Abstract
Sphingosine-1-phosphate (S1P) is a bioactive lipid mediator involved in many biological actions, including vascular homeostasis and immune cell trafficking. S1P activity is mediated by specific G protein-coupled receptors, leading to multiple physiological responses including adherens junction formation in endothelial cells. Here, we describe bioassays for rapidly assessing S1P activity in biological fluids based on ligand-induced receptor internalization in transfected HEK293 cells and consequent adherens junction formation of vascular endothelial cells.
Keywords: Bioassay, Sphingosine-1-phosphate, Receptor, GFP, Internalization, Adherens junction, Immunofluorescence staining
1. Introduction
Sphingosine-1-phosphate (S1P) is a pleiotropic lipid mediator produced from sphingomyelin by the sequential enzymatic actions of sphingomyelinase, ceramidase, and sphingosine kinase (1, 2). S1P is enriched in blood and lymph in the submicromolar range, whereas S1P in interstitial fluids of tissues is much lower, creating a steep S1P gradient (1). This vascular S1P gradient is utilized to regulate trafficking of immune cells, such as lymphocytes, hematopoietic progenitor cells, and dendritic cells (1, 3–6). S1P also plays important roles in vessel maturation, angiogenesis, and vascular permeability both in the developmental stages and in the adult (1, 7). S1P is also involved in cancer (8). Thus, it is critical to know when and where S1P is produced for better understanding of its functions.
Several methods to measure S1P levels have been developed utilizing thin-layer chromatography (9, 10), high-performance liquid chromatography (11–15), or liquid chromatography-mass spectrometry (16, 17). Although these methods can provide reasonable values of S1P concentration, they usually include specialized and time-consuming procedures, such as radiolabeling, S1P extraction from crude samples, and derivatization.
In this chapter, we describe bioassays to rapidly assess S1P activity in biological fluids based on the functions of a specific receptor for S1P. Biological functions of S1P are mediated by cell surface G protein-coupled receptors (18). Among five receptors identified so far (S1P1-S1P5), the prototypical S1P1 receptor is well characterized. S1P1 is rapidly internalized upon ligand stimulation via the endosomal pathway and gradually recycled back to the plasma membrane in HEK293 cells (19). Activation of S1P1 evokes several intracellular signaling cascades leading to proliferation, NO production, rearrangement of actin cytoskeleton, and formation of adherens junctions in endothelial cells (1, 20). Based on these observations, we describe here S1P1 internalization assay and visualization of adherens junction as tools for assessing S1P activity, utilizing the GFP fluorescence fused to the C terminus of the receptor and the standard technique of immunofluorescence staining of VE-cadherin, respectively. Although these bioassays give only rough estimates of S1P activity, the specificity for S1P and the simplicity of the procedures provide good opportunities as an initial assessment of S1P activity. These assays can be also applied for the development of agonist/antagonist of S1P1 receptor.
2. Materials
HEK293 cells.
Dulbecco's modified Eagle medium (DMEM).
Fetal bovine serum (FBS).
Human umbilical vein endothelial cells (HUVECs, passage 4–10).
Medium 199 (M199).
Phosphate-buffered saline (PBS).
Fibronectin solution: 50 μ g/ml in PBS.
Heparin.
Endothelial cell growth supplement (Biomedical Technologies, Inc.).
Expression vector for mammalian cells (see Note 1).
Lipofection reagent.
Charcoal (see Note 2).
Syringe filters, 0.45 and 0.2 μ m pore.
35-mm glass-bottom dishes.
4% Paraformaldehyde solution (see Note 3).
Permeabilization solution: 0.2% Triton X-100 in PBS.
Blocking solution: 2% bovine serum albumin and 0.1% Triton X-100 in PBS.
Anti-VE-cadherin antibody (see Note 4).
Fluorescent dye-conjugated secondary antibody (see Note 5).
Rhodamine phalloidin (see Note 6).
Nuclear staining dye (see Note 7).
Confocal microscope.
3. Methods
3.1. Cell Culture
HEK293 cells are cultured in DMEM supplemented with 10% FBS. HUVECs are cultured on fibronectin-coated dishes in M199 supplemented with 10% FBS, 50 μ g/ml endothelial cell growth supplement, and 5 U/ml heparin. Cells are maintained at 37°C in a humidified 5% CO2 incubator.
3.2. Preparation of HEK293 Cells Stably Expressing S1P1-GFP Fusion Protein (293-S1P1-GFP Cells)
Create an expression vector for S1P1-GFP fusion protein. C-terminal termination codon of S1P1 should be deleted. Transfect HEK293 cells with the S1P1-GFP expression vector by lipofection method according to the manufacturer's instructions. After selection by an antibiotic of choice, isolate several individual clones by limiting dilution method. Further, select the clones that show good surface localization of S1P1 by fluorescent microscopy.
3.3. Preparation of Charcoal-Stripped FBS
Because S1P content is high in serum, FBS should be treated with charcoal to remove S1P.
