Abstract
Study of specific target protein expression is often performed by western blotting, a commonly used method to measure the protein expression in neuroscience research by specific antibodies. Housekeeping proteins are used as an internal control for protein loading as well as reference in the western blotting analysis. This practice is based on the belief that such housekeeping genes are considered to be ubiquitously and constitutively expressed in every tissue and produce the minimal essential transcripts necessary for normal cellular function. The most commonly used housekeeping proteins are β-actin, β-tubulin, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). However, recent studies have shown significant variation in some housekeeping genes both at the mRNA and protein levels in various neuropathological events, such as spinal cord injury and Alzheimer's diseases. Changes of housekeeping genes are also induced by non-neuronal diseases in various tissues. Therefore, these discoveries raise a potential concern regarding whether using a housekeeping protein as an internal standard for target protein analysis is an appropriate practice. This mini review will focus on (I) the effects of neuronal and non-neuronal diseases, experimental condition, and tissues-specific roles on alteration of housekeeping genes, and (II) alternative internal standards for gene and protein expression analysis.
Keywords: β-actin, β-tubulin, GAPDH, protein staining, internal reference, diseases, age, tissue-specificity
Introduction
Analysis of specific neuronal associated protein expression is often performed by western blotting, a commonly used method to semiquantitatively measure the protein expression of target proteins by specific antibodies. Because the expression levels of specific target proteins are estimated by relative densities, which are based on the assumption that samples are loaded with the same amount of proteins, it is always necessary to control equal protein loading which is often done by re-probing the western blot membrane with an antibody that recognize a housekeeping protein, such as β-actin, β-tubline and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The housekeeping proteins are used as reference proteins to normalize the target protein during western blotting analysis. Therefore, to accurately compare western blotting signals, one must compensate for these non-sample-related variations in signal intensity. However, increasing evidence shows that the housekeeping proteins are subject to change in many biological conditions, such as neuronal diseases, tissues type, as well as under some specific experimental conditions (figure 1). Furthermore, those housekeeping proteins were also altered under some drug and experimental treatments and conditions [Aldridge et al. 2008; Greer et al. 2010], cell cycle phase, differentiation [Said et al. 2009] or proliferation status, and age [Lowe et al. 2000]. These findings raise a potentially serious question on whether using housekeeping genes as an internal standard reference indeed results in the description of “false-positive” differences in our “standard” assay.
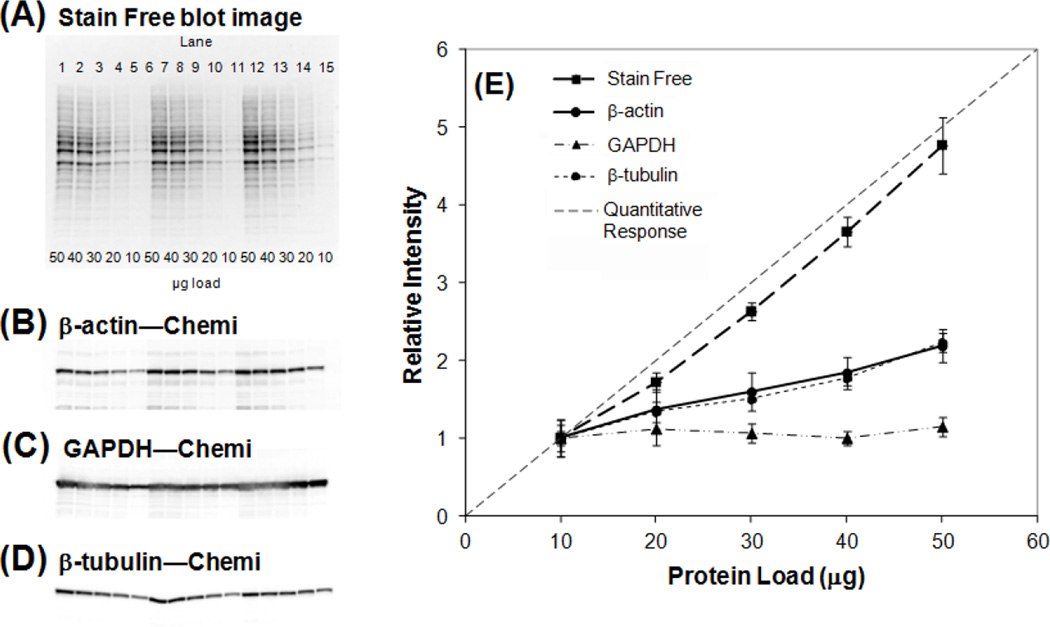
Figure 1.
