Abstract
Few studies exist in the literature investigating the impact of idiopathic Parkinson’s Disease (IPD) on swallow-related quality of life. We therefore aimed in this project to: (1) evaluate swallow-specific quality of life in IPD; (2) delineate potential relationships between IPD duration and severity with swallow-specific quality of life; (3) investigate relationships between swallow-specific quality of life and general health-related quality of life; and (4) investigate relationships between swallow-specific quality of life and depression. Thirty-six patients diagnosed with IPD with and without dysphagia filled out self-report assessments of the SWAL-QOL, Parkinson’s Disease Questionnaire-39 (PDQ-39), and Beck Depression Inventory (BDI). A series of Mann Whitney U tests were performed between non-dysphagic and dysphagic groups for the total SWAL-QOL score and the 10 SWAL-QOL domains. Spearman’s Rho correlation analyses were performed between the SWAL-QOL and (1) PDQ-39; (2) Hoehn and Yahr stage; (3) PD disease duration; (4) UPDRS “on” score; and (5) the BDI. The dysphagia swallowing group reported significant reductions compared to the nondysphagic group for the total SWAL-QOL score (P = 0.02), mental health domain score (P = 0.002) and social domain score (P = 0.002). No relationships existed between swallow-specific quality of life and disease duration or severity. Significant relationships existed between swallow-specific quality of life and general health-related quality of life (rs =−0.56, P = 0.000) and depression (rs = −0.48, P = 0.003). These exploratory data highlight the psychosocial sequelae that swallowing impairment can have in those with IPD and suggest a possible association between swallowing, social function, and depression.
Keywords: swallowing, idiopathic Parkinson’s disease, quality of life, depression
Idiopathic Parkinson’s Disease (IPD) is a chronic and progressive neurodegenerative syndrome associated with substantial morbidity, increased mortality, and a high economic burden on society.1 Ninety percent of individuals with IPD develop dysphagia during their disease course2 with the leading cause of death as aspiration pneumonia.3 Swallowing impairments can occur during any of the swallow phases and are usually attributed to movement dysfunction of affected bulbar structures. These can include lingual tremor,4 repetitive lingual pumping,5 anterior bolus leakage,6 slow or impaired mastication,7 mandible rigidity,7 reduced and delayed pharyngeal constrictor contraction,8 slow and reduced laryngeal excursion,5,9 slowing of true vocal fold closure,9 reduced epiglottic range of movement,10 reduced and delayed opening of the upper esophageal sphincter (UES) and lower esophageal sphincter (LES),8,10 abnormal esophageal motility and esophageal bolus redirection.10 These bulbar movement abnormalities may contribute to functional swallowing deficits that include poor oral bolus control,11 ineffective oral transit,7 increased oral transit time,5,12 oral buccal residue,5 premature spillage of the bolus into the valleculae,5 delay in the execution of the swallow reflex,11 stasis in the valleculae or pyriforms,5,10 penetration and/or aspiration,5,10,13,14 and gastroesophageal reflux.10
Although the physical signs of dysphagia are well documented,15 little attention has been given to the psychosocial impact of dysphagia in individuals with IPD. Only one study has investigated the impact of swallowing function on quality of life in people with IPD and reported significant psychosocial consequences of swallowing abilities.16 In other disease populations dysphagia has been linked to social withdrawal,17–20 decreased self-esteem,17,18,20–22 and increased mealtime anxiety.17,20 Measures from patients’ point of view are important because (1) clinicians and patient perspectives often differ,23 (2) these measures often gauge the impact of a disease or condition in a daily living setting, and (3) this information can guide management strategies. Historically, outcomes from the patient’s point of view have been difficult to measure because they are typically more abstract than physiologic outcomes. The past decade has brought with it the development of many reliable and validated tools to measure quality of life from the patient’s perspective.23,24
Therefore, the aims of this study were to (1) evaluate swallow-specific quality of life in IPD; (2) delineate potential relationships between IPD duration and severity with swallow-specific quality of life; (3) investigate relationships between swallow-specific quality of life and general health-related quality of life; and 4) investigate relationships between swallow-specific quality of life and depression. We hypothesized that (1) swallow dysfunction would adversely impact swallow-related quality of life in IPD; (2) swallow-specific quality of life would be more adversely impacted with increasing disease duration and severity; and (3) because eating is such an important sociocultural function, swallow-specific quality of life would be related to general quality of life and depression in IPD patients.
