pluripotency. Cell. 2011;145:827–30. doi: 10.1016/j.cell.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010 Nov 25;468:521–6. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 65.Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Südhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–82. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–7. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 67.Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, Brüstle O, Edenhofer F. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10:473–9. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song K, Nam Y-J, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]