Stain free total protein measurement reflects the difference in protein load better than the housekeeping proteins blotting signals. Hela cell lysate was loaded at 10, 20, 30, 40, or 50 µg per lane on a Bio-Rad stain free gel, this serial dilution was repeated 6 times in each experiment and the experiment was repeated three times. For each blot, a stain free image (A) and a chemiluminescent image of a house keeping protein such as β-actin (B), GAPDH (C), or beta-tubulin (D) was taken. The image of total protein from staining free gel and the housekeeping protein blotting signals in each lane was measured using Image Lab software following the manufacture’s instruction. The average and the standard deviation of these measurements from 6 repeats were plotted in the graph (E). The intensity of the protein bands or total protein measurement for the lane loaded with 10 µg of the Hela cell lysate was normalized to 1. The dotted line indicates the quantitative response curve where the relative intensity from the lanes with 50 µg of protein load was expected to be 5.
β-actin / β-tubulin / glyceraldehyde 3-phosphate dehydrogenase (GAPDH) – the most commonly used housekeeping proteins
The most commonly used housekeeping gene-coded proteins are β-actin, β-tubulin and GAPDH. Those housekeeping proteins are used as internal controls in the western blot analysis with presumed stability and no changes in physiological condition. However, if these housekeeping proteins DO change in certain biological or pathological condition, then using these housekeeping genes as internal controls may cause problems in data acquisition, analysis, and interpretation.
1. Pathology related cytoskeleton protein changes
Variability of these housekeeping genes has been found in various neuronal diseases and pathological states. For example, studies demonstrated changes of β-actin and DAPDH in various pathological conditions such as in the brain after injury [Liu and Xu 2006] or under neuropathological conditions [Bauer et al. 2009; Moehle et al 2012]. Studies showed that spinal cord injury induced more than a 2 fold increase in β-actin expression while no statistically significant difference was found in β- tubulin expression after the injury compared with sham-operated controls [Liu and Xu 2006]. The variability of housekeeping gene expression after nerve injury was also reported by other independent research groups, that compared β-actin and β-tubulin, GAPDH as the one most stable housekeeping genes for normalizing gene expression for qRT-PCR analysis in the context of peripheral nerve injury [Bangaru et al. 2012]. In contrast, β-actin along with GAPDH are found suitable as western blot loading controls for human postmortem studies of schizophrenia as there was no differences in the expression of those housekeeping proteins between schizophrenia and comparison groups [Bauer et al. 2009], although a recent study demonstrated that β-tubulin protein levels were decreased in the anterior cingulated cortex, increased in the dorsolateral prefrontal cortex and saw no change in the hippocampus in schizophrenia [Moehle et al. 2012], suggesting a disease-associated major cytoskeleton protein. In a study of Alzheimer' diseases (AD), an extremely low expression of GAPDH and β-actin have been reported in AD cases compared to controls [Gebhardt et al. 2010].