METHODS
Participants
Thirty-six individuals referred to the University of Florida (UF) Speech and Hearing Clinic by a Movement Disorders neurologist participated in this study. To be included, participants had to meet the diagnostic criteria for IPD in accordance with the U.K. Brain Bank criteria25 and have no other neurological disease. The study protocol was approved by the UF Institutional Review Board. Each participant signed an informed consent. Table 1 presents participant demographics.
TABLE 1.
Patient demographics for the overall group, non-dysphagic swallowing group, and dysphagic swallowing group
| Overall
|
Non-dysphagic
|
Dysphagic
|
||||
|---|---|---|---|---|---|---|
| Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | |
| Agea | 43–85 | 65.6 (10.8) | 43–85 | 64.2 (10.8) | 50–82 | 69.8 (10.4) |
| PD Durationa | 1–28 | 11.57 (6.5) | 1–22 | 10.3 (5.2) | 4–28 | 15.5 (8.7) |
| Hoehn and Yahr | 1.5–5.0 | 2.7 (1.0) | 1.5–5.0 | 2.4 (0.7) | 2.0–5.0 | 3.6 (1.2) |
| UPDRS “on” | 4–85 | 32.6 (17.9) | 4–85 | 29.2 (17.2) | 11–69 | 42.5 (16.9) |
| UPDRS “off” | 9–70 | 38.7 (13.8) | 9–70 | 34.9 (13.5) | 35–64 | 48.6 (9.4) |
SD, standard deviation; UPDRS, Unified Parkinson’s Disease Rating Scale.
Years.
Outcome Measures
Three validated surveys evaluated swallow-specific quality of life, health-related quality of life, and depression. The quality of life in swallowing disorders (SWAL-QOL),23,26,27 contains 44-items divided into 10 domains (burden, eating duration, eating desire, food selection, communication, fear, mental health, social, sleep, and fatigue). Each item is given a score from 0 to 4 (worst-best). Scoring in each domain is calculated by the sums of scores for each item expressed as a percentage of the maximum possible domain score. A total SWAL-QOL score is derived by summing each domain score and dividing by 10 giving a total SWAL-QOL score that ranges between 0 and 100 (worst–best).
The Parkinson’s Disease Questionnaire-39 (PDQ-39)28 is a PD-specific measure of health-related quality of life containing 39-items divided into 8 domains (mobility, activities of daily living, emotions, stigma, social, cognitive, communication, and body discomfort). Each item has a score from 0 to 4 (best–worst). Scoring in each domain is calculated by the sums of scores for each item expressed as a percentage of the maximum possible domain score. For this study, a total PDQ-39 score was derived by summing each domain score percentage and dividing by 8. Thus, a total score could range between 0 and 80 (best–worst health-related quality of life).
The Beck Depression Inventory (BDI)29 is a self-report measure of depression. This instrument contains 21 self-evaluative comments scored between 0 and 3 (least severe–most severe). The total BDI score is the sum of all items and can range from 0 to 61. A BDI score of 0–13 is considered to be in the minimal range; 14–19, mild; 20–28, moderate; and 29–63, severe.