Take 2.5 g of activated charcoal into a 50-ml conical tube.
Wash the charcoal with distilled water three times.
Add 25 ml FBS to the tube.
Rotate overnight at 4°C.
Centrifuge at 1,200 × g for 15 min.
Filter the supernatant with syringe filters twice, 0.45-μ m pore at first followed by 0.2-μ m pore.
Store the charcoal-stripped FBS at 4°C until use (see Note 8).
3.4. Receptor Internalization Assay
Coat 35-mm glass-bottom dishes with the fibronectin solution for at least 10 min at room temperature (see Note 9).
Prepare the suspension of 293-S1P1-GFP cells at the density of 1.5 × 105/ml in DMEM containing 2% charcoal-stripped FBS (see Note 10).
Aspirate the fibronectin solution from the dishes, and add 1 ml of the cell suspension to each dish.
Incubate the dishes overnight in a CO2 incubator.
Replace the medium with plain DMEM for serum starvation (see Note 11).
Incubate for 2 h in a CO2 incubator.
Aspirate the medium, and add the solution of interest to the dishes (see Note 12).
Incubate for 1 h in a CO2 incubator (see Note 13).
Fix the cells with 1 ml/dish of 4% paraformaldehyde solution for 15 min at room temperature (see Note 3).
Wash the cells twice with PBS (see Note 14).
Observe the cells with a confocal microscope (Fig. 1).
Fig. 1.
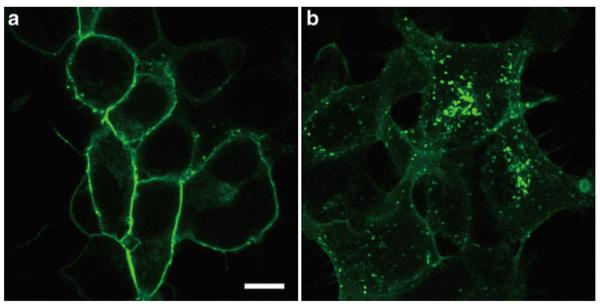
293-S1P1-GFP cells were stimulated with control solution (a) or 100 nM S1P for 1 h (b). Scale bar, 10 μ m.
3.5. Adherens Junction Formation
3.5.1. Preparation and Stimulation of Cells
Coat 35-mm glass-bottom dishes with the fibronectin solution for at least 10 min at room temperature.
Prepare the suspension of HUVEC at the density of 1 × 105/ml in M199 containing 1% charcoal-stripped FBS (see Note 15).
Aspirate the fibronectin solution from the dishes, and add 1 ml of the cell suspension to each dish.
Incubate the dishes overnight in a CO2 incubator.
Replace the medium with plain M199 (see Note 11).
Incubate for 2 h in a CO2 incubator.
Aspirate the medium, and add the solution of interest to the dishes (see Note 12).
Incubate for 1 h in a CO2 incubator (see Note 13).
Fix the cells with 1 ml/dish of 4% paraformaldehyde solution for 15 min at room temperature (see Note 3).
Wash the cells twice with PBS (see Note 14).
3.5.2. Immunofluorescence Staining of VE-Cadherin
All procedures are carried out at room temperature.
Aspirate the PBS, and add 1 ml/dish of the permeabilization solution. Incubate for 3 min.
Aspirate the permeabilization solution, and add 1 ml/dish of the blocking solution. Incubate for 30 min.
Dilute anti-VE-cadherin antibody in the blocking solution (see Note 4).
Aspirate the blocking solution, and add 100 μ l/dish of the primary antibody solution. Incubate for 1 h.
Wash with PBS for three times.
Dilute fluorescent dye-conjugated secondary antibody in the blocking solution (see Note 5).
Add 100 μ l/dish of the secondary antibody solution. Incubate for 1 h.
-
Wash with PBS for three times.
When simultaneous visualization of cortical actin filaments and nuclei is preferable, the following procedures can be carried out before proceeding to microscopic observation.
Dilute rhodamine phalloidin in the blocking buffer (see Note 6).
Add 100 μ l/dish of the rhodamine phalloidin solution. Incubate for 20 min.
Wash with PBS for three times.
Dilute nuclear staining dye in PBS (see Note 7).
Add 100 μ l/dish of the nuclear staining solution. Incubate for 10 min.
Wash with PBS for three times.
Observe the cells with a confocal microscope (Fig. 2).
Fig. 2.
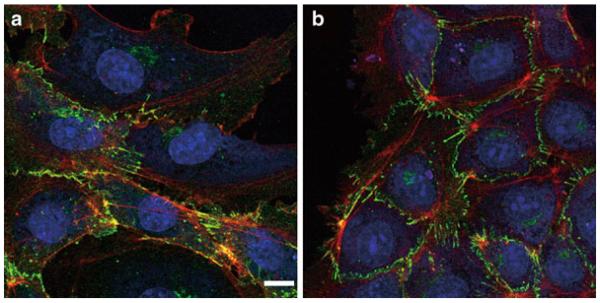
HUVECs were stimulated with control solution (a) or 100 nM S1P for 1 h (b). VE-cadherin (green), cortical actin filaments (red), and nuclei (blue) are visualized. Scale bar, 10 μ m.