The housekeeping genes were affected in other non-neuronal tissues such as placentas from preeclampsia or gestational diabetes mellitus as part of cellular processes in their pathogenesis [Lanoix et al. 2012; Redman and Sargent 2009]. As shown in table 1, Dr. Ferguson and his colleagues study the variability of the β-actin, β-tubulin and GAPDH in number of different established renal cancer cell lines, matched pairs of renal tumor and normal kidney lysates as well as nine different human tissues. They reported that β-actin shows the most variation between lines while all three housekeeping genes are increased in kidney tumor tissue compared to normal kidney. [Ferguson et al. 2005]. Cells transfected with the Von Hippel Lindau (VHL) showed reduction of GAPDH which might be caused by interaction between VHL and hypoxia-induced factor-α gene [Ferguson et al. 2005; Lu et al. 2002]. Recent liver studies also found that β-actin and GAPDH tended to decrease with steatosis and to increase with alcoholic hepatitis while other internal references such as 18S and SFRS4 levels were not significantly changed in the alcohol-induced liver histological lesions [Delbos et al. 2012].
Table 1.
Summary of biological event-related changes in housekeeping genes
| β–αχτιν | GAPDH | β–τυβυλιν | ||
|---|---|---|---|---|
| Pathological condition | in kidney tumor | in alcoholic hepatitis | in anterior cingulated | |
| (Ferguson et al. 2005) | in steatosis | cortex, in dorsolateral | ||
| In spinal injury | (Delbos et al. 2012) | prefrontal cortex of | ||
| (Liu and Xu 2006) | in Alzheimer’s brain | Schizophrenia brain | ||
| in alcoholic hepatitis | (Gebhardt et al. 2010) | (Moehle et al. 2012) | ||
| in steatosis | ||||
| (Delbos et al. 2012) | ||||
| in Alzheimer’s brain | ||||
| (Gebhardt et al. 2010) | ||||
| Experimental condition | in miRNA treatment | in VHL transfection | ||
| (Sikland et al. 2012) | (Ferguson et al. 2005, Lu et al. | |||
| Extensive variable in qPCR | 2002) | |||
| (Albershardt et al. 2012) | in miRNA treatment | |||
| No change with cell confluence | (Sikland et al. 2012) | |||
| (10–100%) | Extensive variable in qPCR | |||
| (Greer et al. 2011) | (Albershardt et al. 2012) | |||
| Unreliable for total loading | with cell confluence (10 – 100%) | |||
| protein 1.8–7.5 µg No signal | (Greer et al. 2011) | |||
| <0.12 µg of total loading protein | Unreliable for total loading | |||
| (Dittmer and Dittmer 2006) | protein 0.9–7.5 µg. No signal | |||
| <0.94 µg of total loading protein | ||||
| (Dittmer and Dittmer 2006) | ||||
| Tissue-specific | with age in leukocytes | Unstable in adipose | Unstable in adipose | |
| condition | (Yu et al. 2011) | tissue | tissue | |
| (Pérez-Pérez et al. 2012) | (Pérez-Pérez et al. 2012) | |||
| in rat muscle with age | with age in leukocytes | |||
| (Lowe et al. 2000) | (Yu et al. 2011) | |||
2. Experimental condition related variation of housekeeping protein changes
One of the biggest challenges that Western blotting faces is to detect a target protein that is being expressed at very low levels. Increasing loading proteins is a commonly used practice. Our studies found that the β-actin antibody failed to detect actin levels when higher total protein loads are needed for the detection of low-abundance proteins (figure 1). Our data is consistent with other publications as Dr. Dittmer found that signal intensity as generated by the anti-GAPDH antibody again gradually decreased from 7.5 to 0.94 µg of total protein loaded, while no signal could be observed below 0.94 µg. On the other hand, β-actin showed no linear change in band intensity in the range of 7.5–1.88 µg of total protein and the signal was undetectable at 0.12 µg or lower [Dittmer and Dittmer, 2006]. In concert with the Dr. Dittmer’s finding, a group from Canada also observed that cell confluence significantly affects the levels of α-actin and GAPDH while the levels of β-actin remained unchanged at a wide range of cell densities, suggesting β-actin is a more reliable loading control for cell culture study [Greer et al. 2010]. However, in many experimental conditions, the housekeeping proteins remain unchanged. For example, studies of 6 different tumor cell lines under hypoxic stimulation, found no change of GAPDH in all of the tested cancer cell lines, including Hep-3-B, HepG2 human hepatocellar carcinoma cell lines, human lung adenocarcinoma epithelial cell line (A-549), Ht-29 and HCT-116 colon cancer cell lines even under hypoxia [Said et al. 2009]. Quantitative western blot analysis with chemiluminescence is a common method to compare protein expression in various biological samples. Given that housekeeping protein bands often appear very thick and dark in intensity on many published papers, it is possible that the chemiluminescence signal of the housekeeping protein can be easily saturated in this range and loses the credibility for quantization [Colella et al. 2012; Suzuki et al. 2011]. Recent studies also reported that microRNA inhibition or over-expression in vitro might also regulate β-actin and GAPDH expression [Sikand et al. 2012], suggesting β-actin and GAPDH should not be used as an internal control for normalization of microRNA targeted mRNA expression. This observation is further confirmed by other independent research groups in mouse lymphocytes by reporting that β-actin is not a suitable reference gene in qPCR analysis due to its extensive variability in expression [Albershardt et al. 2012].