Statistical Analyses
Descriptive statistics tallied the SWAL-QOL scores for each of the 10 SWAL-QOL domains. Additionally, participants were divided into non-dysphagic and dysphagic groups. Those in the non-dysphagic group included 27 individuals with IPD who reported no restrictions in food and liquid intake by answering item “a” to questions 11 and 12 of the SWAL-QOL. The dysphagic group consisted of 9 individuals with IPD who reported being on a restricted diet in questions 11 and 12 by answering with items “b”, “c”, or “d”. These participants included those who were on a puree diet, mechanical soft diet, using thickened liquids, or requiring nonoral feeds. In addition, a clinical diagnosis of dysphagia was verified from a UF Speech-Language Pathologist with 45 years of clinical experience in swallowing disorders. This clinical diagnosis was based on a thorough swallowing history and clinical examination that included movement integrity of the lips, tongue, larynx, and pharynx using nonverbal, maximum performance tasks and liquid swallows.
To examine the impact of swallowing impairment on swallow-specific quality of life a series of Mann-Whitney U tests were performed between the non-dysphagic and dysphagic swallowing groups for the total SWAL-QOL score and each of the 10 SWAL-QOL domain scores. To correct for multiple comparisons, the Holm’s step-down procedure was applied.
To examine the impact of IPD disease duration and severity on swallow-related quality of life (aim two) a series of Spearman’s Rho correlation analyses were performed between the total SWAL-QOL score and (1) IPD disease duration in months, (2) Hoehn and Yahr stage, and (3) UPDRS “on medication” motor score. A Spearman’s Rho correlation analysis examined the relationship between the PDQ-39 score and the total SWAL-QOL score as well as each of the 10 SWAL-QOL domain scores. The Holm’s step-down procedure was applied in this analysis to adjust for multiple testing. Finally, to examine the relationship between depression and swallow-specific quality of life a Spearman’s Rho correlation analysis between the BDI and total SWAL-QOL score was performed.
RESULTS
Patient Reported Swallow-Related Quality of Life
Table 2 presents summary data for the 10 SWAL-QOL domains across overall, non-dysphagic and dysphagic groups. The combined data (overall group) indicated that swallow-specific quality of life was mild to moderately involved with mean SWAL-QOL domain scores ranging between 42 and 76. Group data revealed that swallow dysfunction adversely affected patient reported quality of life with a significant difference observed between the total SWAL-QOL score for the non-dysphagic group (mean, 65.40; SD, 18.98) and dysphagic group (mean, 47.09; SD, 18.37) (Z = 2.17, P = 0.02). The dysphagic swallow group reported lower scores across all SWAL-QOL domains except for the sleep domain and with equivalent mean scores for the eating desire domain across groups. Significant differences existed between nondysphagic and dysphagic swallow groups for mental health (Z = −3.05, P = 0.00) and social (Z = −3.12, P = 0.00) domains, with the burden domain approaching significance (Z = − 2.36, P = 0.02).
TABLE 2.
SWAL-QOL domain data for the overall group, non-dysphagic swallowing group, and dysphagic swallowing group
| Overall
|
Non-dysphagic
|
Dysphagic
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Range | Median | Mean ± SD | Range | Median | Mean ± SD | Range | Median | Median ± SD | P value |