4. Notes
The vector should carry an antibiotic-resistance cassette that allows transfected eukaryotic cells to be selected with an antibiotic of choice. We usually use a pcDNA3.1 vector, and select transfected cells with 0.5 mg/ml G418.
We use granular-activated charcoal (4–8 mesh), which is easier to remove than powder-form charcoal.
Freshly prepare a 4% paraformaldehyde solution.
We use goat anti-VE-cadherin antibody (C-19) from Santa Cruz at the dilution 1:200.
We use Alexa488-conjugated donkey anti-goat IgG antibody from Invitrogen at the dilution 1:1,000.
We use rhodamine phalloidin from Invitrogen at the dilution 1:500.
We use TO-PRO-3 dye from Invitrogen at the dilution 1:1,000.
Keep the charcoal-stripped FBS at −20°C for long-term storage.
Glass-bottom dishes can be coated by other types of adhesion molecules, such as collagen, gelatin, and poly l-lysine.
HEK293 cells become extremely easy to come off the dish when they form sheet-like structure. It is important to keep the cell density low and reduce the number of medium change.
Do not aspirate the entire medium, but leave the medium in the glass-bottom region to avoid cell damage and loss. Wash two to three times with plain DMEM to remove FBS.
No need to cover the whole dish when the ligand solution is precious. To cover the glass-bottom region, 100 μ l/dish is more than enough. When possible, titration analysis of the samples is preferable.
Optimal time point should be determined.
The fixed cells can be stored in PBS at 4°C for several days before proceeding to microscopic observation or immunofluorescent staining.
The cell density and serum-starvation conditions should be optimized so that cells are close enough to each other to make adherens junctions but still do not complete junction formations before stimulation.
Acknowledgment
We are grateful to Catherine H. Liu and Shobha Thangada for their efforts to establish these bioassays. This work is supported by NIH grants HL-67330 and HL-89934.
References
- 1.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007;19:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 4.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 5.Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czeloth N, Bernhardt G, Hofmann F, Genth H, Förster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 7.Kono M, Allende ML, Proia RL. Sphingosine-1-phosphate regulation of mammalian development. Biochim Biophys Acta. 2008;1781:435–441. doi: 10.1016/j.bbalip.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyne N, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 9.Yatomi Y, Ruan F, Ohta J, Welch RJ, Hakomori S, Igarashi Y. Anal Biochem. 1995;230:315–320. doi: 10.1006/abio.1995.1480. [DOI] [PubMed] [Google Scholar]
- 10.Edsall LC, Spiegel S. Enzymatic measurement of sphingosine 1-phosphate. Anal Biochem. 1999;272:80–86. doi: 10.1006/abio.1999.4157. [DOI] [PubMed] [Google Scholar]
- 11.Caligan TB, Peters K, Ou J, Wang E, Saba J, Merrill AH. A high-performance liquid chromatographic method to measure sphingosine 1-phosphate and related compounds from sphingosine kinase assays and other biological samples. Anal Biochem. 2000;281:36–44. doi: 10.1006/abio.2000.4555. [DOI] [PubMed] [Google Scholar]
- 12.Ruwisch L, Schäfer-Korting M, Kleuser B. An improved high-performance liquid chromatographic method for the determination of sphingosine-1-phosphate in complex biological materials. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:358–363. doi: 10.1007/s002100000365. [DOI] [PubMed] [Google Scholar]
- 13.Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem. 2002;303:167–175. doi: 10.1006/abio.2002.5579. [DOI] [PubMed] [Google Scholar]
- 14.Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He X, Huang CL, Schuchman EH. Quantitative analysis of sphingosine-1-phosphate by HPLC after napthalene-2,3-dicarboxaldehyde (NDA) derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:983–990. doi: 10.1016/j.jchromb.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Mano N, Oda Y, Yamada K, Asakawa N, Katayama K. Simultaneous quantitative determination method for sphingolipid metabolites by liquid chromatography/ionspray ionization tandem mass spectrometry. Anal Biochem. 1997;244:291–300. doi: 10.1006/abio.1996.9891. [DOI] [PubMed] [Google Scholar]
- 17.Bielawski J, Pierce JS, Snider J, Rembiesa B, Szulc ZM, Bielawska A. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol Biol. 2009;579:443–467. doi: 10.1007/978-1-60761-322-0_22. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 19.Liu CH, Thangada S, Lee MJ, Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okajima F, Sato K, Kimura T. Anti-atherogenic actions of high-density lipoprotein through sphingosine 1-phosphate receptors and scavenger receptor class B type I. Endocr J. 2009;56:317–334. doi: 10.1507/endocrj.k08e-228. [DOI] [PubMed] [Google Scholar]