3. Tissue specific alteration of housekeeping genes
Several studies indicate that levels of some of the most commonly used housekeeping genes change based on tissue type. For example, a study of human adipose tissue samples from obese and non-obese subjects demonstrated that neither GAPDH nor β-tubulin are at adequate standards in protein expression studies on adipose tissue, while β-actin showed steady expression levels between non-obese and obese individuals [Pérez-Pérez et al. 2012]. Moreover, a recent study determined the eight housekeeping genes, including β-actin, ribosomal protein L17 (RPL17), α-tubulin (TUBA), elongation factor-1-α(EF1A), β-2-Microglobulin (B2M), RNA polymerase II subunit D (RPSD), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and 18S ribosomal RNA (18S rRNA), as internal standards for qRT-PCR analysis of gene expression in turbot before and after bacterial challenge as a function of bacterial infection. They detected these housekeeping genes in the liver, spleen, kidney, gill, heart and brain and reported that RPSD is the most stable gene across tissue types under normal physiological conditions and during bacterial infection, while β-actin might be used as an internal control for the normalization of gene expression in immune relevant organs [Dang and Sun 2011]. Most importantly, their studies suggested that no single gene or single pair of genes among the 8 housekeeping genes can serve as a universal reference across all tissue types under the condition of bacterial infection.
In addition to tissue variation, the three commonly used housekeeping proteins showed different variability between ages. For example, studies of peripheral blood cells found age-related different expression level of β-actin and β-tubulin in leukocyte subpopulations while GAPDH maintains no change during development [Yu et al. 2011]. The age-associated change of housekeeping proteins is also found in rat muscles [Lowe et al. 2000].
Other options for internal reference controls
Due to the nature of western blot analysis, in order to estimate the specific protein concentration, all samples have to be loaded with same amount of total protein and the total protein amount should be confirmed before and after gel transferring using housekeeping genes as internal references or protein loading controls. Since using a single housekeeping protein as the loading control, such as GAPDH and β-actin does not always accurately reflect differences in protein concentration in many cases, using total protein staining instead of a single-protein loading control as alternative sample loading control becomes more acceptable practice during the past decade. Here are a few methods to detect total proteins in addition to the colorimetric assays, such as the Bicinchoninic Acid (BCA) assay.
1. Reversible Ponceau S staining
While β-actin showed variability under various physiological and pathological conditions, studies using Ponceau S staining after transferring showed great correlation between ponceau and β-actin densitometroc signals in rat colon, kidney and liver samples [Romero-Calvo et al 2010]. Moreover, the analysis of the densitometric quantitation of the Ponceau S staining and β-actin signals at protein loading ranges from 10 to140 µg show an enhanced linearity in the Ponceau S staining at higher levels of protein loading than β-actin. The advantage of Ponceau S staining is that the staining does not rely on a single protein for normalization or loading control, especially when the housekeeping proteins may vary in some conditions or get saturated while loading the amount which is necessary for detecting low-expression target products. However, when comparing this method to other protein staining methods such as western blot, Ponceasu S staining has lower sensitivity with regards to a minimum amount in detecting proteins, only 200 ng, while Amido Black and Coomassie Blue can detect proteins when only 50 ng are loaded [Harper and Speicher 2001].