| Burden | 25–100 | 62.5 | 62.1 ± 24.7 | 25–100 | 75.0 | 67.5 ± 23.7 | 25–75 | 37.5 | 42.9 ± 18.9 | 0.019 |
| Eating duration | 0–100 | 62.5 | 60.7 ± 31.1 | 0–100 | 62.5 | 65.3 ± 28.9 | 0–100 | 50.0 | 45.3 ± 35.3 | 0.134 |
| Eating desire | 0–100 | 83.3 | 75.9 ± 27.2 | 16.7–100 | 83.3 | 76.8 ± 26.6 | 0–91.7 | 83.3 | 72.9 ± 30.8 | 0.483 |
| Symptom freq | 25–89.3 | 64.3 | 59.9 ± 30.8 | 27.3–89.3 | 64.3 | 63.0 ± 15.2 | 25–71.1 | 43.9 | 49.2 ± 16.5 | 0.0.87 |
| Food selection | 12.5–100 | 75.0 | 67.5 ± 27.1 | 12.5–100 | 75.0 | 70.8 ± 28.4 | 25–87.5 | 56.2 | 56.2 ± 20.0 | 0.141 |
| Communication | 0–100 | 50.0 | 51.8 ± 31.8 | 0–100 | 50.0 | 54.2 ± 32.5 | 0–100 | 43.7 | 43.7 ± 29.9 | 0.360 |
| Fear | 6.2–100 | 68.7 | 65.5 ± 23.2 | 31.2–100 | 68.7 | 69.6 ± 21.2 | 6.2–100 | 63.5 | 51.8 ± 25.9 | 0.220 |
| Mental health | 0–100 | 65.0 | 61.5 ± 28.4 | 0–100 | 75.0 | 69.9 ± 24.1 | 0–65 | 37.5 | 33.1 ± 24.2 | 0.002a |
| Social | 15–100 | 70.0 | 67.6 ± 27.0 | 25–100 | 80.0 | 75.3 ± 23.5 | 15–85 | 42.5 | 40.6 ± 21.4 | 0.002a |
| Sleep | 0–100 | 68.7 | 60.4 ± 26.3 | 0–100 | 62.5 | 58.5 ± 31.0 | 0–100 | 75.0 | 67.2 ± 31.3 | 0.419 |
| Fatigue | 0–100 | 50.0 | 47.4 ± 26.3 | 0–100 | 50.0 | 48.2 ± 26.2 | 8.3–75 | 41.7 | 44.8 ± 28.5 | 0.804 |
To correct for multiple comparisons, the Holm’s step-down procedure was applied, in which the kth smallest P value is compared with 0.05/(11-k).
Significant difference between non-dysphagic and dysphagic groups after adjustment of multiple testing.
SD, standard deviation.
Relationship Between Swallow-Related Quality of Life and IPD Severity
No significant correlations existed between months with symptoms and total SWAL-QOL score (rs = − 0.02, P = 0.93), Hoehn and Yahr score and total SWAL-QOL score (rs = −0.10, P = 0.59) or UPDRS “on” score and total SWAL-QOL score (rs = −0.12, P = 0.50).
Swallow-Related Quality of Life and General Quality of Life
A significant negative correlation was found between total SWAL-QOL and PDQ-39 scores (rs =−0.56, P = 0.000). Because these tests are scored on an opposite scale, this meant that the better the reported swallow-specific quality of life (i.e. higher the SWAL-QOL score), the better the reported health-related quality of life (i.e. lower the PDQ-39 score). Figure 1 depicts a scatter plot of this negative linear trend.
FIG. 1.

Scatterplot with regression line depicting the relationship between the total SWAL-QOL and total PDQ-39 Score in this group of patients with IPD.
To investigate which specific SWAL-QOL domains were related to general patient-reported quality of life, a series of correlation analyses were performed between the PDQ-39 and each of the ten SWAL-QOL domains with an adjustment for multiple testing. This revealed four significant negative correlations that are presented in Table 3 in the order of relatedness.
TABLE 3.
Significant correlations between the PDQ-39 and SWAL-QOL domains
| SWAL-QOL domain | Spearmans Rho (rs) | P value |
|---|---|---|
| Fatigue | −0.569 | 0.000 |
| Food Selection | −0.525 | 0.001 |
| Social | −0.508 | 0.002 |
| Communication | −0.483 | 0.004 |
Relationship Between Swallow-Related Quality of Life and Depression
A significant negative correlation was found between total SWAL-QOL score and BDI (rs =−0.48, P = 0.003). Because these tests are scored on an opposite scale, this meant that the worse the swallow-specific quality of life (i.e. lower the SWAL-QOL score), the worse the depression (i.e. higher the BDI score). Figure 2 shows a scatter plot of this relationship.
FIG. 2.