2. Coomassie Blue staining
is another method to stain total protein as an internal standard in the western blot. Recent studies showed that Coomassie Blue staining can also be used on membranes to detect total protein as a loading control after transferring and even after immunoblotting. However, this is not a reversible staining and the protein bands can be further analyzed by peptide mass fingerprinting [Welinder and Ekblad 2011].
3. SYPRO Ruby and Amido Black gel staining
Sypro Ruby and Amido Black staining are two traditional methods of protein gel detection. The Sypro Ruby family of fluorescent dyes has high sensitivity and broad linear dynamic ranges. Studies showed that both staining have detection sensitivity around 50 ng/protein band [Harper and Speicher 2001; Ren et al. 2012]. The sensitivity of newly developed “visible Sypro” staining is compatible with mass spectrometry [Yang et al. 2011] such as detection protein range improving up to 2.5 ng on SDS-PAGE gel. The Sypro Ruby and Amido Black staining showed much better linearity with various protein loading amounts (up to 40 µg protein) than β-actin and GAPDH [Aldridge et al. 2008]. While reversible Ponceau S has a low-sensitivity in general protein stain and most of gels after classical Sypro Ruby and Amido Black staining are no longer able to be used for target protein blotting, recent modified Sypro Ruby- and Flamingo-staining methods provide an application of completed western blotting after gel staining [Hagiwara et al. 2010], suggesting a potential usage of Sypro Ruby and Amido Black for protein loading control in western blot.
4. Staining free gel
One of the most recently developed technologies on detecting total protein as internal reference for western blotting is a staining free method using a pre-stained gel and measured by UV detection [Gurtler et al. 2012]. Investigation of the staining free gel under 5 different conditions such as post-transfer; post-blocking, first antibody detection, post-stripping and re-blocking, and second antibody detection, showed the quantification of protein load was linear from 10–40 µg total protein in all tested conditions [Colella et al. 2012]. Interestingly enough, we compared the traditional housekeeping proteins with the free staining gel and showed a better stable and less variability of protein loading by using staining free gel than regular β-actin, β-tubulin and GAPDH as showed in figure 1. The staining free gel method has been used as loading control in several recent publications [Mollica et al. 2012; Larkins et al. 2012; Elliott et al. 2012]. However, further investigations are needed to verify whether the advantage of staining free gel method on protein loading control could be applied to various diseases conditions.
Conclusion
The Western blot method is widely used for the study of the relative expression of interest targeting proteins by specific antibodies. Housekeeping proteins such as β-actin, β-tubulin and GAPDH are used most often as protein loading controls or internal references to normalize the target protein expression. For a long time, these housekeeping proteins were believed to express in every tissue ubiquitously and constitutively with minimal essential transcripts necessary for normal cellular function. During the past decade, increasing evidence shows that using a single housekeeping protein as a loading control might lead to a pseudo-positive or false negative expression of target proteins because of the instability and variability of the housekeeping proteins under many biological and pathological conditions. Recent studies suggest that total protein detection is more reliable and sensitive protein loading control or internal reference for study relative expression of interesting proteins. At the same time, the use of housekeeping proteins as internal standards should be examined carefully in relation to the cell or tissue types, the experimental conditions and disease state.
Acknowledgements
This work was supported by grants from the Alzheimer’s Association IIRG-07-59510 and IIRG-09-61521, American Health Assistance Foundation Grant G2006-118, and National Institute of Health (NIH R01AG032441-01, R01AG025888-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Albershardt TC, Iritani BM, Ruddell A. Evaluation of reference genes for quantitative PCR analysis of mouse lymphocytes. J Immunol Methods. 2012;384(1–2):196–199. doi: 10.1016/j.jim.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172(2):250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangaru ML, Park F, Hudmon A, McCallum JB, Hogan QH. Quantification of gene expression after painful nerve injury: validation of optimal reference genes. J Mol Neurosci. 2012;46(3):497–504. doi: 10.1007/s12031-011-9628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Expression of four housekeeping proteins in elderly patients with schizophrenia. J Neural Transm. 2009;116(4):487–491. doi: 10.1007/s00702-008-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella AD, Chegenii N, Tea MN, Gibbins IL, Williams KA, Chataway TK. Comparison of Stain-Free gels with traditional immunoblot loading control methodology. Anal Biochem. 2012;430(2):108–110. doi: 10.1016/j.ab.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Dang W, Sun L. Determination of internal controls for quantitative real time RT-PCR analysis of the effect of Edwardsiella tarda infection on gene expression in turbot (Scophthalmus maximus) Fish Shellfish Immunol. 2011;30(2):720–728. doi: 10.1016/j.fsi.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Delbos L, Cassard-Doulcier AM, Njiké-Nakseu M, Maitre S, Prévot S, Dagher I, Agostini H, Voican CS, Emilie D, Perlemuter G, Naveau S. Housekeeping gene variability in the liver of alcoholic patients. Boujedidi H, Bouchet-Alcohol Clin Exp Res. 2012;36(2):258–266. doi: 10.1111/j.1530-0277.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- Dittmer A, Dittmer J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27(14):2844–2845. doi: 10.1002/elps.200500785. [DOI] [PubMed] [Google Scholar]
- Elliott S, Busse L, Swift S, McCaffery I, Rossi J, Kassner P, Begley CG. Lack of expression and function of erythropoietin receptors in the kidney. Nephrol Dial Transplant. 2012;27(7):2733–2745. doi: 10.1093/ndt/gfr698. [DOI] [PubMed] [Google Scholar]
- Ferguson RE, Carroll HP, Harris A, Maher ER, Selby PJ, Banks RE. Housekeeping proteins: a preliminary study illustrating some limitations as useful references in protein expression studies. Proteomics. 2005;5(2):566–571. doi: 10.1002/pmic.200400941. [DOI] [PubMed] [Google Scholar]
- Gebhardt FM, Scott HA, Dodd PR. Housekeepers for accurate transcript expression analysis in Alzheimer's disease autopsy brain tissue. Alzheimers Dement. 2010;6(6):465–474. doi: 10.1016/j.jalz.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Greer S, Honeywell R, Geletu M, Arulanandam R, Raptis L. Housekeeping genes; expression levels may change with density of cultured cells. J Immunol Methods. 2010;355(1–2):76–79. doi: 10.1016/j.jim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Gürtler A, Kunz N, Gomolka M, Hornhardt S, Friedl AA, McDonald K, Kohn JE, Posch A. Stain-Free technology as a normalization tool in Western blot analysis. Anal Biochem. 2012 Oct 19; doi: 10.1016/j.ab.2012.10.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Kobayashi K, Tadokoro T, Yamamoto Y. Application of SYPRO Ruby- and Flamingo-stained polyacrylamide gels to Western blot analysis. Anal Biochem. 2010;397(2):262–264. doi: 10.1016/j.ab.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Harper S, Speicher DW. Detection of proteins on blot membranes. Curr Protoc Protein Sci. 2001;Chapter 10(Unit 10.8) doi: 10.1002/0471140864.ps1008s00. [DOI] [PubMed] [Google Scholar]