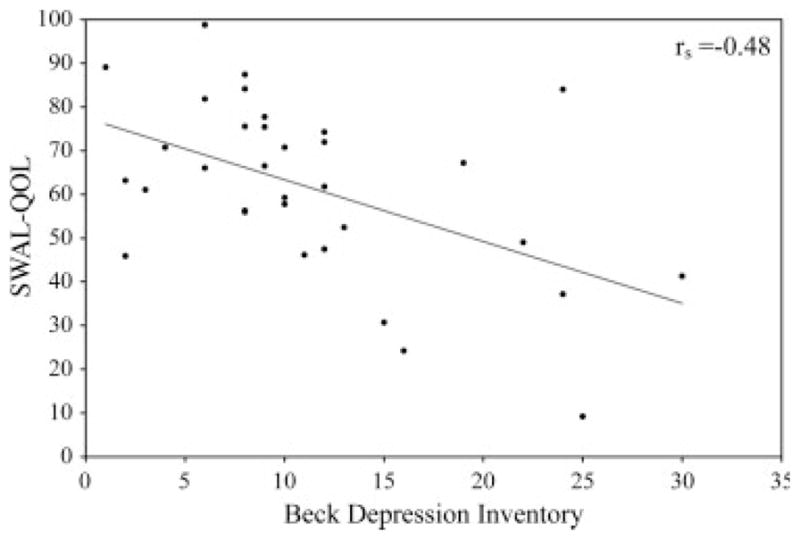
Scatterplot with regression line depicting the relationship between the total SWAL-QOL and total BDI Scores in this group of patients with IPD.
DISCUSSION
The combined dataset indicated that swallow-specific quality of life was mild to moderately reduced, with the dysphagic swallowing group reporting worse swallow-specific quality of life. No relationships were found between swallow-specific quality of life and disease duration or disease severity; however, significant relationships were observed between swallow-specific quality of life and general health-related quality of life and also with depression.
The modest reductions in overall swallow-related quality of life are likely related to the fact that only 9 participants were on nonoral or altered diets. After partitioning the participants into non-dysphagic or dysphagic swallowing groups it became clearer that swallowing dysfunction affected swallow-related quality of life domains in different ways. Although sleep and eating desire were not adversely affected by swallowing dysfunction, the domains of burden, social function, and mental health were most detrimentally impacted as indicated by large differences in domain scores between swallowing groups.
The detrimental impact of swallowing dysfunction on the burden domain may be related to learning and implementing safe swallow strategies such as a chin tuck or swallow maneuver; preparation of foods and liquids that conform to a certain texture or consistency; financial obligations to pay for medical services, and special dietary needs as well as continual maintenance of nonoral feeding devices. These additional responsibilities on a patient with potentially reduced capacity could be burdensome.
The dysphagic group perceived their role with family and friends, ability to eat out, or engage in social, vocational and leisure activities as reduced. The current finding is consistent with reports in other patient populations where dysphagia has been reported to adversely impact social functioning. Nguyen et al.21 reported embarrassment and reductions in self-esteem and confidence in a head and neck cancer population, and concluded that the inability to eat ultimately represented a social handicap. In a nursing home setting, Chow et al.17 reported reductions in social activities, while Ekberg et al.20 observed that 41% of dysphagic individuals experienced anxiety or panic during meals. Also 36% of the sample avoided eating with others leading to increased feelings of social isolation and loss of self-esteem. In a post-surgical dysphagic population, Farri et al.18 reported reductions in self-confidence, social relations, and increased isolation. Finally, in a group of patients with esophageal dysphagia, Gustafsson and Tibbling22 reported decreased levels of self-esteem, security, work capacity, exercise, and leisure time. This dataset contributes to these findings for those with IPD providing additional emerging evidence that swallowing dysfunction can have pervasive social implications that negatively impact quality of life.
In this study, those with dysphagia also reported significant reductions in the mental health domain. This finding may be related to the aforementioned worsening in social functioning and feelings of burden. The inability to fully engage in society, the isolation, mealtime anxiety, and increased burden frequently encountered in individuals with dysphagia likely contribute to mental well-being. Additionally, the finding that all individuals retained a strong desire to eat, regardless of swallowing abilities or dietary intake, likely contributed to feelings of frustration for those with dysphagia who had been prescribed either a nonoral or altered diet. Further, a significant correlation was found between the BDI and SWAL-QOL scores. This relationship must be interpreted with caution, since it is known that depressed individuals are more likely to report lower scores in tests involving self-report. Yet, one would expect if this were true in this study, that these individuals would have rated all items across all domains consistently low and not just the mental health and social function domains.
Interestingly, other investigators have reported a link between depression and dysphagia. Via logistic regression, Eslick et al.30 reported that in a large general adult population intermitant dysphagia was independently associated with anxiety while progressive dysphagia was independently associated with depression. In a head and neck cancer population, Nguyen et al.21 reported statistically significant increases in anxiety and depression in individuals with dysphagia compared to those with no dysphagia. Chow et al.17 identified swallowing impairment to be an independent risk factor for depression in a nursing home setting. Likewise, Lin et al.17 reported the prevalence for depression as 52% of patients and identified swallow function as an independent risk factor. Depression has also been associated with dysphagia in neurologic groups. In a group of patients with Amyotrophic Lateral Sclerosis (ALS), Hillemacher et al.31 noted that while general quality of life [as measured by the ALS Functional Rating Scale (ALS-FRS)] was not associated with self-rated depression, impairment of swallowing correlated significantly with depression. These authors also noted a trend for the “bulbar score” of the ALS-FRS (composed of speech, saliva, and swallowing items) to be correlated with depression and concluded that patients with bulbar symptoms should be carefully screened for depression. Bretan et al.32 presented five cases of patients with multiple sclerosis and dysphagia with emotional distress. Four of the five patients had anxiety and one depression. This small but growing body of evidence underscores the notion that the sequelae of dysphagia are far reaching and include psychosocial domains. Further information regarding the psychosocial impact of dysphagia in those with IPD warrants attention to better guide treatment strategies.
Next, patient reported swallow-specific quality of life was not significantly related to disease duration or severity and suggests that the clinician needs to be cognizant in attempting to identify reductions in swallow-related quality of life domains throughout all stages of the disease, not just in advanced cases.
As hypothesized, swallow-specific quality of life and general health-related quality of life measures were significantly related. In addition, the specific SWAL-QOL domains of fatigue, food selection, social function, and communication were highly related to the overall total health-related quality of life score indicating their contribution to overall feelings of quality of life. Notably, both the SWAL-QOL and PDQ-39 instruments contain social and communication domains, and although they have different items that related to either swallow specific functions or general functions respectively, they are still measuring a similar construct which may account, in part, for the strong relationship observed.
Because the literature to date has focused on the physiologic aspects of swallowing in IPD13,33,34 it will be important for clinicians to consider emerging data, and therefore also assess psychosocial functioning. The results of this study also highlight the need for neurologists and speech-language pathologists to collaborate closely with mental health practitioners in the treatment of those with IPD and dysphagia. Clinicians should incorporate education and counseling regarding the high prevalence of reductions in social and mental health domains so that patients and caregivers can be informed to deal with any reductions in these areas of functioning. Finally, these findings highlight the need for rehabilitation specialists to incorporate treatment strategies that maximize social functioning, minimize burden on the patient and caregiver, and thereby reduce the likelihood of reductions in mental well-being.
This study provides much-needed information regarding the impact of swallowing alterations in IPD on quality of life. As a retrospective pilot investigation using a small convenience sample of patients with IPD the data do provide direction for future work to investigate the psychosocial impact of dysphagia in a larger and more general IPD population where a more rigorous verification of swallowing impairment can be performed.