- Larkins NT, Murphy RM, Lamb GD. Influences of temperature, oxidative stress, and phosphorylation on binding of heat shock proteins in skeletal muscle fibers. Am J Physiol Cell Physiol. 2012;3(6):C654–C665. doi: 10.1152/ajpcell.00180.2012. [DOI] [PubMed] [Google Scholar]
- Lanoix D, St-Pierre J, Lacasse AA, Viau M, Lafond J, Vaillancourt C. Stability of reference proteins in human placenta: general protein stains are the benchmark. Placenta. 2012;33(3):151–156. doi: 10.1016/j.placenta.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Liu NK, Xu XM. Beta-tubulin is a more suitable internal control than beta-actin in western blot analysis of spinal cord tissues after traumatic injury. J Neurotrauma. 2006;23(12):1794–1801. doi: 10.1089/neu.2006.23.1794. [DOI] [PubMed] [Google Scholar]
- Lowe DA, Degens H, Chen KD, Always SE. Glyceraldehyde-3-phosphate dehydrogenase varies with age in glycolytic muscles of rats. J Gerontol A Biol Sci Med Sci. 2000;55(3):B160–B164. doi: 10.1093/gerona/55.3.b160. [DOI] [PubMed] [Google Scholar]
- Lu S, Gu X, Hoestje S, Epner DE. Identification of an additional hypoxia responsive element in the glyceraldehyde-3-phosphate dehydrogenase gene promoter. Biochim Biophys Acta. 2002;1574(2):152–156. doi: 10.1016/s0167-4781(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Moehle MS, Luduena RF, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Regional differences in expression of β-tubulin isoforms in schizophrenia. Schizophr Res. 2012;135(1–3):181–186. doi: 10.1016/j.schres.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J Physiol. 2012;590(Pt 6):1443–1463. doi: 10.1113/jphysiol.2011.224535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez R, López JA, García-Santos E, Camafeita E, Gómez-Serrano M, Ortega-Delgado FJ, Ricart W, Fernández-Real JM, Peral B. Uncovering suitable reference proteins for expression studies in human adipose tissue with relevance to obesity. PLoS One. 2012;7(1):e30326. doi: 10.1371/journal.pone.0030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Ren G, Okerberg CK, Mathews ST. Ultrasensitive protein detection and imaging: comparison of Lumitein™, ProteoSilver™, SYPRO(®) Ruby, and Coomassie (®) Brilliant Blue gel stains. Methods Mol Biol. 2012;869:621–632. doi: 10.1007/978-1-61779-821-4_57. [DOI] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401(2):318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Said HM, Polat B, Hagemann C, Anacker J, Flentje M, Vordermark D. Absence of GAPDH regulation in tumor-cells of different origin under hypoxic conditions in - vitro. BMC Res Notes. 2009;2:8. doi: 10.1186/1756-0500-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand K, Singh J, Ebron JS, Shukla GC. Housekeeping gene selection advisory: glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin are targets of miR-644a. PLoS One. 2012;7(10):e47510. doi: 10.1371/journal.pone.0047510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki O, Koura M, Noguchi Y, Uchio-Yamada K, Matsuda J. Use of sample mixtures for standard curve creation in quantitative western blots. Exp Anim. 2011;60(2):193–196. doi: 10.1538/expanim.60.193. [DOI] [PubMed] [Google Scholar]
- Welinder C, Ekblad L. Coomassie staining as loading control in Western blot analysis. J Proteome Res. 2012;10(3):1416–1419. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang J, Bu D, Zhang L, Li S, Zhou L, Wei H. A fluorescencebased Coomassie Blue protocol for twodimensional gel-based proteomics. Biotechnol Lett. 2011;33:119–121. doi: 10.1007/s10529-010-0404-8. [DOI] [PubMed] [Google Scholar]
- Yu HR, Kuo HC, Huang HC, Huang LT, Tain YL, Chen CC, Liang CD, Sheen JM, Lin IC, Wu CC, Ou CY, Yang KD. Glyceraldehyde-3-phosphate dehydrogenase is a reliable internal control in Western blot analysis of leukocyte subpopulations from children. Anal Biochem. 2011;413(1):24–29. doi: 10.1016/j.ab.2011.01.037. [DOI] [PubMed] [Google Scholar]