Acknowledgments
The project described was supported by Grant Number T32 DC008768 from the National Institute of Deafness and Other Communication Disorders (NIH-NIDCD) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH-NIDCD.
Footnotes
Potential conflict of interest: None reported.
Author Roles: Emily Plowman-Prine: Research project: A. Conception, B. Organization, C. Execution; Statistical Analysis: A. Design, B. Execution; Manuscript: A. Writing of the first draft and subsequent revisions. Christine Sapienza: Research project: A. Conception, B. Organization, C. Execution; Statistical Analysis: A. Design, C. Review and Critique; Manuscript: B. Extensive Review and Critique. Michael Okun: Research project: A. Conception, B. Organization, C. Execution; Statistical Analysis: A. Design, B. Execution, C. Review and Critique; Manuscript: B. Review and Critique. Stephenie Pollock: Research project: A. Conception, B. Organization, C. Execution. Manuscript: B. Review and Critique. Charles Jacobson IV: Research project: A. Conception, B. Organization, C. Execution; Manuscript: B. Review and Critique. Sammual Wu: Statistical Analysis: A. Design, B. Execution, C. Review and Critique; Manuscript: B. Review and Critique of methods section. John Rosenbek: (senior author) Research project: A. Conception, B. Organization, C. Execution; Statistical Analysis: A. Design, B. Execution, C. Review and Critique; Manuscript: B. Review and Critique.
References
- 1.Weintraub D, Comella CL, Horn S. Parkinson’s disease, Part 1. Pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care. 2008;14(2 Suppl):S40–S48. [PubMed] [Google Scholar]
- 2.Sapir S, Ramig L, Fox C. Speech and swallowing disorders in Parkinson disease. Curr Opin Otolaryngol Head Neck Surg. 2008;16:205–210. doi: 10.1097/MOO.0b013e3282febd3a. [DOI] [PubMed] [Google Scholar]
- 3.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 4.Durham TM, Hodges ED, Henry MJ, Geasland J, Straub P. Management of orofacial manifestations of Parkinson’s disease with splint therapy: a case report. Spec Care Dentist. 1993;13:155–158. doi: 10.1111/j.1754-4505.1993.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 5.Nagaya M, Kachi T, Yamada T, Igata A. Videofluorographic study of swallowing in Parkinson’s disease. Dysphagia. 1998;13:95–100. doi: 10.1007/PL00009562. [DOI] [PubMed] [Google Scholar]
- 6.Chou KL, Evatt M, Hinson V, Kompoliti K. Sialorrhea in Parkinson’s disease: a review. Mov Disord. 2007;22:2306–2313. doi: 10.1002/mds.21646. [DOI] [PubMed] [Google Scholar]
- 7.Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia. 1996;11:14–22. doi: 10.1007/BF00385794. [DOI] [PubMed] [Google Scholar]
- 8.Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110:383–392. doi: 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- 9.Leopold NA, Kagel MC. Laryngeal deglutition movement in Parkinson’s disease. Neurology. 1997;48:373–376. doi: 10.1212/wnl.48.2.373. [DOI] [PubMed] [Google Scholar]
- 10.Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12:11–18. doi: 10.1007/pl00009512. discussion 19–20. [DOI] [PubMed] [Google Scholar]
- 11.Ertekin C, Tarlaci S, Aydogdu I, et al. Electrophysiological evaluation of pharyngeal phase of swallowing in patients with Parkinson’s disease. Mov Disord. 2002;17:942–949. doi: 10.1002/mds.10240. [DOI] [PubMed] [Google Scholar]
- 12.Nagaya M, Kachi T, Yamada T. Effect of swallowing training on swallowing disorders in Parkinson’s disease. Scand J Rehabil Med. 2000;32:11–15. doi: 10.1080/003655000750045677. [DOI] [PubMed] [Google Scholar]
- 13.Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson’s disease. Dysphagia. 2008;23:26–32. doi: 10.1007/s00455-007-9090-7. [DOI] [PubMed] [Google Scholar]
- 14.Stroudley J, Walsh M. Radiological assessment of dysphagia in Parkinson’s disease. Br J Radiol. 1991;64:890–893. doi: 10.1259/0007-1285-64-766-890. [DOI] [PubMed] [Google Scholar]
- 15.Hind JA, Nicosia MA, Gangnon R, Robbins J. The effects of intraoral pressure sensors on normal young and old swallowing patterns. Dysphagia. 2005;20:249–253. doi: 10.1007/s00455-005-0020-2. [DOI] [PubMed] [Google Scholar]
- 16.Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson’s disease. Age Ageing. 2006;35:614–618. doi: 10.1093/ageing/afl105. [DOI] [PubMed] [Google Scholar]
- 17.Chow ES, Kong BM, Wong MT, et al. The prevalence of depressive symptoms among elderly Chinese private nursing home residents in Hong Kong. Int J Geriatr Psychiatry. 2004;19:734–740. doi: 10.1002/gps.1158. [DOI] [PubMed] [Google Scholar]
- 18.Farri A, Accornero A, Burdese C. Social importance of dysphagia: its impact on diagnosis and therapy. Acta Otorhinolaryngol Ital. 2007;27:83–86. [PMC free article] [PubMed] [Google Scholar]
- 19.Perry L. Dysphagia: the management and detection of a disabling problem. Br J Nurs. 2001;10:837–844. doi: 10.12968/bjon.2001.10.13.837. [DOI] [PubMed] [Google Scholar]
- 20.Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17:139–146. doi: 10.1007/s00455-001-0113-5. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61:772–778. doi: 10.1016/j.ijrobp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Gustafsson B, Tibbling L. Dysphagia, an unrecognized handicap. Dysphagia. 1991;6:193–199. doi: 10.1007/BF02493525. [DOI] [PubMed] [Google Scholar]
- 23.McHorney CA, Bricker DE, Kramer AE, et al. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults. I. Conceptual foundation and item development. Dysphagia. 2000;15:115–121. doi: 10.1007/s004550010012. [DOI] [PubMed] [Google Scholar]
- 24.Watkins K, Connell CM. Measurement of health-related QOL in diabetes mellitus. Pharmacoeconomics. 2004;22:1109–1126. doi: 10.2165/00019053-200422170-00002. [DOI] [PubMed] [Google Scholar]
- 25.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHorney CA, Bricker DE, Robbins J, Kramer AE, Rosenbek JC, Chignell KA. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults. II. Item reduction and preliminary scaling. Dysphagia. 2000;15:122–133. doi: 10.1007/s004550010013. [DOI] [PubMed] [Google Scholar]
- 27.McHorney CA, Robbins J, Lomax K, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults. III. Documentation of reliability and validity. Dysphagia. 2002;17:97–114. doi: 10.1007/s00455-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 28.Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4:241–248. doi: 10.1007/BF02260863. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 30.Eslick GD, Talley NJ. Dysphagia: epidemiology, risk factors and impact on quality of life—a population-based study. Aliment Pharmacol Ther. 2008;27:971–979. doi: 10.1111/j.1365-2036.2008.03664.x. [DOI] [PubMed] [Google Scholar]
- 31.Hillemacher T, Grassel E, Tigges S, et al. Depression and bulbar involvement in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:245–249. doi: 10.1080/14660820410021294. [DOI] [PubMed] [Google Scholar]
- 32.Bretan O, Henry MA, Kerr-Correa F. Dysphagia and emotional distress. Arq Gastroenterol. 1996;33:60–65. [PubMed] [Google Scholar]
- 33.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23:297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins JA, Logemann JA, Kirshner HS. Swallowing and speech production in Parkinson’s disease. Ann Neurol. 1986;19:283–287. doi: 10.1002/ana.410190310. [DOI] [PubMed] [Google Scholar]


